Abstract
Sarcomas infrequently arise in the larynx where the vast majority of tumors are of epithelial origin. Given their rarity, studies of these lesions are limited in number. In this series, we describe our institutional experience with ten primary sarcomas of the larynx encountered over an 18 year period, comprising 1.9% of all laryngeal malignancies observed in this timeframe. The cases include four chondrosarcomas and one example each of osteosarcoma, embryonal rhabdomyosarcoma, undifferentiated spindle cell sarcoma, well-differentiated liposarcoma, Kaposi sarcoma, and synovial sarcoma. Patients included nine males and one female, with a mean age of 59 years (range 34–75). The mean clinical follow-up time was 3.4 years (range 0–12 years). Clinically, all patients presented with vocal and/or respiratory symptoms, and all received surgical treatment with the exception of the case of Kaposi sarcoma. Of the nine patients who underwent surgical excision, two, both chondrosarcomas, experienced local recurrence. No instances of distant metastasis or death of disease had occurred at the time of preparation of this manuscript. In conclusion, primary sarcomas of the larynx are rare but tend to present with early symptoms. This likely allows for earlier detection and intervention as compared to their counterparts in other deep soft tissue locations. Pathologically, it is important, although difficult in some cases, to distinguish these neoplasms from sarcomatoid carcinoma and reactive processes. Careful morphologic and immunohistochemical evaluation, as well as correlation with the clinical and radiologic findings, is important for accurate tumor classification.
Keywords: Sarcoma, Larynx, Osteosarcoma, Chondrosarcoma, Undifferentiated sarcoma, Rhabdomyosarcoma, Synovial sarcoma
Introduction
Primary sarcomas of the larynx are rare and represent less than 1% of malignant neoplasms arising at this site [1, 2]. Chondrosarcoma is the most common sarcoma subtype primary to the larynx and typically arises within the multiple cartilaginous structures [3]. Other sarcomas are less common and most often arise in the submucosal connective tissue [4]. Studies of laryngeal sarcoma are limited in number, necessitating further analysis of their characteristics and clinical outcomes. In this series, we describe our institutional experience of ten cases of laryngeal sarcomas encountered over an 18-year period to add to the limited data of these rare entities.
Methods
Case Selection
With prior approval from our Institutional Review Board, a retrospective search of the electronic surgical pathology records was conducted to identify examples of sarcoma arising in the larynx received between 2000 and 2018. Search terms included sarcoma, spindle, malignant, etc. in combination with larynx or laryngectomy. All available histologic slides from each sarcoma case, including relevant immunohistochemical stains, were retrieved from the institutional pathology archives. Clinical data was reviewed using the electronic medical records system.
The total number of patients diagnosed with non-sarcomatous malignant neoplasms of the larynx during this time frame was identified by searching the surgical pathology records using terms such as carcinoma, malignant, and lymphoma in combination with larynx or laryngectomy.
Immunohistochemistry
All immunohistochemical staining was performed in a College of American Pathologists (CAP)-certified laboratory according to standardized protocols. An automated immunostainer (Bond III, Leica Biosystems, Germany) and polymer anti-mouse or anti-rabbit poly-HRP-IgG were used. The chromogen was 3300-diaminobenzidine tetrahydrochoride. Antibody clones, dilutions, and sources are reported in Table 1.
Table 1.
Immunohistochemistry antibodies used in this series
| Antigen | Clone | Dilution | Source |
|---|---|---|---|
| CK | AE1/AE3 | 1:100 | Dako |
| LMWK | Cam5.2 | 1:50 | Becton–Dickinson |
| CEA | CEA31 | 1:400 | Cell Marque |
| CK5 | EP1601Y | 1:50 | Cell Marque |
| EMA | E29 | 1:1000 | Dako |
| ERG | EPR3864 | 1:100 | Abcam |
| p40 | BC28 | 1:200 | BioCare Medical |
| p63 | BC4A4 | 1:125 | BioCare Medical |
| SOX10 | BC34 | 1:50 | BioCare Medical |
| Desmin | D33 | 1:800 | Dako |
| Myogenin | F5D | 1:25 | Cell Marque |
| MyoD1 | EP212 | 1:20 | Cell Marque |
| CD21 | 1F8 | 1:20 | Dako |
| CD35 | EP197 | 1:400 | Cell Marque |
| ALK | ALK-1 | 1:20 | Cell Marque |
| SATB2 | CL0276 | 1:100 | Sigma-Aldrich |
| CD34 | QBEnd 10 | 1:200 | Dako |
| SMA | 1A4 | 1:200 | Cell Marque |
| STAT6 | YE361 | 1:400 | Abcam |
| HHV-8 | 13B10 | 1:200 | Cell Marque |
CK indicates cytokeratin; LMCK low molecular weight keratin; EMA epithelial membrane antigen
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH) for MDM2 amplification was performed according to a clinically validated laboratory protocol using the SpectrumOrange Vysis LSI (Abbott) MDM2 FISH probe. After identifying histologic regions appropriate for analysis, a minimum of 100 cell nuclei per case were evaluated under oil immersion at 1000× magnification. Overlapping nuclei were excluded. Amplification was defined as an average of 5 or more fluorescent signals per nucleus.
