Abstract
Hyalinizing trabecular tumor (HTT) is a benign, follicular-derived neoplasm composed of thick trabeculae with round or elongated cells having irregular and clear nuclei, and containing intra-trabecular hyaline material. The cytological features of HTT resemble those of papillary carcinoma, which helps explain why these lesions are usually classified as indeterminate/suspicious according to the Bethesda system for reporting thyroid cytology. A review of the literature indicates that reaching the correct preoperative cytologic diagnosis of HTT remains elusive, as the correct interpretation was achieved in only 8% of cases. In contrast, the correct diagnosis posed a less significant diagnostic challenge in the majority of histological series, despite the reported controversy on the relationship of this tumor with papillary and medullary thyroid carcinomas. The aim of this review is to highlight the cytological and histological clues in the diagnosis of HTT, as well as its molecular profile.
Keywords: Diagnostic pitfall, Thyroid, Immunocytochemistry, Molecular testing, Hyalinizing trabecular tumor, PAX8/GLIS 1-3 rearrangement
Introduction
Hyalinizing trabecular tumor (HTT) is a distinct and rare thyroid neoplasm of follicular cell origin characterized by a trabecular pattern associated with conspicuous intra-trabecular (rather than inter-trabecular) hyaline material [1–5]. Despite these peculiar features, recognizing HTT in pathology samples can be challenging [6–9]. In cytological specimens, HTT is often misinterpreted due to its morphological overlapping characteristics with other thyroid neoplasms including papillary thyroid carcinoma (PTC), medullary thyroid carcinoma (MTC) and with all the rare trabecular patterned tumors encountered within the thyroid gland including trabecular fetal-type follicular adenoma, poorly differentiated carcinoma (PDC), intrathyroid parathyroid tumors and metastases [10]. This negatively impacts the clinical management of these lesions as an accurate pre-surgical diagnosis of HTT is critical to avoid unnecessary overtreatment of this indolent tumor. A review of the literature shows that the correct preoperative cytologic diagnosis of HTT was rendered in only 8% of published series [10]. Although the identification of HTT on resected thyroid specimens is more straightforward, the differential diagnosis also includes PTC and MTC. The purpose of this review is to highlight the diagnostic clues for HTT in cytological and histological samples as well as by ultrasound imaging. The emerging role of molecular testing to reach the correct diagnosis of HTT is also discussed.
Ultrasound Diagnosis
Although a detailed evaluation of the ultrasonographic features of HTT has been previously reported [10–14], it is important to point out that a diagnosis of HTT cannot be reliably rendered based on ultrasound findings alone. Moreover, Jang et al. and Lee et al. note the lack of malignant features of HTT based upon ultrasound evaluation [12, 13]. However, Choi et al. found that 29% of their HTT cases showed malignant ultrasound features [14]. The majority of cases demonstrate a solitary, well-defined, oval/round, solid hypoechoic nodule without microcalcifications (Fig. 1). Since HTT is frequently “cold” on a radioactive scan the probability of a malignant lesion remains in the differential diagnosis. High intralesional blood flow may be present using power Doppler ultrasonography that is also concerning for malignancy.
Fig. 1.
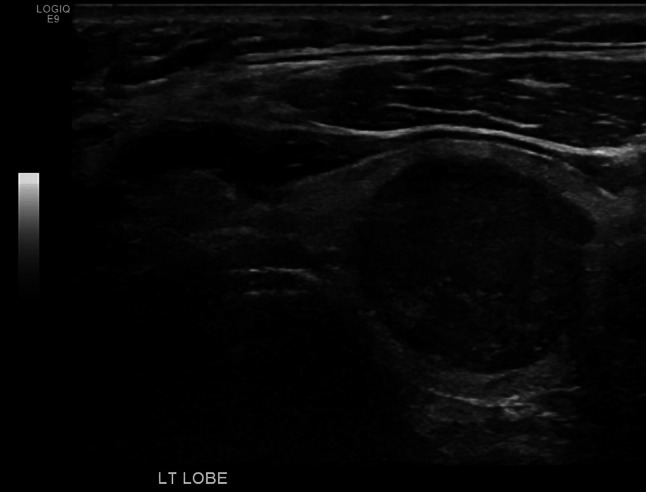
Ultrasound of a left lobe thyroid HTT showing a solid, well-circumscribed hypoechoic nodule measuring 2.7 × 1.9 × 2.1 cm in the mid-portion of the lobe
Cytological Diagnosis
One of the first publications regarding the cytological characteristics of HTT was authored by Goellner and Carney in which they underlined the morphological overlap with PTC and MTC [15]. Cytologically, HTT shows cells that may be isolated, arranged in cohesive clusters, or form syncytial clusters with a trabecular pattern [10]. The neoplastic cells are characterized by a polygonal to spindled shape with moderate or abundant pale to dense cytoplasm and containing oval nuclei with finely granular chromatin and micronucleoli.
