Abstract
Salivary gland secretory carcinoma, also termed mammary analogue secretory carcinoma (MASC), is a recently described salivary gland neoplasm with characteristic histomorphologic findings similar to those of secretory carcinoma of the breast and harboring recurrent ETV6–NTRK3 fusions. Recent findings have expanded the molecular profile of salivary gland secretory carcinoma to include multiple novel ETV6 fusion partners, including RET, MET, and MAML3. Here, we report a case of cystic MASC with cribriform and papillary histology harboring two gene fusions, ETV6–RET and EGFR–SEPT14, identified by targeted RNA sequencing. The presence of the rearrangements was confirmed by FISH, RT-PCR, and Sanger sequencing. This is the first EGFR–SEPT14 fusion reported in secretory carcinoma as a single event or in association with an ETV6 rearrangement. This finding adds to the expanding molecular profile of this tumor entity, and may translate into novel treatment strategies.
Keywords: Secretory carcinoma, Mammary analogue secretory carcinoma, Salivary gland carcinoma, ETV6 fusion, EGFR–SEPT14
Introduction
Mammary analogue secretory carcinoma (MASC), also termed secretory carcinoma of the salivary gland, is a distinct salivary gland neoplasm first described in 2010 [1]. MASC resembles secretory carcinoma of the breast histologically, immunohistochemically, and genetically [2]. Histologically, these are non-encapsulated circumscribed tumors divided by fibrous septa with areas of microcystic, tubular, and solid architecture. The microcystic areas contain abundant secretory material that stains with PAS, PAS-D, mucicarmine, MUC1, and MUC4 [1]. Tumor nuclei are low-grade, homogeneous, and vesicular with prominent central nucleoli and pale eosinophilic vacuolated cytoplasm. Immunohistochemical characterization shows that MASC generally stains strongly and diffusely for CK7, CK8, CK18, CK19, GCDFP-15, EMA, S-100, GATA3, vimentin, and mammaglobin [1, 3]. MASC may also show variable expression of myoepithelial markers, including calponin, p63, and CD10 [4].
Secretory carcinomas of both the breast and salivary gland were found to harbor a recurrent chromosomal translocation, t(12;15) (p13;q25) ETV6–NTRK3, which results in a fusion of the ETV6 and NTRK3 genes on chromosomes 12 and 15, respectively [1, 5]. It was later reported that ETV6 can also demonstrate non-NTRK3 fusion partners in MASC [6, 7]. In 2018, Skalova et al. reported a subset of MASC tumors with ETV6–RET translocations [8]. In August 2018, Rooper et al. reported a novel ETV6–MET fusion in a case of MASC with high-grade histology and aggressive behavior [9]. More recently, Guilmette et al. reported a novel ETV6–MAML3 fusion present in a case of secretory carcinoma with concurrent ETV6–NTRK3 fusion [10].
Clinically, MASC can present as a slow-growing nodule or as rapidly-growing mass. While this tumor can arise in any salivary gland tissue, it most often occurs in the parotid gland [1]. MASC demonstrates a wide range of clinical behavior ranging from indolent tumors to aggressive tumors with rapid growth followed by metastases and death [1]. Treatment ranges from simple excision to radical resection with neck dissection with or without adjuvant radio- and/or chemotherapy. There are recent reports suggesting the use of targeted tyrosine kinase inhibitors as a useful treatment strategy in aggressive cases of MASC with ETV6–NTRK3 or ETV6–RET translocations [11].
We report a case of a young man with MASC of the parotid gland that harbors an ETV6–MET translocation associated to a novel gene fusion, EGFR–SEPT14, which may be potentially targeted by tyrosine kinase inhibitors.
Case Report
An 18 year old man with no significant past medical history presented for evaluation of a mass near his right ear. The mass had been present for about 1 year and had increased in size over 4 months prior to the clinical encounter. The mass was slightly tender, and there were no overlying skin changes or prior infections or trauma. The patient history is negative for smoking and alcohol consumption. Physical examination revealed a 2.5 cm firm, mobile, discrete right parotid mass. Ultrasound guided fine needle aspiration (FNA) was performed, and 2 mL of colorless watery fluid were obtained. Diff-Quik and Thin Prep staining revealed cystic contents with rare clusters of benign epithelial cells and macrophages. Given the non-malignant cytologic appearance and complete sonographic resolution of the cyst, the patient was advised to return if his symptoms returned or progressed.