Results
Patient Characteristics
We identified a total of 528 patients with malignant neoplasms arising in the larynx, ten of which were sarcomas. These cases represented 1.9% of total laryngeal malignancies in our pathology archives from 2000 to 2018 and comprised four examples of chondrosarcoma and one each of osteosarcoma, embryonal rhabdomyosarcoma, well-differentiated liposarcoma, Kaposi sarcoma, synovial sarcoma, and undifferentiated spindle cell sarcoma. Patients included nine males and one female with a mean age of 59 years (range 34–75). The mean clinical follow-up time was 3.4 years (range 0–12). The clinical and pathologic characteristics of all sarcoma patients are summarized in Table 2.
Table 2.
Clinicopathologic features of ten laryngeal sarcomas
| Patient # | Diagnosis | Age (years)/sex | Size (cm) | Site of tumor Epicenter | Symptoms | Treatment (margins) | Recurrence/metastasis (months) | Status & total follow-up (years) |
|---|---|---|---|---|---|---|---|---|
| 1 | Chondrosarcoma, grade I | 71/M | 2.5 | L Arytenoid | Hoarseness, dysphagia | Total laryngectomy (−) | N/N | DOC (3) |
| 2 | Chondrosarcoma, grade I | 65/F | 4.6 | R Arytenoid | Hoarseness, dysphagia | Fragmented local excision (N/A); Laryngectomy (−) | Y(6)N/N | NED (12) |
| 3 | Chondrosarcoma, grade II | 54/M | 3.5 | Cricoid | Hoarseness, discomfort | Partial laryngectomy (+); Total laryngectomy (−) | Y(24)Y(15)/N | AWD (7) |
| 4 | Chondrosarcoma, grade I | 58/M | 2.6 | Subglottis | Stridor | Partial laryngectomy (−) | N/N | NED (2) |
| 5 | Osteosarcoma, high grade | 75/M | 2.1 | Anterior commisure | Hoarseness | Laryngectomy (−) | N/N | LOST |
| 6 | Embryonal rhabdomyosarcoma | 61/M | <1 | R Arytenoid | Dyspnea, stridor | Fragmented local excisions x2 (N/A), CT, RADx | N/N | NED (1) |
| 7 | Well-differentiated liposarcoma | 36/M | 2.4 | Epiglottis/vallecula | Sleep apnea, dysphagia | Partial laryngectomy (−), RADx | N/N | NED (1) |
| 8 | Kaposi Sarcoma | 34/M | N/A | Epiglottis, aryepiglottic folds | Vocal changes | CT | – | NED (9) |
| 9 | Synovial sarcoma, FNCLCC grade 3 | 69/M | 6.8 | Vallecula | Cough | Partial laryngectomy (−), RADx | N/N | LOST |
| 10 | Undifferentiated spindle cell sarcoma, FNCLCC grade 3 | 68/M | 2.5 | Subglottis posterior | Dyspnea, stridor, hemoptysis | Laryngectomy (−); RADx | N/N | LOST (0.25) |
AWD indicates alive with disease; CT chemotherapy; DOC died of other causes; NED no evidence of disease; RADx, radiation
We identified 508 patients (96%) with carcinoma: 488 squamous cell carcinomas (92%), 10 adenoid cystic carcinomas (1.9%), 6 sarcomatoid carcinomas (1.1%), 2 adenosquamous carcinomas (0.4%), 1 mucoepidermoid carcinoma (0.2%), and 1 atypical carcinoid tumor (0.2%). Ten cases of lymphoma were identified which represented 1.9% of all cases. These included 5 diffuse large B cell lymphomas, 2 extranodal NK/T-cell lymphomas, 2 peripheral T-cell lymphomas, and 1 Hodgkin lymphoma.
