Abstract
Background
Selected patients with advanced non-small cell lung cancer (NSCLC) benefit from immunotherapy, especially immune checkpoint inhibitors such as PD-1 (programmed cell death protein 1) inhibitor. Peripheral blood biomarkers would be most convenient to predict treatment outcome and immune-related adverse events (irAEs) in candidate patients. This study explored associations between inflammation-related peripheral blood markers and onset of irAEs and outcome in patients with advanced NSCLC receiving PD-1 inhibitors.
Methods
A retrospective analysis was conducted of 102 patients with advanced NSCLC receiving PD-1 inhibitors from January 2017 to May 2019. Cox regression models were employed to assess the prognostic effect of low/high neutrophil/lymphocyte ratio (NLR), lactate dehydrogenase (LDH), and prognostic nutrition index (PNI) on overall survival (OS) and progression-free survival (PFS). Logistic regression models were used to analyze the correlation between peripheral blood markers and the onset of irAEs.
Result
NLR < 5, LDH < 240 U/L, or PNI ≥ 45 was favorably associated with significantly better outcomes compared with higher, higher, or lower values, respectively. The multivariate analysis determined that these parameters were independently associated with both better PFS (p = 0.049, 0.046, 0.014, respectively) and longer OS (p = 0.007, 0.031, < 0.001, respectively). Patients with three favorable factors among NLR, LDH, and PNI had better PFS and OS than did those with two, one, or none. PNI and NLR were associated with the onset of irAEs.
Conclusion
In patients with advanced NSCLC treated with PD-1 inhibitors, pretreatment NLR, LDH, and PNI may be useful predictive markers of clinical outcome and irAEs.
Keywords: Lung cancer, Immunotherapy, Immune-related adverse events, Neutrophil-to-lymphocyte ratio, Lactate dehydrogenase, Prognostic nutrition index
Introduction
Whether across the globe or in China, lung cancer is the primary cause of cancer-related deaths [1]. Non-small cell lung cancer (NSCLC) accounts for ~ 85% of cases, and a majority include distant metastasis at diagnosis.
In recent years, immunotherapy has been associated with improved long-term survival in advanced NSCLC. The treatment options of patients have vastly expanded, especially through the use immune checkpoint inhibitors [2]. However, only a minority of patients have benefited [3]. There are predictive biomarkers that may be helpful for selecting patients for immunotherapy. Among the most recently recognized are PD-L1 (programmed death-ligand 1), TMB (tumor mutational burden), and MSI-H (microsatellite instability-high). However, these markers are costly, and the testing technology is not yet mature. On the other hand, if peripheral blood markers were known, the test would be clinically convenient and practically noninvasive.
Inflammation is closely linked to cancer, as it promotes a favorable microenvironment for cancer cell growth and spread, and activation of carcinogenic signaling pathways [4]. The prognostic value of some inflammation-related peripheral blood parameters has been investigated, including the neutrophil-to-lymphocyte ratio (NLR), lactate dehydrogenase (LDH), and prognostic nutritional index (PNI). Calculation of the NLR depends on the absolute neutrophil count and the absolute lymphocyte count within the peripheral blood; some studies have shown that NLR is associated with worsened prognosis in patients with melanoma receiving immunotherapy [5–7]. Similarly, a high pretreatment NLR is putatively a poor prognostic indicator in NSCLC [8–10]. So too, high serum LDH is reportedly a risk factor of poor prognosis in patients with NSCLC [11–13].
The PNI is based on serum albumin level and total lymphocyte count. It is easily calculated in daily routine, and a simple means to assess perioperative immunological and nutritional condition and stratify the risk of postoperative complications [14]. The preoperative or pretreatment PNI status is a good prognostic indicator in various cancers, including lung [15, 16], gastric [17], and colorectal [18] cancers, and glioblastoma [19]. However, sufficient data regarding the application of the PNI in the field of cancer immunotherapy are lacking.
Although patients can better tolerate immune checkpoint inhibitors compared with traditional chemotherapy, some patients still suffer adverse events which were known as immune-related adverse events (irAEs) that may cause treatment discontinuation or even death. Markers that may predict the onset of irAEs are unknown.