Two months after the FNA, the patient returned for evaluation because the cyst had returned and slowly enlarged since the procedure. The cyst was now more painful; a second FNA was performed, and 5 mL of clear fluid were aspirated, no specimen was sent for cytology. A follow-up MRI performed 1 month later showed a right parotid cystic lesion with subtle wall enhancement and thickening posteromedially (Fig. 1). The lesion was felt to be radiologically and clinically benign, and surgical excision was executed. Follow-up MRIs performed at 3 and 9 months after surgical excision revealed no evidence of recurrent parotid mass of lymphadenopathy. US-guided FNA of two palpable non-tender lymph nodes in the right neck was performed 9 months after surgery, and results were consistent with benign reactive lymph nodes. The patient remains asymptomatic, and he will be followed with surveillance physical examination and imaging.
Fig. 1.
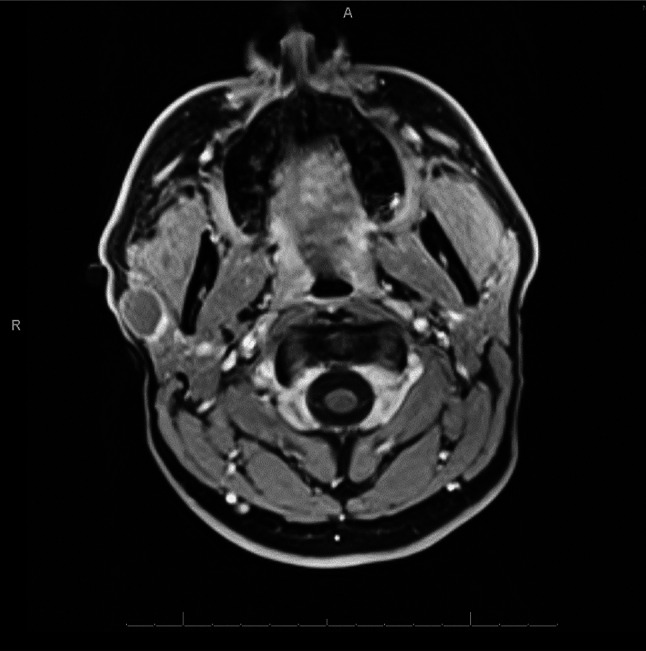
MRI shows right cystic parotid lesion (2 × 2 × 1.6 cm) with subtle wall enhancement and thickening posteriomedially
Pathologic Findings
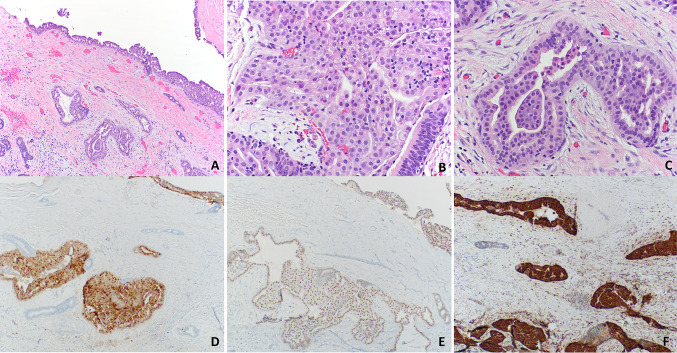
Pathologic examination of the resection specimen showed a 2.0 cm tan-pink deflated cystic mass with 0.1–0.2 cm wall thickness. Cut sections revealed a smooth unilocular and hemorrhagic cyst with a 1.2 cm area of papillary excrescences. Histologic examination showed small groups of infiltrative and intraductal tumor cells with focal cribriform and papillary growth patterns (Fig. 2). Tumor cells were low-grade, and there was no necrosis, perineural invasion, or prominent mitotic activity. Infiltrative cells focally extended to the resection margin, and the tumor was staged as pT1 according to AJCC 8th Edition.
Fig. 2.