Chondrosarcoma
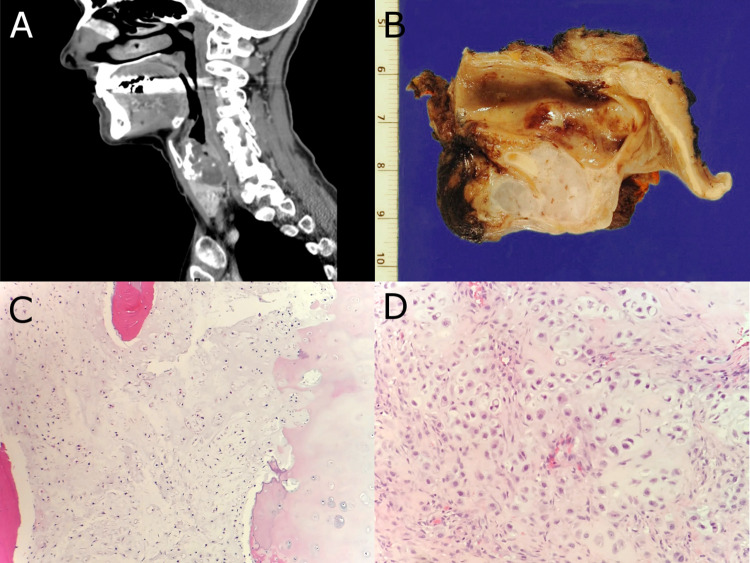
Primary laryngeal chondrosarcoma accounted for four cases in our series and represented the only sarcoma subtype with multiple examples. All patients were symptomatic upon presentation with reports of hoarseness, dysphagia, and/or stridor. Of the four patients, only one had a history of tobacco smoking, and two reported moderate alcohol use. Histologically, all tumors were conventional type chondrosarcomas and showed multinodular growth of variably hypercellular hyaline cartilage. Features of aggressive growth, including invasion into adjacent soft tissue and destruction of laryngeal cartilaginous structures, were present. Using the grading system described in the WHO Classification of Tumours of Soft Tissue and Bone, three tumors were grade I and one was grade II [5]. The mean tumor size was 3.3 cm with a range of 2.5–4.6 cm. Two patients (patients #2 and #3) experienced local recurrence after initial conservative excision at 6 months and 2 years, respectively, and both subsequently underwent total laryngectomy. Patient #3 had a second local recurrence 15 months after total laryngectomy. No distant metastases were observed in any of the patients. Representative radiologic, gross, and microscopic features of chondrosarcoma are provided in Fig. 1.
Fig. 1.
a, b Sagittal CT image and gross resection specimen from patient #2 demonstrating a destructive cartilaginous lesion which involves the cricoid cartilage. c H&E stained histologic section showing grade I conventional chondrosarcoma from patient #2. d H&E stained histologic section of grade II conventional chondrosarcoma from patient #3
Osteosarcoma
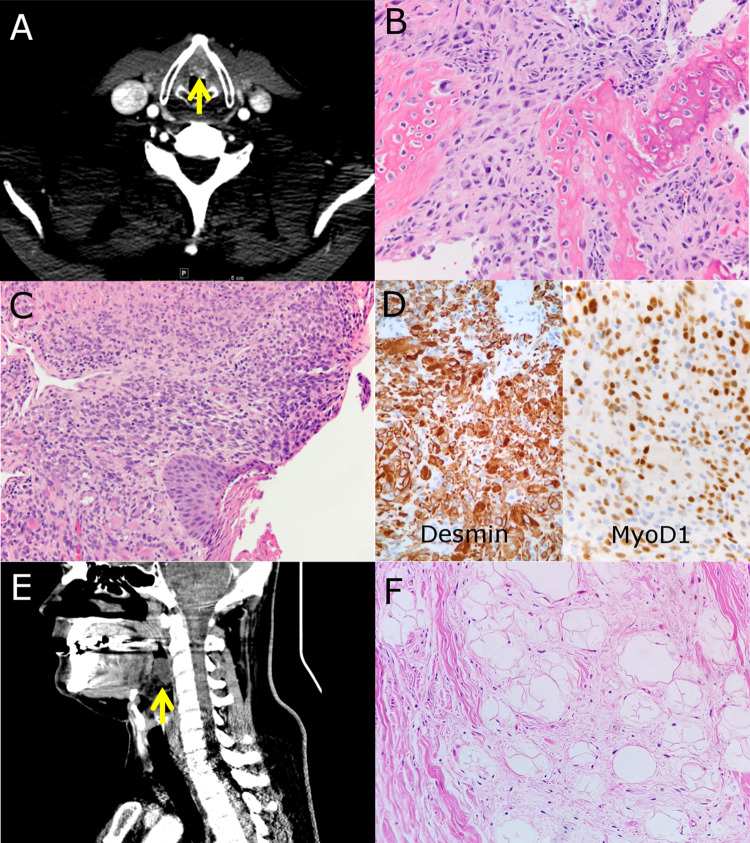
A 75-year-old male former smoker presented with progressive hoarseness. A mass was identified within the submucosal connective tissue of the anterior commissure underlying the vocal folds. Computed tomography (CT) imaging showed scattered areas of calcification (Fig. 2a). Biopsy of the lesion showed a high-grade malignant spindle cell neoplasm with bone matrix production. No evidence of epithelial differentiation was present. Subsequent laryngectomy revealed a neoplasm with osteosarcomatous differentiation centered within the submucosal connective tissue (Fig. 2b). The malignancy abutted, but did not invade, the thyroid cartilage. There was no morphologic evidence of an overlying or pre-existing epithelial neoplasm to include dysplasia of the overlying mucosa or invasive squamous cell carcinoma. The possibility of spindle cell/sarcomatoid carcinoma with heterologous osteosarcomatous differentiation was ruled out with multiple negative immunohistochemical studies for squamous differentiation (AE1/AE3, Cam5.2, CK5, p63, and p40). SATB2 demonstrated strong nuclear staining, further supporting the morphologic impression of osteoblastic differentiation. The tumor was ultimately classified as high-grade osteosarcoma. No other sites of disease were identified after clinical work-up to suggest metastasis from another primary location. The patient ultimately transferred care to another institution, and no further follow-up information was available.