This retrospective study explored associations between inflammation-related peripheral blood markers (NLR, LDH, and PNI), and outcome and onset of irAEs in patients with advanced NSCLC receiving PD-1 inhibitors.
Materials and methods
Patients
The Research Ethics Board of First Affiliated Hospital of Nanchang University approved this retrospective study. The study population comprised 102 patients with a histologically or cytologically proven diagnosis of advanced NSCLC (IIIB/IV), who were treated with anti-PD-1 antibodies at our hospital from January 2017 to May 2019. Further inclusion criteria were an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2, and ≥ 4 cycles of immunotherapy treatment. Patients with any of the following were excluded from this study: autoimmune disease; pulmonary interstitial disease; adrenal insufficiency; or systemic immunosuppression.
Treatment and data collection
Patients received the following, until tumor progression, development of unacceptable drug toxicity, withdrawal, or death: nivolumab and toripalimab (intravenously 3 mg/kg every 2 weeks); sintilimab (200 mg every 3 weeks); and pembrolizumab (2 mg/kg every 3 weeks). The patients’ clinical characteristics were collected, including age, gender, histology, sensitive gene mutation status and so on.
Baseline measurements were defined as those taken within 1 week before receiving PD-1 inhibitors. The baseline peripheral blood data including absolute neutrophil count, LDH, absolute lymphocyte count, total lymphocyte count, and serum albumin level were mostly used to compute the NLR (absolute neutrophil count divided by absolute lymphocyte count) and the PNI (10 × serum albumin value, g/dL + 0.005 × total lymphocyte count/mm3).
Study assessments
Drug efficacy was assessed every 8–12 weeks based on the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1) by computed tomography (CT) scan. The data deadline was August 2019. The Common Terminology Criteria for Adverse Events (CTCAE) of the National Cancer Institute (version 4.03) was used to assess patients’ adverse events. The irAEs were defined as adverse events that reflected a disorder of the immune system, such as rash, colitis, liver dysfunction, thyroid disorder, and other conditions. The irAEs were evaluated for 3 months, because the incidence of irAEs has been reported to be highest within the first 12 weeks [20].
Progression-free survival (PFS) was defined as the first day of immunotherapy to the date of disease progression. Overall survival (OS) was from the time of initial anti-PD-1 immunotherapy to death from any cause or last follow-up.
Statistical analysis
Patients were stratified as low- and high-NLR (< 5 and ≥ 5, respectively) [10], low- and high-LDH (< 240 and ≥ 240 U/L) [12], or low- and high-PNI (< 45 and ≥ 45) groups [21]. These cutoffs were selected according to literature references 10, 12, 21 and their median, respectively.
PFS and OS curves were calculated using the Kaplan–Meier method, and the log-rank test was employed to assess differences. Cox regression models were applied to find independent indicators associated with PFS and OS. Factors which were statistically significant in the univariate analysis were incorporated into the multivariate analysis. Logistic regression analysis was applied to explore the correlation between peripheral blood markers and the onset of irAEs. SPSS 25.0 software (SPSS, Chicago, IL) was used for all the statistical tests. A p value< 0.05 was considered statistically significant.
Results
Patient characteristics
In our study, 102 patients were enrolled who accepted at least four cycles of immunotherapy (Table 1). Every patient was administered monotherapy with PD-1 inhibitor; 19 patients accepted PD-1 inhibitors as first-line treatment. The median age was 62 years. Most were men (87/102, 85.3%); most had no or undetected sensitive gene mutations (94/102, 92.2%); and most had an ECOG performance status of 0–1 (89/102, 87.3%).
Table 1.