Secretory carcinoma of the salivary gland. Cyst wall with infiltrating glands showing focal cribriform and papillary architecture (a). Tumor cells are round, monomorphic, and have ample eosinophilic cytoplasm (b). Invasive gland surrounded by demosplastic stroma (c). Tumor cells are immunoreactive for S-100 (d), GATA3 (e), and mammaglobin (f)
Immunohistochemical stains were performed on a Ventana Benchmark XT Autostainer (Ventana Medical Systems, Tuscon, AZ) to support the diagnosis. Tumor cells were diffusely positive for mammaglobin (clone 31A5; Ventana Medical Systems, Tucson, AZ), CK7 (clone OV-TL 12/30; Cell Marque, Rocklin, CA), GATA3 (clone L50-823; Cell Marque, Rocklin, CA), and S-100 (clone 4C4.9; Ventana Medical Systems, Tucson, AZ) and focally positive for p63 (clone 4A4; Ventana Medical Systems, Tucson, AZ) and GCDFP-15 (clone D6; BioLegend, Dedham, MA) (Fig. 2). Tumor cells were negative for androgen receptor (clone SP107; Ventana Medical Systems, Tucson, AZ), CK5/6 (clone D5/16 B4; Ventana Medical Systems, Tucson, AZ), and calponin (clone EP798Y; Ventana Medical Systems, Tucson, AZ). Although rare, cystic salivary secretory carcinomas have been described [12, 13]. The gross and histomorphologic features were consistent with secretory carcinoma, and molecular tests were performed to confirm the diagnosis.
Molecular Findings
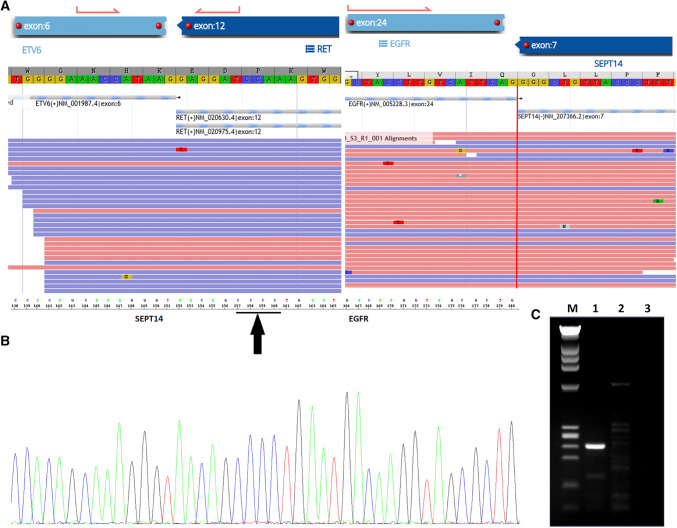
Fluorescence in situ hybridization (FISH) using a dual color break-apart probe (Mayo Clinical Laboratories, Rochester, MN) revealed rearrangement of the ETV6 gene at 12p13.2. Archer FusionPlex Custom Solid Panel (ArcherDX Inc., Boulder, CO) [14], a next-generation targeted RNA sequencing assay utilizing Anchored Multiplex PCR technology to detect gene fusions and oncogenic isoforms in selected protein-coding exons of 86 genes, was performed. Tumor RNA was extracted from 5 μm, formalin-fixed, paraffin-embedded tumoral tissue sections followed by cDNA synthesis and library preparation. Final targeted amplicons were sequenced on an Illumina NextSeq. Data were analyzed using the Archer Software pipeline. Two gene fusions, ETV6–RET and EGFR–SEPT14 (Fig. 3), were detected. Both fusions showed good read support and were in-frame. RT-PCR was performed with two sets of primers to target and amplify the EGFR–SEPT14 fusion gene. The presence of the EGFR–SEPT14 gene product was verified by gel electrophoresis (Fig. 3). Sanger sequencing was also performed to demonstrate the presence of the EGFR–SEPT14 gene product (Fig. 3). The histopathologic, immunohistochemical, and genetic molecular features are consistent with mammary analogue secretory carcinoma with a novel EGFR–SEPT14 gene fusion.
Fig. 3.
ETV6–RET and EGFR–SEPT14 translocations. Archer FusionPlex Custom Solid Panel demonstrates ETV6–RET translocation (ETV6 breakpoint, exon 6; RET breakpoint, exon 12) and EGFR–SEPT14 translocation (EGFR breakpoint, exon 24; SEPT14 breakpoint, exon 7) (a). RT-PCR amplification with 2 sets of primers verifies EGFR–SEPT14 fusion by both gel electrophoresis (b) [M, DNA ladder; Lane 1, amplified 237 bp fusion gene; Lane 2, HAPMAP Normal RNA control; Lane 3, water] and Sanger sequencing (c)
Discussion
Mammary analogue secretory carcinoma was first described as a distinct salivary gland neoplasm harboring recurrent ETV6–NTRK3 gene rearrangements [1], which also define secretory carcinomas of the breast [2]. The ETV6–NTRK3 translocation is not specific to secretory carcinoma of the breast or salivary gland, as it has also been identified in a range of neoplasms, including acute myeloid leukemia and congenital fibrosarcomas [15]. Nonetheless, in breast and salivary gland tumors, this translocation appears to be most common in secretory carcinomas. Initial reports of MASC included ETV6–NTRK3 as a diagnostic criterion, but recent identification of translocations between ETV6 and non-NTRK3 genes in MASC have indicated that wider molecular criteria may be warranted for this entity. In addition to NTRK3, reported ETV6 fusion partners now include RET and MET [6–9]. Recently, a case with dual-fusion ETV6–NTRK3 and ETV6–MAML3 fusions was identified in a tumor with two distinct histomorphologic growth patterns [10].