Fig. 2.
a Osteosarcoma. CT imaging demonstrates a partially mineralized soft tissue mass located in the anterior commissure. b Histologic sections demonstrate malignant spindle cells with osteoblastic differentiation, including bone matrix deposition. c Embryonal rhabdomyosarcoma. Histologic section showing a submucosal proliferation of atypical spindled to round neoplastic cells with subepithelial condensation and scattered cells showing features of rhabdomyoblastic differentiation. d Immunohistochemical stains show strong immunoreactivity for desmin and MyoD1. e Well-differentiated liposarcoma. CT imaging demonstrates a mass involving the epiglottis/vallecula. f Histologic section demonstrating a neoplasm composed of adipose tissue and loose fibrous stroma containing atypical spindle cells characteristic of well-differentiated liposarcoma
Rhabdomyosarcoma, Embryonal Type
A 61-year-old man presented with a 2 year history of dyspnea and stridor associated with vocal cord immobility following prior intubation. He reported former cigarette smoking for over 30 years as well as chronic opioid dependence. Laryngoscopy revealed an exophytic, ulcerated lesion of the right posterior arytenoid region measuring less than one centimeter. The clinical impression was of granulation tissue. The mass was excised and histologic sections revealed a submucosal proliferation of highly atypical spindled to round neoplastic cells with subepithelial condensation. Scattered cells demonstrated rhabdomyoblastic differentiation (Fig. 2c). The neoplastic cells showed diffuse expression of desmin and MyoD1 and focal myogenin expression (Fig. 2d). Cytokeratins (AE1/AE3, Cam5.2, CK5), CEA, EMA, ERG, p40, p63, and SOX10 were all negative in the neoplastic population. The lesion was classified as rhabdomyosarcoma with features suggesting an embryonal subtype. Although there was no clinically apparent residual tumor, conservative re-excision showed microscopic residual neoplasm. The patient was treated with systemic chemotherapy and radiation without further surgical intervention. No evidence of recurrent disease or metastasis was present after 1 year of follow-up.
Well-Differentiated Liposarcoma
A 36-year-old male non-smoker presented with a 3 year history of sleep apnea and dysphagia. Laryngoscopy and CT imaging revealed a 4.0 cm fatty mass arising in the base of the epiglottis/vallecula (Fig. 2e). Biopsy demonstrated a fat-forming neoplasm with interspersed fibrous bands containing atypical spindle cells (Fig. 2f). FISH studies demonstrated MDM2 gene amplification which supported the diagnosis of well-differentiated liposarcoma. Supraglottic partial laryngectomy was performed followed by adjuvant radiation therapy. No evidence of dedifferentiation was found in the excision specimen. At 1 year of follow-up, no local recurrence or metastatic disease was clinically identified.