Patient characteristics
| Feature | N | Percentage (%) |
|---|---|---|
| Gender | 102 | 100 |
| Female | 15 | 14.7 |
| Male | 87 | 85.3 |
| Age (years) | ||
| < 60 | 39 | 38.2 |
| ≥ 60 | 63 | 61.8 |
| Histology | ||
| Adenocarcinoma | 58 | 56.9 |
| Squamous carcinoma | 42 | 41.2 |
| Large cell carcinoma | 2 | 1.9 |
| Clinical stage | ||
| IIIB | 40 | 39.2 |
| IV | 62 | 60.8 |
| ECOG PS | ||
| 0–1 | 89 | 87.3 |
| 2 | 13 | 12.7 |
| Smoking status | ||
| Never | 41 | 40.2 |
| Former/current | 61 | 59.8 |
| Line of immunotherapy | ||
| First | 19 | 18.6 |
| Second | 51 | 50.0 |
| ≥ Third | 32 | 31.4 |
| Actionable mutation | ||
| (–)/undetected | 94 | 92.2 |
| EGFR or ALK/ROS1(+) | 8 | 7.8 |
| Number of metastatic sites | ||
| < 3 | 46 | 45.1 |
| ≥ 3 | 56 | 54.9 |
| Immune adverse events | ||
| No | 63 | 61.8 |
| Yes | 39 | 38.2 |
| Type of immunotherapy | ||
| Nivolumab | 11 | 10.8 |
| Pembrolizumab | 26 | 25.5 |
| Toripalimab | 30 | 29.4 |
| Sintilimab | 35 | 34.3 |
ECOG PS Eastern Cooperative Oncology Group performance status, EGFR epidermal growth factor receptor, ALK anaplastic lymphoma kinase, ROS1 c-ros oncogene 1
Univariate and multivariate analyses of biomarkers for OS and PFS
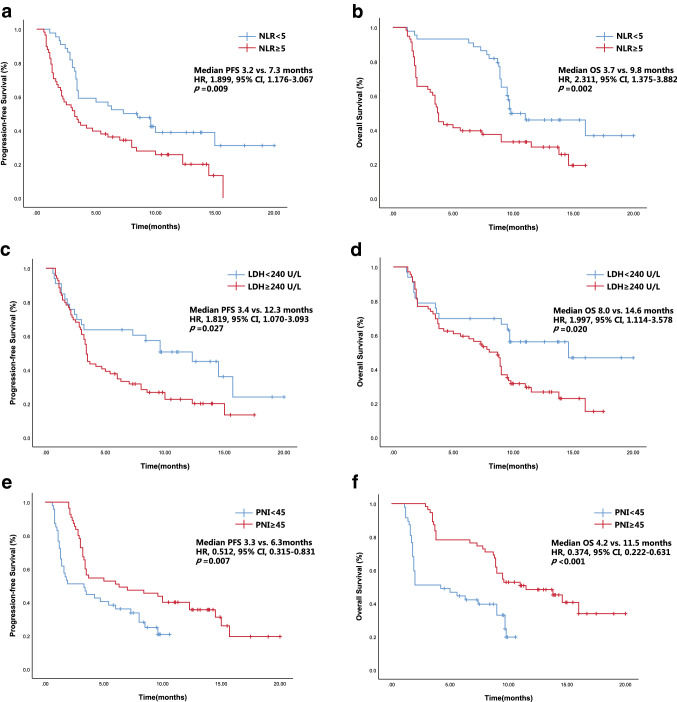
For the population overall, the median OS and PFS were 9 months and 3.7 months, respectively. According to the univariate analysis, the high-NLR group had a significantly worse median OS (3.7 months) and median PFS (3.2 months) compared with the low-NLR group (9.8 months and 7.3 months, respectively; Table 2). The high-LDH group had a significantly worse median OS (8.0 months) and median PFS (3.4 months) compared with the low-NLR group (14.6 months and 12.3 months). The high-PNI group had a significantly better median OS (11.5 months) and median PFS (6.3 months) compared with the low-PNI group (4.2 months and 3.3 months). The multivariate analysis showed that the following factors were significantly associated with OS and PFS (Table 2): NLR ≥ 5, LDH ≥ 240 U/L, and PNI ≥ 45 (Fig. 1).
Table 2.