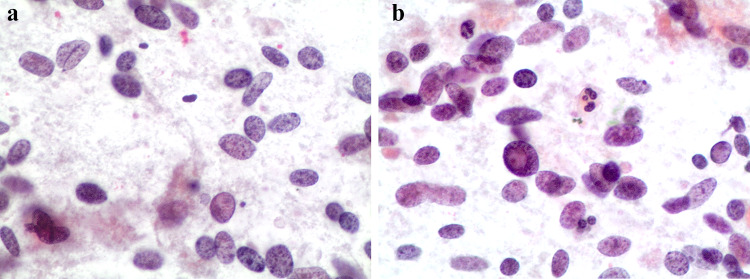
Despite the presence of nuclear grooves (Fig. 2a), pseudoinclusions (Fig. 2b) and irregular nuclear borders (Fig. 3), all of which mimic PTC, the following diagnostic clues favor HTT including the presence of hyaline or amyloid-like material (Fig. 4), loosely cohesive groups of tumor cells with a trabecular or syncytial pattern, radiating arrangement of neoplastic cells around a hyaline core, abundant eosinophilic or amphophilic cytoplasm, lack of papillae and calcifications [3, 4, 16, 17]. Although several published series have confirmed that the cytological features of HTT in fine needle aspirates often result in a suspicious or positive for PTC diagnosis [6–10], many HTT cases are classified as indeterminate/suspicious (categories three–four for the British Thyroid Association or III–V for the Bethesda System guidelines for thyroid aspirates), thus implying the need for subsequent surgical resection [18, 19]. The absence of papillary structures or fibrovascular stalks and the presence of elongated nuclei associated with hyaline stromal components are critical diagnostic clues for HTT.
Fig. 2.
a, b Cytological features of a HTT showing the oval–spindle nuclei with nuclear grooves (Fig. 2a), elongations, nuclear pseudoinclusion (Fig. 2b) and pale nuclei (conventional cytology-smears, H&E 200 ×)
Fig. 3.
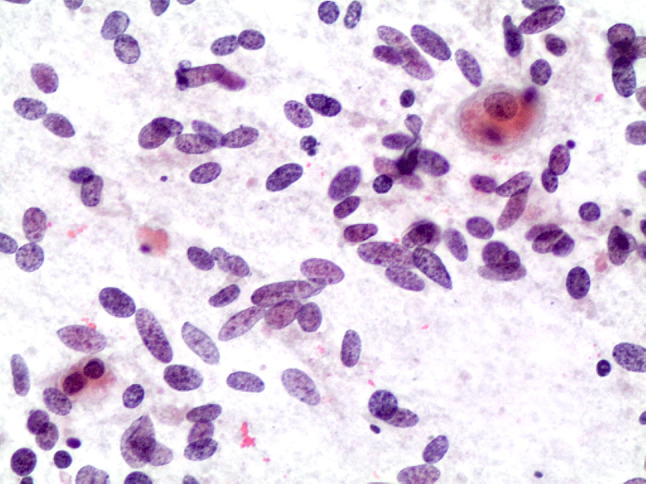
Cytological features of a HTT showing the oval–spindle nuclei with focal mild atypia (conventional cytology-smears, H&E 200 ×)
Fig. 4.