Undifferentiated Spindle Cell Sarcoma
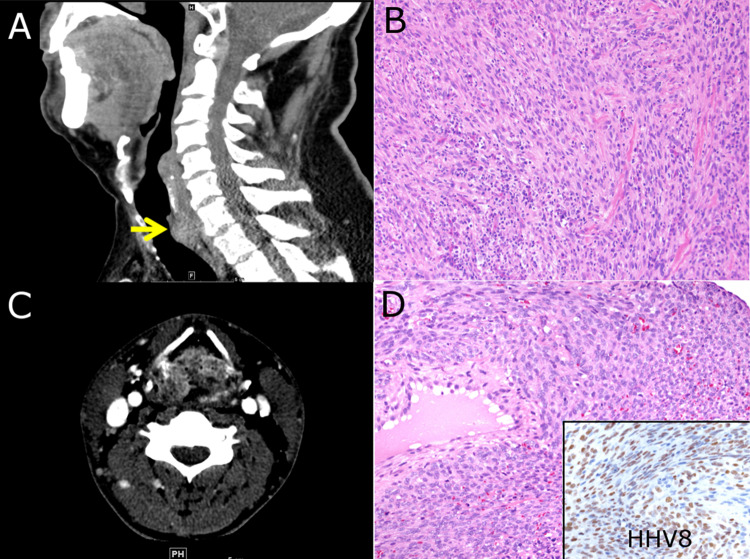
A 68-year-old man with no history of tobacco, alcohol, or other substance use presented with rapidly worsening dyspnea, stridor, and hemoptysis requiring intubation. CT imaging showed a 2.7 cm mass involving the soft tissues of the posterior subglottis (Fig. 3a). No regional lymphadenopathy was identified on imaging studies. Histologic sections from a biopsy showed a malignant spindle cell neoplasm with vague fascicular growth, severe nuclear atypia, brisk mitotic activity, and intermixed inflammatory cells (Fig. 3b). The subsequent total laryngectomy demonstrated a mass centered in the soft tissues external to the posterior subglottis with extension into the larynx and proximal trachea. The histologic features remained similar to those seen in the biopsy and there was no morphologic evidence of pre-existing surface epithelial dysplasia or carcinoma. Immunohistochemical stains demonstrated variable expression of SMA and focal Cam5.2. Negative stains included AE1/AE3, CK5, p40, p63, S100, desmin, ALK-1, CD21, CD34, CD35, STAT6, and ERG. MDM2 gene amplification was not identified by FISH. The neoplasm was ultimately classified as undifferentiated spindle cell sarcoma. A limited cervical lymph node dissection was performed yielding only one lymph node for evaluation which showed no evidence of metastasis. After resection, the patient was treated with additional radiation and no evidence of recurrence or distant metastasis was seen after three months of follow-up.
Fig. 3.
a Undifferentiated spindle cell sarcoma. CT imaging demonstrates an ill-defined mass within the posterior subglottis. b Histologic section demonstrates a malignant neoplasm with highly atypical spindle cells and intermixed inflammatory cells. c Kaposi sarcoma. CT imaging shows a mass involving the epiglottis and aryepiglottic folds. d Histologic examination demonstrates a spindle cell neoplasm with nuclear staining for HHV-8 immunohistochemistry (inset)
Kaposi Sarcoma
A 34-year-old male non-smoker with a history of moderate alcohol use, HIV/AIDS, and cutaneous Kaposi sarcoma presented after 2 months of progressive voice changes. He was found to have a large mass involving the epiglottis and aryepiglottic folds (Fig. 3c). A biopsy showed a spindle cell neoplasm forming vascular spaces and blood extravasation. Immunohistochemistry revealed nuclear staining with HHV8, supportive of Kaposi sarcoma (Fig. 3d). Initial treatment with HAART and Doxorubicin resulted in tumor regression; however, after 1 year, his tumor recurred. He received additional chemotherapy with complete clinical resolution and no further recurrence after 9 years.
Synovial Sarcoma
A 69-year-old male non-smoker presented with several months of persistent cough. CT demonstrated a 6.8 cm mass arising in the vallecula and invading into the thyroid cartilage. Histologic sections of the biopsy specimen showed a highly cellular monotonous spindle cell neoplasm. RT-PCR, performed at an outside laboratory, detected a SYT-SSX2 fusion which confirmed a diagnosis of monophasic synovial sarcoma. The patient underwent partial laryngectomy with postoperative radiation therapy. He subsequently transferred care to another institution and was lost to follow-up.
Discussion
Sarcomas arising in the larynx are rare, corresponding to < 1% of malignant neoplasms at this site [1–4, 6]. There are few series describing multiple cases and clinical outcomes in the literature. A search of our institutional archives revealed that sarcomas comprised 1.9% of laryngeal malignancies, a proportion slightly higher than reported. As our institution is a relatively large sarcoma referral center, the increase is likely due to an overrepresentation of sarcomas in our cases.