The majority of MASC tumors are low-grade and have an indolent progression [16]. However, a subset are reported to have high-grade histologic features and aggressive clinical behavior that may require additional treatment after surgical resection [11, 17–19]. Recently developed tyrosine kinase inhibitors entrectinib and larotrectinib can be used in tumors expressing NTRK gene rearrangements, such as the ETV6–NTRK3 fusion seen in classical secretory carcinoma of the breast and salivary gland [20]. However, as the molecular landscape of MASC expands, alternate treatment strategies may be needed for aggressive or metastatic tumors that do not express the ETV6–NTRK3 gene fusion. Tumors with the rare ETV6–MET fusion may benefit from the use of cabozantinib or crizotinib, targeted c-Met inhibitors [9]. In addition, tumors may benefit from multiple targeted therapies to overcome the development of resistance. A case of metastatic MASC with ETV6–NTRK3 fusion was treated with the pan-tyrosine kinase inhibitor entrectinib; after initial improvement, the disease later acquired a NTRK3 G623R mutation followed by disease progression [11].
We report a case of mammary analogue secretory carcinoma of the parotid gland with dual in-frame fusions involving ETV6–RET and EGFR–SEPT14. The novel EGFR–SEPT14 fusion was identified by targeted RNA sequencing and validated by both RT-PCR and Sanger sequencing. To the best of our knowledge, EGFR–SEPT14 translocation has never been reported in salivary secretory carcinoma and has only previously been reported in glioblastomas [21]. In glioma cells, this in-frame fusion was found to constitutively activate STAT3 signaling to confer growth without mitogen activation. A study by Frattini et al. showed that tumors harboring the EGFR–SEPT14 fusion are sensitive to EGFR inhibition by lapatinib and erlotinib, causing significant delay in tumor growth [21]. Interestingly, in glioblastomas, the SEPT14 fusion partner was found in exon 10, whereas in the currently reported case of MASC, the SEPT14 rearrangement involved exon 7. Both the ETV6–RET and EGFR–SEPT14 fusions may act as oncogenic drivers in the current case, and simultaneous therapy targeting both fusions may have clinical utility.
The reported case showed unusual cystic histology. A previous report of unicystic MASC mimicking cystadenoma demonstrated similar histomorphology to the currently reported case and showed complex ETV6 rearrangement with duplication of the distal ETV6 probe in 61.7% of analyzed tumor cells [22]. EGFR–SEPT14 is a novel fusion reported in mammary analogue secretory carcinoma of the salivary gland, and the only non-ETV6 translocation reported in this entity to date. While the prognostic implications of the novel EGFR–SEPT14 translocation in MASC remain unclear, it may be related to a cystic histomorphologic subtype and may have implications for treatment in clinically aggressive cases. In gliomas, tumors with the EGFR–SEPT14 fusion were sensitive to EGFR inhibition by lapatinib and erlotinib [21]. Expanded studies of the histology, immunohistochemistry, and genetic alterations of salivary gland secretory carcinomas are currently ongoing in a larger cohort.
Funding
None.