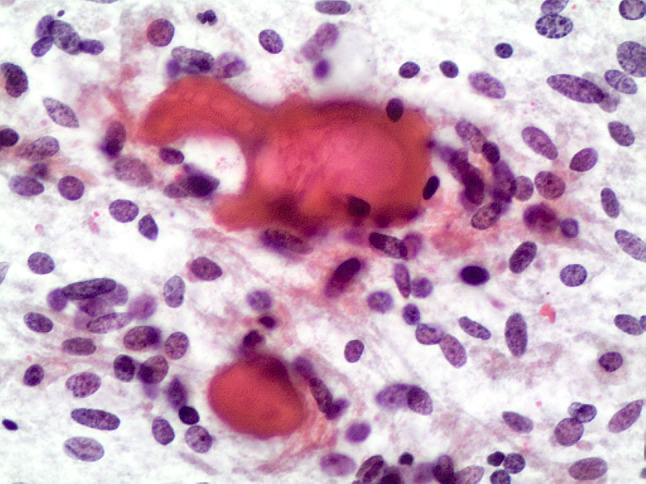
Cytological features of a HTT showing the amyloid-like material and the clusters of cells with oval–spindle nuclei with irregular nuclear membranes (conventional cytology-smears, H&E 200 ×)
The recent introduction of NIFTP poses some additional diagnostic issues [20], as both entities show nuclear features suggestive of PTC [10, 21–23]. The presence of hyaline material may be misinterpreted as amyloid, therefore leading to an incorrect diagnosis of MTC or may even thought to be colloid causing a false negative diagnosis [6–10, 18, 24]. It is important to remember that in MTC the tumor cells have eccentrically located nuclei with granular “salt and pepper” chromatin and no nucleoli. Whilst nuclear pseudoinclusions may be encountered in MTC, nuclear grooves and psammoma bodies are not [10, 11, 18, 25, 26].
The hyaline material is positive for periodic acid-Schiff stain (PAS) but negative using a Congo Red stain. The application of immunocytochemical staining may be of help in discriminating the aforementioned tumors [6, 10, 15, 17]. HTT cells are positive for thyroglobulin, TTF-1 and low-molecular weight cytokeratins. They are negative for calcitonin. Staining with cytokeratin 19, galectin-3 and HBME-1 may vary. A peculiar cell membrane reactivity of the monoclonal MIB1 to Ki67 (run at room temperature conditions), rather than nuclear staining, can further support the diagnosis of HTT [5, 24–33].
In a recent series by Dell’Aquila et al. 18 HTT with cyto-histological correlation were analyzed with the support of immunocytochemistry and BRAFV600E molecular testing. The authors demonstrated that the majority of HTTs (83.3%) were diagnosed in the indeterminate Bethesda categories, suggesting that their cytomorphological features pose issues for reaching a conclusive cytological diagnosis. The ancillary test results in the series support the fact that HTT is a benign neoplasm [34].