Chondrosarcoma is the most common laryngeal primary sarcoma, with most cases representing low grade, conventional-type lesions [1–4]. Laryngeal chondrosarcoma has a reported male predominance and a mean age at presentation of 64 years [3]. The reported risk of recurrence is between 18 and 40%, and the disease-related mortality appears to be lower as compared to the skeletal counterparts [3]. Amongst the few cases of dedifferentiated chondrosarcoma in the larynx reported, neoplasms at the laryngeal site also appears to have a more favorable clinical behavior than those of the skeleton [7]. Our institutional experience is similar to previous reports. In our series, chondrosarcoma was the most common type of sarcoma and constituted 4 of 10 primary laryngeal sarcomas. All cases were conventional type, grade I to II tumors, and the mean patient age was 62 years. Local recurrence was observed in two patients, one of which had a grade II tumor. No patients died of their disease during the follow-up time.
Sarcoma subtypes other than chondrosarcoma have been described in the larynx, mainly as individual case reports or small series and include synovial sarcoma [1, 2, 4], well-differentiated and dedifferentiated liposarcoma [8–10], osteosarcoma [11, 12], alveolar soft part sarcoma [13], low grade fibromyxoid sarcoma [14], embryonal rhabdomyosarcoma [15–19], Kaposi sarcoma [20] and undifferentiated sarcoma [21–23]. In our series, we report an additional example each of well-differentiated liposarcoma, embryonal rhabdomyosarcoma, osteosarcoma, Kaposi sarcoma, synovial sarcoma, and undifferentiated spindle cell sarcoma. With the exception of the Kaposi sarcoma, all patients were treated with surgical resection, with or without additional systemic therapy. From a diagnostic standpoint, a majority of the tumors in our series showed morphologic, immunohistochemical, and/or molecular features typical for each given tumor type and did not pose a particular diagnostic challenge despite the unusual site. The osteosarcoma, undifferentiated spindle cell sarcoma, and embryonal rhabdomyosarcoma were diagnostically difficult due to the site and overlapping features with more common entities.
For osteosarcoma and undifferentiated spindle cell sarcoma, the most relevant entity in the differential diagnosis to exclude is the more common sarcomatoid carcinoma which represented 1.1% of total laryngeal malignancies in our archives. Sarcomatoid carcinoma can harbor various heterologous components, including osteosarcomatous elements, that add to the difficulty in interpretation [12, 24–26]. Generous sampling and careful histologic evaluation to search for co-existing intra-epithelial neoplasia/dysplasia or invasive carcinoma is necessary. Immunostaining for cytokeratins, p63, and/or p40 are helpful in evaluating for evidence of epithelial or squamous differentiation; however, sarcomatoid carcinoma may lose expression of these markers, emphasizing the need for careful morphologic evaluation [12, 27]. Additionally, the presence of some keratin staining does not absolutely exclude sarcoma since a subset of sarcomas may express keratins [28]. When only provided with limited biopsy material, definitive classification may remain challenging and even impossible. Once sarcomatoid carcinoma is excluded, additional ancillary testing, such as further immunostaining and/or molecular studies as are often used in the diagnosis of sarcomas at other anatomic sites, may be needed for definitive tumor classification.
Radiologic studies may be helpful to provide information about the tumor’s location and relationship to adjacent structures. Generally, sarcomatoid carcinoma shows an association with the mucosal surface whereas sarcomas are more likely be centered within the soft tissue or cartilaginous structures [3]. Matrix formation may also be a diagnostic clue gleaned from radiologic studies, for example calcification patterns associated with osteoid or cartilage matrix or the presence of fat within a tumor as in our case of liposarcoma [3]. While the radiologic features are helpful, careful pathologic evaluation of biopsy or resection material is required for definitive tumor classification and grading.
The distinction between mesenchymal neoplasms, sarcomatoid carcinoma, and reactive stromal changes, such as those underlying an ulcer or in granulation tissue, may present additional diagnostic challenges. In the latter, reactive fibroblasts/myofibroblasts with enlarged nuclei, visible nucleoli, and noticeable mitotic activity may raise concern for a spindle cell neoplasm. There is often accompanying prominent vasculature with reactive endothelium and intermixed inflammatory cells. True nuclear hyperchromasia, pleomorphism, and atypical mitoses should not be present in reactive processes and would indicate the presence of a more ominous entity [29]. Correlation with clinical and/or radiologic findings is often indispensable. On the other hand, spindle cell neoplasms may mimic reactive tissue [30]. For example, the embryonal rhabdomyosarcoma presented as a small, exophytic, ulcerated glottic lesion in a 61-year-old man with prior laryngeal injury. The history and clinical presentation closely mimicked reactive granulation tissue with an overlying ulcer. Careful histologic evaluation revealed highly atypical round to spindled cells with morphologic and immunohistochemical features of rhabdomyoblastic differentiation, ultimately allowing for appropriate classification. In general, embryonal rhabdomyosarcoma is more common in children and adolescents, and, when it does occur in adults, it is typically associated with a worse prognosis than in younger patients [31]. Few prior cases of embryonal rhabdomyosarcoma arising in the larynx are described in adults. Two such cases showed no evidence of recurrence or metastatic disease after combination treatment [16, 17]. A third case was reported in a patient who refused surgical treatment and received only chemotherapy and radiation. Persistent disease was seen at 34 months from the initial biopsy diagnosis [18]. An additional case report described local recurrence and late lymph node metastasis 13 years after multimodal therapy in a patient initially diagnosed during adolescence [19].