Univariate and multivariate analyses of PFS and OS
| Variable | Category | PFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | ||||||||||
| HR | 95%CI | p value | HR | 95%CI | p value | HR | 95%CI | p value | HR | 95%CI | p value | ||
| Gender | Female | 0.780 | 0.418–1.457 | 0.436 | 0.945 | 0.493-1.810 | 0.865 | ||||||
| Age | ≥ 60 | 1.585 | 0.970–2.590 | 0.066 | 1.581 | 0.936–2.671 | 0.087 | ||||||
| Histology | Non-adenocarcinoma | 0.999 | 0.792–1.262 | 0.997 | 0.929 | 0.726–1.190 | 0.562 | ||||||
| Clinical stage | IV | 0.916 | 0.723–1.160 | 0.468 | 0.916 | 0.710–1.180 | 0.497 | ||||||
| ECOG PS | 2 | 1.503 | 0.769–2.936 | 0.233 | 1.496 | 0.738–3.036 | 0.264 | ||||||
| Smoking status | Former/Current | 1.231 | 0.977–1.551 | 0.078 | 1.240 | 0.967–1.589 | 0.090 | ||||||
| Line of immunotherapy | ≥3 | 0.957 | 0.751–1.220 | 0.722 | 0.970 | 0.747–1.261 | 0.822 | ||||||
| Actionable mutations | Undetected/(–) | 0.712 | 0.481–1.055 | 0.090 | 0.713 | 0.480–1.059 | 0.093 | ||||||
| Metastatic sites | ≥ 3 | 1.021 | 0.810–1.285 | 0.863 | 1.008 | 0.788–1.291 | 0.947 | ||||||
| Adverse events | Yes | 1.344 | 1.051–1.717 | 0.018 | 1.011 | 0.730–1.402 | 0.945 | 1.426 | 1.096–1.855 | 0.008 | 0.957 | 0.675–1.356 | 0.804 |
| NLR | ≥ 5 | 1.899 | 1.176–3.067 | 0.009 | 1.845 | 1.002–3.399 | 0.049 | 2.311 | 1.375–3.882 | 0.002 | 2.491 | 1.288–4.819 | 0.007 |
| PNI | ≥ 45 | 0.512 | 0.315–0.831 | 0.007 | 0.520 | 0.308–0.877 | 0.014 | 0.374 | 0.222–0.631 | <0.001 | 0.358 | 0.202–0.636 | <0.001 |
| LDH | ≥ 240 | 1.819 | 1.070–3.093 | 0.027 | 1.730 | 1.010–2.963 | 0.046 | 1.997 | 1.114–3.578 | 0.020 | 1.922 | 1.061–3.481 | 0.031 |
PFS progression-free survival, HR hazard ratio, CI confidence interval, ECOG PS Eastern Cooperative Oncology Group performance status, NLR neutrophil-to-lymphocyte ratio, PNI prognostic nutrition index, LDH lactate dehydrogenase
Statistically significant values are in bold (p < 0.05)
Fig. 1.
PFS (a, c, e) and OS (b, d, f) curves of patients stratified according to peripheral blood markers (NLR, LDH, and PNI)
Multifactor model for survival outcome of patients treated with PD-1 inhibitors
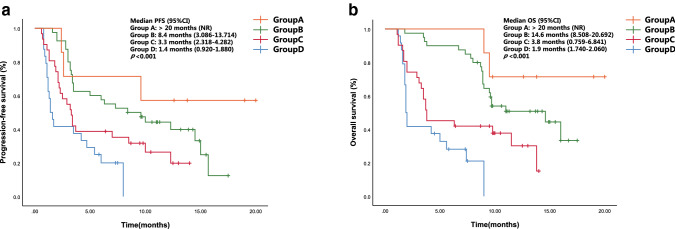
We explored the OS and PFS by the number of advantaged factors (i.e., NLR < 5, LDH < 240 U/L, and PNI ≥ 45; Fig. 2). Of the 102 patients, 24 (23.5%) had no favorable factors (group D, in Fig. 2). The OS and PFS of this group were significantly shorter than that of patients with 1, 2, or 3 of the favorable factors (groups C, B, and A; p < 0.001).
Fig. 2.