Histological Diagnosis and Differential Diagnoses
The differential diagnosis of HTT on histology includes follicular adenoma, trabecular fetal-like follicular adenoma, NIFTP, PTC, MTC, PDC, intrathyroidal parathyroid, metastases and paraganglioma (1–5, 10, Tables 1 and 2). On gross examination, HTT typically presents as a solid, well-circumscribed or more rarely as an encapsulated tumor without capsular, vascular or thyroid parenchyma invasion (Fig. 5). However, Gowrishankar reported a case where there was documented invasion and malignant behavior [35]. HTT are characterized by wide, mostly straight trabeculae (Fig. 6), or more rarely may be composed of small nests of tumor cells delimited by thin stromal bundles imparting a “zellballen” or lobulated appearance to some tumors [1–5, 10, 24, 36–38]. HTT cells are usually polygonal or elongated and of large/medium size. They exhibit a peculiar orientation that is perpendicular to the axis of the trabeculae (Fig. 6). Tumor cell cytoplasm is variably eosinophilic,finely granular, occasionally clear, and typically harbors paranuclear bodies which are sometimes yellow and are of unknown significance [39]. Based on their nuclear features which are vesicular and contain grooves, vacuoles and membrane irregularities, both NIFTP and PTC have to be excluded because of their overlapping nuclear features (Fig. 7, [1–5], [9], [20], Table 1). However, the presence of extensive stromal hyaline material, especially intratrabecular hyalinization, is extremely rare in PTC and NIFTP. The presence of a prominent papillary growth pattern, colloid, occasional psammoma bodies, numerous nuclear grooves and pseudoinclusions would favor PTC. The use of ancillary techniques including immunohistochemistry may be required (Table 2). The presence of membranous and cytoplasmic positivity for MIB-1 (Fig. 8) and the lack of diffuse positivity for HBME-1 and galectin-3 staining favor HTT [24–33]. Furthermore, BRAFV600E and RAS mutations go against the diagnosis of HTT [11, 25]. The discrimination between HTT and follicular adenoma is mostly based on the finding of hyaline stromal material, the presence of oval and spindle nuclei, nuclear grooves and pseudoinclusions in HTT. Cases with a dominant spindle cell component and stromal amyloid are indicative of MTC. Immunoreactivity for calcitonin and CEA in the absence of thyroglobulin staining supports a diagnosis MTC [10, 18]. Primary thyroid paraganglioma is extremely rare, but may be difficult to differentiate from HTT. Paraganglioma demonstrates positivity for synaptophysin, chromogranin A and S100 and is negative for thyroglobulin and cytokeratin [9, 11, 24]. Trabecular fetal type adenomas generally have thinner cords and do not contain hyaline material, while poorly differentiated carcinoma of the trabecular/solid type has clear signs of malignancy (i.e. capsular and or vascular invasion) and in addition does not contain the classical PTC type nuclear changes. Intrathyroidal parathyroid lesions are generally solid rather than trabecular, completely lack PTC nuclear features and a specific immunoprofile with positivity for PTH and negativity for thyroglobulin can discriminate from an intrathyroid parathyroid tumor. A combination of morphological features, clinical history and immunopanel is helpful in the differential diagnosis with metastatic lesions. Concerning PDC, a majority of these carcinomas have an architectural growth pattern that is characterized by large, well-defined solid nests, composed of cells which are usually small and uniform in size, and there is mitotic activity with three or more mitoses per 10 high-power fields. The cells of PDC are positive for keratins, TTF-1, Pax8 and thyroglobulin (sometimes weak and focal), confirming the follicular cell differentiation of PDC. Furthermore, molecular studies have identified a high incidence of RAS mutations in PDC and up to 25% harbor mutations of the CTNNB1 gene, resulting in nuclear translocation of β-catenin and activation of a number of downstream targets of the WNT signaling pathway. These mutations are not found in well-differentiated thyroid carcinomas nor in HTT.
Table 1.
Morphology and molecular findings in overlapping thyroid lesions
| Thyroid tumor | Morphology | IHC | Genetic alterations |
|---|---|---|---|
| HTT | Nests and cords of cells showing oval-spindle nuclei with nuclear pseudoinclusions and abundant intratrabecular hyaline material | KI67/ MIB-1 [cell membrane positivity] Thyroglobulin |
PAX8/GLIS1 PAX8/GLIS3 Wild type BRAF and RAS |
| PTC | Papillary and follicular structures with nuclei having irregular border, grooves and pseudoinclusions | HBME-1, Galectin-3 and CK19 | BRAF V600E mutation |
| NIFTP | Encapsulated tumor made of follicular structures (with no papillae) and more or less prominent nuclear irregularities and pseudoinclusions (as for PTC) | Thyroglobulin and PAX8 |
RAS mutations THADA fusion PAX8/PPARγ BRAF K601E mutation [Wild type BRAF V600E] |
| MTC | Spindle or plasmacytoid cells with round oval eccentric nuclei containing salt and pepper chromatin in a more or less abundant amorphous stroma (amyloid-rich) |
Calcitonin CEA |
Different alterations according to sporadic or familial MTC involving RET gene |
| Paraganglioma | Circumscribed tumor with nesting (zell ballen) or trabecular architecture made of roundish or spindle cells with minimal atypia | Neuroendocrine markers and S100 | SDHA (or B or C or D) germline mutations |
| FA | Encapsulated tumor made of follicular structures with round homogeneous nuclei | Thyroglobulin | RAS mutation |
HTT hyalinizing trabecular tumor, PTC papillary thyroid carcinoma, NIFTP non-invasive follicular thyroid neoplasm with papillary-like nuclear features, MTC medullary thyroid carcinoma, FA follicular adenoma, IHC immunohistochemistry
Table 2.