Compliance with Ethical Standards
Conflict of interest
The authors report no conflicts of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Skalova A, Vanecek T, Sima R, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6–NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 2.Diallo R, Schaefer KL, Bankfalvi A, et al. Secretory carcinoma of the breast: a distinct variant of invasive ductal carcinoma assessed by comparative genomic hybridization and immunohistochemistry. Hum Pathol. 2003;34:1299–1305. doi: 10.1016/S0046-8177(03)00423-4. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz LE, Begum S, Westra WH, Bishop JA. GATA3 immunohistochemical expression in salivary gland neoplasms. Head Neck Pathol. 2013;7:311–315. doi: 10.1007/s12105-013-0442-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laco J, Svajdler M, Jr, Andrejs J, et al. Mammary analog secretory carcinoma of salivary glands: a report of 2 cases with expression of basal/myoepithelial markers (calponin, CD10 and p63 protein) Pathol Res Pract. 2013;209:167–172. doi: 10.1016/j.prp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Tognon C, Knezevich SR, Huntsman D, et al. Expression of the ETV6–NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2:367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Ishibashi K, Masaki A, et al. Mammary analogue secretory carcinoma of salivary glands: a clinicopathologic and molecular study including 2 cases harboring ETV6-X fusion. Am J Surg Pathol. 2015;39:602–610. doi: 10.1097/PAS.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 7.Skalova A, Vanecek T, Simpson RH, et al. Mammary analogue secretory carcinoma of salivary glands: molecular analysis of 25 ETV6 gene rearranged tumors with lack of detection of classical ETV6–NTRK3 fusion transcript by standard RT-PCR: report of 4 cases harboring ETV6-X gene fusion. Am J Surg Pathol. 2016;40:3–13. doi: 10.1097/PAS.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 8.Skalova A, Vanecek T, Martinek P, et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6–RET translocation: report of 10 cases. Am J Surg Pathol. 2018;42:234–246. doi: 10.1097/PAS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 9.Rooper LM, Karantanos T, Ning Y, Bishop JA, Gordon SW, Kang H. Salivary secretory carcinoma with a novel ETV6–MET fusion: expanding the molecular spectrum of a recently described entity. Am J Surg Pathol. 2018;42:1121–1126. doi: 10.1097/PAS.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 10.Guilmette J, Dias-Santagata D, Nose V, Lennerz JK, Sadow PM. Novel gene fusions in secretory carcinoma of the salivary glands: enlarging the ETV6 family. Hum Pathol. 2019;83:50–58. doi: 10.1016/j.humpath.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Drilon A, Li G, Dogan S, et al. What hides behind the MASC: clinical response and acquired resistance to entrectinib after ETV6–NTRK3 identification in a mammary analogue secretory carcinoma (MASC) Ann Oncol. 2016;27:920–926. doi: 10.1093/annonc/mdw042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sethi R, Kozin E, Remenschneider A, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. 2014;124:188–195. doi: 10.1002/lary.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shigeta R, Orgun D, Mizuno H, Hayashi A. Mammary analogue secretory carcinoma arising in the parotid gland of child. Plast ReconstrSurg Glob Open. 2018;6:e2059. doi: 10.1097/GOX.0000000000002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Z, Liebers M, Zhelyazkova B, et al. Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20:1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]
- 15.Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6–NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Can Res. 1998;58:5046–5048. [PubMed] [Google Scholar]
- 16.Chiosea SI, Griffith C, Assaad A, Seethala RR. Clinicopathological characterization of mammary analogue secretory carcinoma of salivary glands. Histopathology. 2012;61:387–394. doi: 10.1111/j.1365-2559.2012.04232.x. [DOI] [PubMed] [Google Scholar]
- 17.Cipriani NA, Blair EA, Finkle J, et al. Salivary gland secretory carcinoma with high-grade transformation, CDKN2A/B Loss, distant metastasis, and lack of sustained response to crizotinib. Int J Surg Pathol. 2017;25:613–618. doi: 10.1177/1066896917709350. [DOI] [PubMed] [Google Scholar]
- 18.Luo W, Lindley SW, Lindley PH, Krempl GA, Seethala RR, Fung KM. Mammary analog secretory carcinoma of salivary gland with high-grade histology arising in hard palate, report of a case and review of literature. Int J Clin Exp Pathol. 2014;7:9008–9022. [PMC free article] [PubMed] [Google Scholar]
- 19.Skalova A, Vanecek T, Majewska H, et al. Mammary analogue secretory carcinoma of salivary glands with high-grade transformation: report of 3 cases with the ETV6–NTRK3 gene fusion and analysis of TP53, beta-catenin, EGFR, and CCND1 genes. Am J Surg Pathol. 2014;38:23–33. doi: 10.1097/PAS.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 20.Khotskaya YB, Holla VR, Farago AF, Mills Shaw KR, Meric-Bernstam F, Hong DS. Targeting TRK family proteins in cancer. Pharmacol Ther. 2017;173:58–66. doi: 10.1016/j.pharmthera.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Frattini V, Trifonov V, Chan JM, et al. The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 2013;45:1141–1149. doi: 10.1038/ng.2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams L, Chiosea SI. Mammary analogue secretory carcinoma mimicking salivary adenoma. Head Neck Pathol. 2013;7:316–319. doi: 10.1007/s12105-013-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]