PFS (a) and OS (b) curves of the multifactor model according to the number of advantageous factors at baseline (NLR < 5, LDH < 240 U/L, and PNI ≥ 45). Abbreviation: NR, not reached
Immune-related adverse events (irAEs)
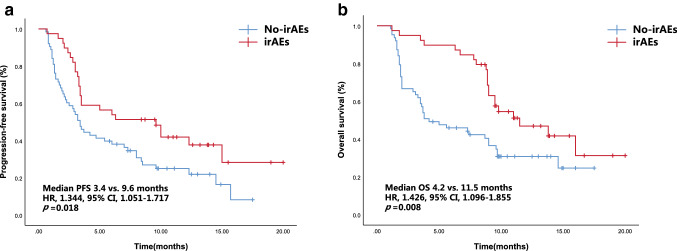
Thirty-nine patients (38.2%) experienced, in all, 6 different irAEs of any grade, and 6 patients (5.8%) experienced high-grade irAEs. The commonly seen irAEs (any grade) were rash (n = 13, 33.3%), liver dysfunctions (n = 9, 23.1%), and hypothyroidism (n = 7, 17.9%). The most common severe irAEs (grade ≥ 3) were rash (n = 2, 5.1%). The median OS of the 39 patients with irAEs (11.5 months) was significantly preferable to that of the 63 patients without irAEs (4.2 months; Table 2, Fig. 3). Similarly, the median PFS of the patients with irAEs (9.6 months) was significantly better than that of patients without irAEs (3.4 months). However, irAEs were not an independent prognostic risk factor of OS and PFS in the multivariate analysis.
Fig. 3.
PFS (a) and OS (b) curves of patients according to the onset of irAEs
The low-NLR, low-LDH, and high-PNI groups consisted of 32 (72.7%), 15 (45.5%), and 30 (54.5%) patients, respectively (Table 3). The univariate analysis indicated associations between low-NLR or high-PNI and any grade of irAEs (p < 0.001, both). The multivariate logistic regression analysis showed that high PNI (p = 0.001) and low NLR (p < 0.001) were independent predictors for the onset of irAEs.
Table 3.
Levels of the peripheral blood markers by irAE development
| Blood parameter | irAEs, n (%) | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| OR (95%CI) | p value | OR (95%CI) | p value | ||
| L-NLR (n = 44) | 32 (72.7) | 0.051(0.02–0.14) | <0.001 | 0.04 (0.01–0.13) | <0.001 |
| H-NLR (n = 58) | 7 (12.1) | 1 | 1 | ||
| L-LDH (n = 33) | 15 (45.5%) | 0.64(0.28–1.49) | 0.301 | 0.45 (0.14–1.41) | 0.169 |
| H-LDH (n = 69) | 24 (34.8%) | 1 | 1 | ||
| H-PNI (n = 55) | 30 (54.5%) | 5.07(2.06–12.4) | <0.001 | 7.61 (2.20–26.3) | 0.001 |
| L-PNI (n = 47) | 9 (19.1%) | 1 | 1 | ||
OR odds ratio, CI confidence interval, H-NLR high NLR, H-LDH high LDH, H-PNI high PNI, irAEs immune-related adverse events, NLR neutrophil-to-lymphocyte ratio, LDH lactate dehydrogenase, PNI prognostic nutrition index, L-NLR low NLR, L-LDH low LDH, L-PNI low PNI
Discussion
Although the preciseness of lung cancer treatment has improved significantly in recent years, NSCLC remains challenging. The emergence of PD-1 inhibitors has brought hope to patients with advanced NSCLC, but many clinical studies have shown that no more than 20% of patients benefit. Therefore, effective predictive biomarkers are urgently needed for screening potential beneficial groups.
PD-L1 is highly expressed on the cell membranes of NSCLC. Anti-PD-1 immunotherapy of NSCLC is designed to block the signal between PD-1 on T cells and PD-L1 on tumor cells [22]. Graves et al. [23] reported that the PD-1 level on CD4+ T cells in the blood of melanoma patients who responded to anti-PD-1 therapy was higher than that of non-responders. Currently, the PD-L1 level is a commonly used marker for predicting the efficacy of immunotherapy. As reported by CheckMate-057 [24] and Keynote-010 [25], patients with high PD-L1 levels in tumor tissues, and who received PD-1/PD-L1 inhibitors, had better survival outcomes compared with those who were not given this treatment. Nevertheless, CheckMate-017 [26] reported that patients who were PD-L1-negative also responded well. Therefore, PD-L1 level is not sufficient as the sole decisive predictor of immunotherapy. TMB is another potential predictive biomarker that has received much attention, but has been considered only as a reference marker; TMB should be explored further in clinical research. In May 2017, pembrolizumab received approval by the United States Food and Drug Association for the treatment of metastatic or advanced solid tumors with mismatch repair deficiency (i.e., high levels of microsatellite instability, or MSI-H). However, the American Society of Clinical Oncology (ASCO) reported in 2016 that MSI-H occurs in only 0.4–0.8% of lung cancer. The predictive markers discussed above are limited by cumbersome detection protocols and high cost. Hence, it is necessary to explore for markers that can effectively predict the benefit of therapy, but which are also clinically practical and without serious drug toxicity.