Morphological features in various thyroid tumors
| Morphologic feature | HTT | PTC | MTC | NIFTP |
|---|---|---|---|---|
| Circumscribed nodule | + | −/+ | +/− | + |
| Trabecular pattern | + | − | +/− | − |
| Spindle nuclei | + | − | +/− | − |
| Nuclear pseudoinclusions | + | + | +/− | + |
| Nuclear grooves | + | + | Rare | + |
| Hyaline material | + | − | + | − |
| Amyloid material | − | − | + | − |
| Cytoplasmic yellow bodies | + | − | − | − |
| Psammoma bodies | +/− | + | −/+ | − |
| MIB-1a | + | − | − | − |
| Cytokeratin-19 | − | + | − | +/− |
| Galectin-3 | −/+ | + | + | +/− |
HTT hyalinizing trabecular tumor, PTC papillary thyroid carcinoma, NIFTP non-invasive follicular thyroid neoplasm with papillary-like nuclear features, MTC medullary thyroid carcinoma
aMembranous and cytoplasmic expression
Fig. 5.
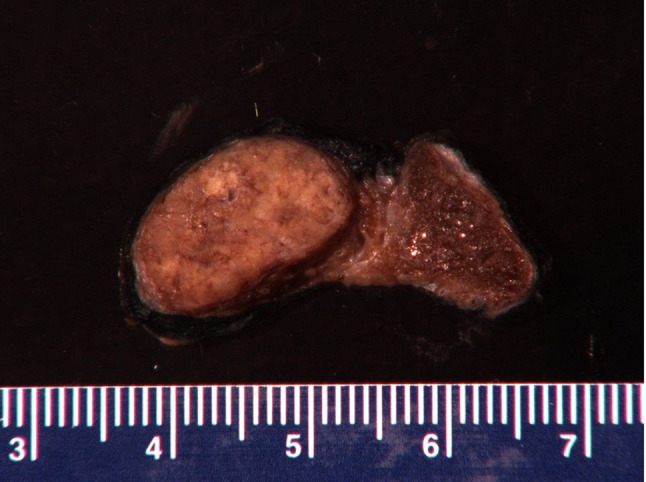
Gross features of a HTT lesions. It shows the case of a 57 years man with hypothyroidism. FNA of a suspicious right side thyroid nodule was called PTC. Total thyroidectomy was then performed and showed a 2.0 cm circumscribed mass
Fig. 6.
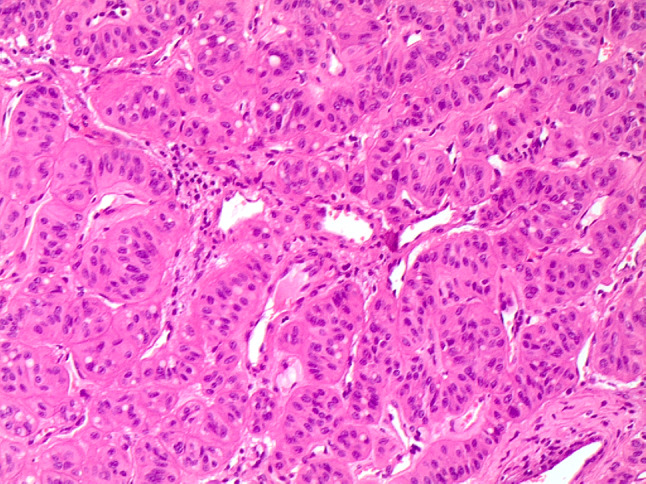
Histological trabecular pattern of HTT with the peculiar cellular features of HTT (H&E 100 ×)
Fig. 7.