Prior case series of laryngeal sarcoma, when compared to their counterparts in other body sites, have demonstrated a lower disease-related mortality and recurrence rate in most types [1, 3, 7, 9, 10, 21–23]. Given the fact that all of the tumors in our series and the majority in the existing literature are symptomatic upon presentation, it seems likely that they come to clinical attention and receive treatment earlier than their counterparts in deep soft tissue, bone, and body cavities. A notable exception is osteosarcoma of the larynx which resulted in distant metastasis and subsequent death of disease in a significant number of cases [11, 12].
Conclusion
While sarcoma of the larynx is rare, a wide variety of sarcoma types may occur at this location with chondrosarcoma reported most commonly. The histologic features and relevant ancillary studies are identical to those of sarcomas affecting other sites. The main diagnostic difficulty arising in some cases is the distinction of sarcoma from sarcomatoid carcinoma and/or reactive stromal processes. Incorporation of clinical and radiologic findings, along with extensive sampling and careful histologic evaluation, is necessary for appropriate classification. The possibility that the laryngeal lesion represents a metastasis from another site should also be excluded based on clinical and radiologic findings. Most laryngeal sarcomas, with the possible exception of osteosarcoma, appear to have a more favorable survival when compared to sarcomas arising at other anatomic locations. This may be due to early symptoms, likely as a direct consequence of the affected location, and patients seeking timely clinical attention.
Compliance with Ethical Standards
Conflicts of interest
All authors declare that have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fernandez-Acenero MJ, Larach F, Ortega-Fernandez C. Non-epithelial lesions of the larynx: review of the 10-year experience in a tertiary Spanish hospital. Acta Otolaryngol. 2009;129(1):108–112. doi: 10.1080/00016480802008207. [DOI] [PubMed] [Google Scholar]
- 2.Karatayli-Ozgursoy S, et al. Non-epithelial tumors of the larynx: a single institution review. Am J Otolaryngol. 2016;37(3):279–285. doi: 10.1016/j.amjoto.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Thompson LD, Gannon FH. Chondrosarcoma of the larynx: a clinicopathologic study of 111 cases with a review of the literature. Am J Surg Pathol. 2002;26(7):836–851. doi: 10.1097/00000478-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Friedman AD, et al. Submucosal neoplasms of the laryngeal introitus. J Laryngol Otol. 2012;126(7):706–713. doi: 10.1017/S0022215112000928. [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CDM, W.H. Organization . WHO classification of tumours of soft tissue and bone. Lyon: IARC Press; 2013. [Google Scholar]
- 6.AbdullGaffar B, Keloth T. Laryngeal sarcomas: a case series of 5 cases. Ann Diagn Pathol. 2018;37:35–41. doi: 10.1016/j.anndiagpath.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Garcia RE, Gannon FH, Thompson LD. Dedifferentiated chondrosarcomas of the larynx: a report of two cases and review of the literature. Laryngoscope. 2002;112(6):1015–1018. doi: 10.1097/00005537-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Wenig BM, Weiss SW, Gnepp DR. Laryngeal and hypopharyngeal liposarcoma. A clinicopathologic study of 10 cases with a comparison to soft-tissue counterparts. Am J Surg Pathol. 1990;14(2):134–141. doi: 10.1097/00000478-199002000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Makeieff M, et al. Laryngeal dedifferentiated liposarcoma. Eur Arch Otorhinolaryngol. 2010;267(6):991–994. doi: 10.1007/s00405-010-1234-y. [DOI] [PubMed] [Google Scholar]
- 10.Zhu H, et al. Well-differentiated laryngeal/hypopharyngeal liposarcoma in the MDM2 era report of three cases and literature review. Head Neck Pathol. 2017;11(2):146–151. doi: 10.1007/s12105-016-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosalleum E, et al. A review of primary osteosarcoma of the larynx and case report. Head Neck Pathol. 2015;9(1):158–164. doi: 10.1007/s12105-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madrigal FM, et al. Laryngeal osteosarcoma: a clinicopathologic analysis of four cases and comparison with a carcinosarcoma. Ann Diagn Pathol. 2002;6(1):1–9. doi: 10.1053/adpa.2002.30604. [DOI] [PubMed] [Google Scholar]