It has been reported that nutritional status and inflammatory status have prognostic relevance in patients with a variety of cancers [27, 28]. The markers evaluated in the present study (NLR, LDH, and PNI) reflect well the inflammation and nutritional status. The association between baseline NLR and the prognosis of melanoma patients treated with immune checkpoint inhibitors has been demonstrated [29, 30]. Bagley et al. [10] studied 175 patients with advanced NSCLC treated with nivolumab and concluded that NLR ≥ 5 at baseline was a risk factor of inferior OS (HR 1.83, 95% CI 1.2–2.8; p = 0.006) and inferior PFS (HR 1.42, 95% CI 1.02–2.0; p = 0.04), compared with NLR < 5. In the multivariate analysis, NLR ≥ 5 was also independently linked to worse outcomes. In addition, another retrospective study showed that baseline NLR > 5 was associated with poor OS [31]. In the present study, we also concluded that an NLR of 5 was the optimal cutoff: NLR ≥ 5 was associated with worse OS (HR 2.311, 95% CI 1.375–3.882; p = 0.002) and PFS (HR 1.899, 95% CI 1.176–3.067; p = 0.009). Furthermore, NLR ≥ 5 was an independent prognostic risk indictor in the multivariate analysis model.
LDH is produced by rapidly growing tumors and therefore reflects the tumor burden. LDH was found associated with outcomes in melanoma patients treated with immune checkpoint inhibitors [32, 33]. Several studies used LDH to forecast PFS [12, 34] and OS [6, 11] in patients with NSCLC treated with immune checkpoint inhibitors. Taniguchi et al. [12] discovered that among patients with advanced NSCLC treated with nivolumab, those with baseline LDH > 240 U/L had a significantly worse PFS compared with those with LDH ≤ 240 U/L. Similarly, the present analysis showed that a baseline LDH ≥ 240 U/L was associated with worse OS and PFS.
In 1984, Japanese scholars Onodera et al. [35] proposed a simplified version of the PNI, which is based only on serum albumin and total the lymphocyte count. Serum albumin concentration can indicate the nutritional status of patients and chronic inflammation. In addition to reflected inflammatory status, the lymphocyte count is also an important parameter of the immune function [36]. Several studies have reported a significant association between the PNI and the prognosis of patients with a variety of malignant cancers. However, there has been little research regarding the value of the PNI in cancer immunotherapy. In the present study, a pretreatment PNI ≥ 45 was associated with better OS (HR 0.374, 95% CI 0.222–0.631; p < 0.001) and PFS (HR 0.512, 95% CI 0.315–0.831; p = 0.007) compared with PNI < 45. In the multivariate analysis, PNI ≥ 45 was also independently associated with worse PFS and OS. An evaluation of baseline PNI may provide meaningful information for selecting suitable patients in immunotherapy.
The increasing use of immune checkpoint inhibitors has been accompanied by a rise in unique adverse events, known as irAEs, which can lead to troubling morbidity and treatment discontinuations [37]. Nakaya et al. [38] reported that patients with NSCLC treated with nivolumab and who experienced an associated irAEs had better PFS compared with those who had no irAEs. Similarly, the present analysis showed that the patients who suffered from irAEs had longer PFS and OS, although this did not rise to the level of an independent prognosis marker for PFS and OS. Moreover, the rate of irAEs in our research also resembled the study that reported by Nakaya et al. [38]. The current study also explored an association between irAEs and the peripheral blood markers NLR, LDH, and PNI and found that low NLR and high PNI were significantly associated with the onset of irAEs. So, the baseline NLR and PNI may be used as a convenient tool to identify irAEs timely, which is essential for reducing the risk of hospitalization and the costs of treatment.