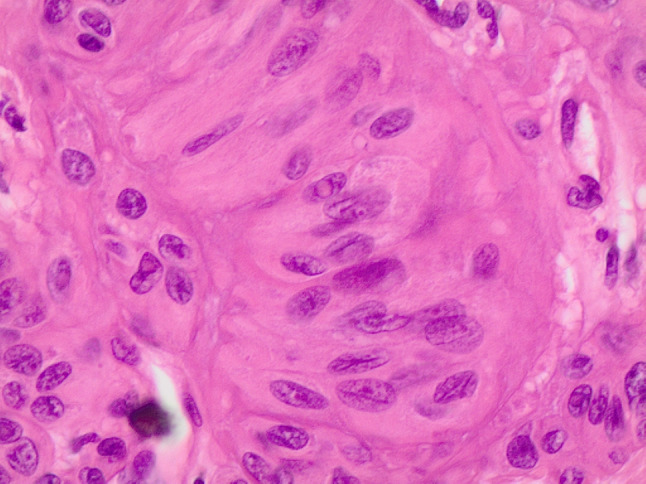
Histological details of cells with nuclear elongation and nuclear pseudoinclusions (H&E 200 ×)
Fig. 8.
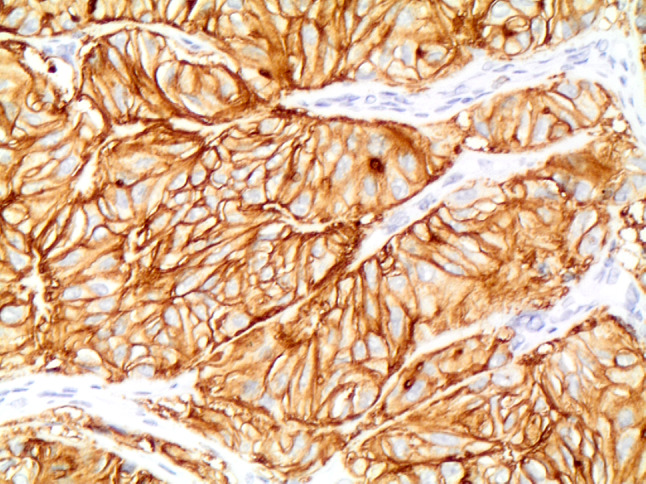
Strong membranous expression of MIB-1 in HTT (A&B 400 ×)
Role of Molecular Analysis
Since its original description [3], the molecular mechanisms of HTT have been unknown. The possible biological correlation between HTT and PTC was supported by different publications demonstrating that HTT frequently harbors RET/PTC rearrangements [40–44]. Although a few early studies demonstrated that HTT had RET/PTC fusions in 29–63% of cases [8, 41, 42], in a series of 18 HTT cases, Sheu et al. failed to detect any RET/PTC rearrangement; this was also confirmed by Nikiforov et al. in a series of 10 HTT [43, 44]. Prior research assessed the wild type status of BRAF and RAS genes, providing clear evidence that there is a distinct molecular pattern involved in the development of HTT [41]. Recent studies have demonstrated the molecular role of the GLIS family in HTT, including PAX8-GLIS3 fusion [45]. Concerning GLIS3, a member of the GLI-similar zinc finger protein family encoding a nuclear protein with five C2H2-type zinc finger domains, it acts as both a repressor and activator of transcription and it is involved in the development of the thyroid gland [45].