- 13.Tamaki A, Wasman J, Weidenbecher M. Laryngeal alveolar soft part sarcoma: a case report of a rare malignancy in an atypical location. Am J Otolaryngol. 2017;38(2):260–262. doi: 10.1016/j.amjoto.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Cowan ML, et al. Low-grade fibromyxoid sarcoma of the head and neck: a clinicopathologic series and review of the literature. Head Neck Pathol. 2016;10(2):161–166. doi: 10.1007/s12105-015-0647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dikbas O, et al. Embryonal rhabdomyosarcoma of the larynx. Otolaryngol Head Neck Surg. 2005;133(1):160–162. doi: 10.1016/j.otohns.2004.09.115. [DOI] [PubMed] [Google Scholar]
- 16.Jain A, et al. A rare case of subglottic embryonal rhabdomyosarcoma: managed with the aim of organ preservation. J Laryngol Otol. 2015;129(1):106–109. doi: 10.1017/S002221511400293X. [DOI] [PubMed] [Google Scholar]
- 17.Kukwa W, et al. Laryngeal embryonal rhabdomyosarcoma in an adult—a case presentation in the eyes of geneticists and clinicians. BMC Cancer. 2011;11:166. doi: 10.1186/1471-2407-11-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell JO, et al. Failed organ preservation strategy for adult laryngeal embryonal rhabdomyosarcoma. Am J Otolaryngol. 2015;36(2):277–279. doi: 10.1016/j.amjoto.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Sivanandan R, et al. Laryngeal embryonal rhabdomyosarcoma: a case of cervical metastases 13 years after treatment and a 25-year review of existing literature. Arch Otolaryngol Head Neck Surg. 2004;130(10):1217–1222. doi: 10.1001/archotol.130.10.1217. [DOI] [PubMed] [Google Scholar]
- 20.Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190. doi: 10.1186/1471-2407-8-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeong CY, Kim CS. Undifferentiated pleomorphic sarcoma of the vocal fold. Ear Nose Throat J. 2016;95(12):E12–e14. [PubMed] [Google Scholar]
- 22.Kim JP, et al. A rare case of malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma NOS) of the vocal fold. Ear Nose Throat J. 2015;94(7):270–272. doi: 10.1177/014556131509400707. [DOI] [PubMed] [Google Scholar]
- 23.Cambruzzi E, et al. Undifferentiated high-grade pleomorphic sarcoma of the larynx treated with partial laryngectomy. Braz J Otorhinolaryngol. 2016 doi: 10.1016/j.bjorl.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy S, Purgina B, Seethala RR. Spindle cell carcinoma of the larynx with rhabdomyoblastic heterologous element: a rare form of divergent differentiation. Head Neck Pathol. 2013;7(3):263–267. doi: 10.1007/s12105-012-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furuta Y, et al. A rare case of carcinosarcoma of the maxillary sinus with osteosarcomatous differentiation. Auris Nasus Larynx. 2001;28(Suppl):S127–S129. doi: 10.1016/S0385-8146(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 26.Klijanienko J, et al. True carcinosarcoma of the larynx. J Laryngol Otol. 1992;106(1):58–60. doi: 10.1017/S0022215100118626. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan S, et al. Sarcomatoid (spindle cell) carcinoma of the head and neck mucosal region: a clinicopathologic review of 103 cases from a tertiary referral cancer centre. Head Neck Pathol. 2010;4(4):265–275. doi: 10.1007/s12105-010-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miettinen M. Keratin subsets in spindle cell sarcomas. Keratins are widespread but synovial sarcoma contains a distinctive keratin polypeptide pattern and desmoplakins. Am J Pathol. 1991;138(2):505–513. [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg AE. Pseudosarcomas of soft tissue. Arch Pathol Lab Med. 2008;132(4):579–586. doi: 10.5858/2008-132-579-POST. [DOI] [PubMed] [Google Scholar]
- 30.Hollowood K, Fletcher CD. Soft tissue sarcomas that mimic benign lesions. Semin Diagn Pathol. 1995;12(1):87–97. [PubMed] [Google Scholar]
- 31.Van Gaal JC, et al. The impact of age on outcome of embryonal and alveolar rhabdomyosarcoma patients. A multicenter study. Anticancer Res. 2012;32(10):4485–4497. [PubMed] [Google Scholar]