The limitations of this research are its retrospective nature and relatively small sample size. Although the results of our study are interesting, the predictive value of the peripheral blood markers (NLR, LDH, and PNI) on PFS, OS or irAEs requires further validation by randomized studies with an untreated control group. Furthermore, most of the patients received PD-1 inhibitors as their second-line treatment or beyond. Thus, the degree of baseline inflammation may be affected by previous treatments, although the number of lines of immunotherapy was not associated with clinical outcomes. Additionally, the PD-L1 status was known in so few patients that we could not include in our study and was conducted using different methods. Finally, immune-related response criteria were not applied in this study, because it was not a prospective research and most physicians are unfamiliar with the criteria. Despite these limitations, a unique aspect of this study was the combined model of three baseline peripheral blood markers for the outcome of PD-1 inhibitors. In addition, to our best knowledge, it is the first study to explore a correlation between peripheral blood markers (LDH and PNI) and irAEs in patients with advanced NSCLC accepting PD-1 inhibitors, or an evaluation of the effect of irAEs on both PFS and OS.
In conclusion, the pretreatment peripheral blood markers analyzed herein (NLR, LDH, and PNI) may correlate with outcomes and the onset of irAEs in patients with advanced NSCLC accepting PD-1 inhibitors. This provides some directions for clinical research on immunotherapy of lung cancer. With the rising attention to health-related costs and precision medicine, the predictive role of peripheral blood markers in cancer immunotherapy is very meaningful. These preliminary results warrant further research.
Abbreviations
- AE
Adverse event
- ALK
Anaplastic lymphoma kinase
- CI
Confidence interval
- CT
Computed tomography
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EGFR
Epidermal growth factor receptor
- HR
Hazard ratio
- irAEs
Immune-related adverse events
- LDH
Lactate dehydrogenase
- MSI-H
Microsatellite instability-high
- NLR
Neutrophil-to-lymphocyte ratio
- NR
Not reached
- NSCLC
Non-small cell lung cancer
- OR
Odds ratio
- OS
Overall survival
- PD-1
Programmed cell death-1
- PD-L1
Programmed death-ligand 1
- PFS
Progression-free survival
- PNI
Prognostic nutrition index
- ROS1
c-ros oncogene 1
- TMB
Tumor mutational burden
Author contributions
LP, YW, FL, XQ, XZ, CF, and XQ contributed to the acquisition, analysis and interpretation of data, and statistical analysis and drafting of the manuscript. LP, YW, and YL were responsible for the study concept and design, analysis and interpretation of the data, drafting of the manuscript, and obtaining funding and study supervision. All authors read and approved the final manuscript.
Funding
This study was supported by Grants from the National Natural Science Foundation of China (Nos. 81560379, 81460292, 81660315), the Surface Project of the Natural Science Foundation of Jiangxi Province (No. 20181BAB205046), the Technology Supporting Program of Jiangxi Province (No. 2015BBG70236), the Key Project of the Education Department of Jiangxi Province (No. GJJ170012), and the Guiding Science and Technology Project of Ganzhou (No. GZ2018ZSF306).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and ethical standards
The study was performed in accordance with the Declaration of Helsinki.
Informed consent
All patients from First Affiliated Hospital of Nanchang University provided signed informed consent for the treatments performed and for the anonymous use of their clinical data for research and publication purposes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lihong Peng and Yong Wang have contributed equally to this work.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iwai Y, Hamanishi J, Chamoto K, Honjo T. Cancer immunotherapies targeting the PD-1 signaling pathway. J Biomed Sci. 2017;24:26. doi: 10.1186/s12929-017-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philip M, Rowley DA, Schreiber H. Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol. 2004;14:433–439. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol. 2018;29:524. doi: 10.1093/annonc/mdx059. [DOI] [PubMed] [Google Scholar]
- 6.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6:74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa Y, Yoshino K, Otsuka A, et al. Baseline neutrophil to lymphocyte ratio combined with serum lactate dehydrogenase level associated with outcome of nivolumab immunotherapy in a Japanese advanced melanoma population. Br J Dermatol. 2018;179:213–215. doi: 10.1111/bjd.16427. [DOI] [PubMed] [Google Scholar]
- 8.Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. 2018;6:129. doi: 10.1186/s40425-018-0447-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ, Fruh M. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Bagley SJ, Kothari S, Aggarwal C, et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Mezquita L, Auclin E, Ferrara R, et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4:351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniguchi Y, Tamiya A, Isa SI, et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res. 2017;37:5857–5862. doi: 10.21873/anticanres.12030. [DOI] [PubMed] [Google Scholar]
- 13.Kataoka Y, Hirano K, Narabayashi T, Hara S, Fujimoto D, Tanaka T, Ebi N, Tomii K, Yoshioka H. Carcinoembryonic antigen as a predictive biomarker of response to nivolumab in non-small cell lung cancer. Anticancer Res. 2018;38:559–563. doi: 10.21873/anticanres.12259. [DOI] [PubMed] [Google Scholar]
- 14.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139:160–167. doi: 10.1016/0002-9610(80)90246-9. [DOI] [PubMed] [Google Scholar]
- 15.Hong S, Zhou T, Fang W, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36:3389–3397. doi: 10.1007/s13277-014-2973-y. [DOI] [PubMed] [Google Scholar]
- 16.Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. 2017;104:296–302. doi: 10.1016/j.athoracsur.2017.01.085. [DOI] [PubMed] [Google Scholar]
- 17.Eo WK, Chang HJ, Suh J, et al. The prognostic nutritional index predicts survival and identifies aggressiveness of gastric cancer. Nutr Cancer. 2015;67:1260–1267. doi: 10.1080/01635581.2015.1082112. [DOI] [PubMed] [Google Scholar]
- 18.Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum. 2015;58:1048–1057. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 19.Rigamonti A, Imbesi F, Silvani A, et al. Prognostic nutritional index as a prognostic marker in glioblastoma: data from a cohort of 282 Italian patients. J Neurol Sci. 2019;400:175–179. doi: 10.1016/j.jns.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, Jordan K, Committee EG. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119-iv42. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe I, Kanauchi N, Watanabe H. Preoperative prognostic nutritional index as a predictor of outcomes in elderly patients after surgery for lung cancer. Jpn J Clin Oncol. 2018;48:382–387. doi: 10.1093/jjco/hyy014. [DOI] [PubMed] [Google Scholar]
- 22.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graves M, CelliMarchett G, van Zyl B, Tang D, Vilain RE, van der Westhuizen A, Bowden NA. Monitoring patient response to pembrolizumab with peripheral blood exhaustion marker profiles. Front Med (Lausanne) 2019;6:113. doi: 10.3389/fmed.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 26.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kos FT, Hocazade C, Kos M, Uncu D, Karakas E, Dogan M, Uncu HG, Ozdemir N, Zengin N. Assessment of prognostic value of “neutrophil to lymphocyte ratio” and “prognostic nutritional index” as a sytemic inflammatory marker in non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16:3997–4002. doi: 10.7314/apjcp.2015.16.9.3997. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Sakai M, Sohda M, et al. Prognostic significance of inflammatory and nutritional parameters in patients with esophageal cancer. Anticancer Res. 2016;36:6557–6562. doi: 10.21873/anticanres.11259. [DOI] [PubMed] [Google Scholar]
- 29.Ferrucci PF, Gandini S, Battaglia A, et al. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904–1910. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaragoza J, Caille A, Beneton N, Bens G, Christiann F, Maillard H, Machet L. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174:146–151. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 31.Kiriu T, Yamamoto M, Nagano T, et al. The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS ONE. 2018;13:e0193018. doi: 10.1371/journal.pone.0193018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelderman S, Heemskerk B, van Tinteren H, et al. Lactate dehydrogenase as a selection criterion for ipilimumab treatment in metastatic melanoma. Cancer Immunol Immunother. 2014;63:449–458. doi: 10.1007/s00262-014-1528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diem S, Kasenda B, Spain L, Martin-Liberal J, Marconcini R, Gore M, Larkin J. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114:256–261. doi: 10.1038/bjc.2015.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oya Y, Yoshida T, Kuroda H, et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017;8:103117–103128. doi: 10.18632/oncotarget.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 36.Lee YJ, Kim WR, Han J, Han YD, Cho MS, Hur H, Lee KY, Kim NK, Min BS. Prognostic impact of immunonutritional status changes during preoperative chemoradiation in patients with rectal cancer. Ann Coloproctol. 2016;32:208–214. doi: 10.3393/ac.2016.32.6.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi: 10.1016/j.ejca.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Nakaya A, Kurata T, Yoshioka H, et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23:634–640. doi: 10.1007/s10147-018-1250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]