A recent publication by Nikiforova et al. investigating 14 HTT cases by next generation sequencing analysis and other techniques, revealed that 13 out of 14 of their cases were positive for the novel PAX8-GLIS3 fusion and one case for PAX8-GLIS1 fusion [45]. Of note, in their control series of 220 histologically proven PTC, no PAX8-GLIS3 and/or PAX8-GLIS1 fusions were found. The authors thus concluded that the morphological features of HTT, including the deposition of collagen and hyaline matrix, are the result of these fusions that lead to overexpression of GLIS and upregulation of extracellular matrix genes. According to their study, this molecular testing can be performed with reliable results on cytological samples. In addition, the specific fusion involving GLIS can be detected by immunochemical analysis. This novel molecular finding has been recently validated in a larger series of 34 HTT, in which PAX8-GLIS3 fusion (but not PAX8-GLIS1) was identified in all cases, but not in any control thyroid tumor histotype analysed [46]. Interestingly, the control group included a series of 15 thyroid trabecular neoplasms of follicular derivation that morphologically resembled HTT and indeed some of these were originally reported as HTT. Upon revision of the morphology and the immunoprofile (namely the Ki67 pattern), these were eventually reclassified as classified as trabecular/embryonal variant of follicular adenoma, solid/trabecular Hurthle cell adenoma or carcinoma, solid/trabecular variant of PTC and poorly differentiated carcinoma [46]. Nonetheless, the molecular features are a work in progress, but the PAX8-GLIS1 or GLIS3 fusions are intriguing and may be followed by additional markers in the future.
Conclusion
HTT are rare tumors of the thyroid defined by a trabecular architecture associated with extensive intratrabecular hyaline stromal material. HTT is considered a benign neoplasm managed by conservative surgical excision, only [45–49]. The fine needle aspiration cytological diagnosis of HTT is challenging due to the presence of nuclear clearing, grooves and intranuclear pseudoinclusions that mimic PTC, and may not be conclusive, rather falling into the indeterminate categories. Although the identification of HTT on histological samples is apparently straightforward, due to morphological similarities, it may still be mistaken for PTC and MTC [44–48]. Ancillary techniques including cell membrane immunoreactivity of MIB1 monoclonal to Ki67 and the molecular identification of the novel PAX8-GLIS1 or PAX8-GLIS3 fusions may aid in making the correct diagnosis and help avoid overtreatment [50]. In conclusion, a definitive diagnosis of HTT can be only made on resected specimens.
Funding
This work did not receive any specific funding.
Compliance with Ethical Standards
Conflict of interest
The authors have no conflict of interest to declare.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Esther Diana Rossi, Mauro Papotti, William Faquin, Luigi Maria Larocca and Liron Pantanowitz have equally contributed to the paper.
References
- 1.Carney JA. Hyalinizing trabecular tumors of the thyroid gland: quadruply described but not by the discoverer. Am J Surg Pathol. 2008;32(4):622–634. doi: 10.1097/PAS.0b013e3181608545. [DOI] [PubMed] [Google Scholar]
- 2.Carney JA, Hirokawa M, Lloyd RV, Papotti M, Sebo TJ. Hyalinizing trabecular tumors of the thyroid gland are almost all benign. Am J Surg Pathol. 2008;32(12):1877–1889. doi: 10.1097/PAS.0b013e31817a8f1b. [DOI] [PubMed] [Google Scholar]
- 3.Carney JA, Ryan J, Goellner JR. Hyalinizing trabecular adenoma of the thyroid gland. Am J Surg Pathol. 1987;11(8):583–591. doi: 10.1097/00000478-198708000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Bakuła-Zalewska E, Cameron R, Gałczyński JP, Domanski HA. Hyaline matrix in hyalinizing trabecular tumor: findings in fine-needle aspiration smears. Diagn Cytopathol. 2015;43(9):710–713. doi: 10.1002/dc.23224. [DOI] [PubMed] [Google Scholar]
- 5.Papotti MG, Volante M, et al. Hyalinizing trabecular tumor. In: Lloyd RV, et al., editors. WHO classifications of tumors of the endocrine organs. 4. Lyon: IARC Press; 2017. [Google Scholar]
- 6.Casey MB, Sebo TJ, Carney JA. Hyalinizing trabecular adenoma of the thyroid gland: cytologic features in 29 cases. Am J Surg Pathol. 2004;28(7):859–867. doi: 10.1097/00000478-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Casey MB, Sebo TJ, Carney JA. Hyalinizing trabecular adenoma of the thyroid gland identification through MIB-1 staining of fine-needle aspiration biopsy smears. Am J Clin Pathol. 2004;122(4):506–510. doi: 10.1309/5KJU-9LV8-LLA6-CL76. [DOI] [PubMed] [Google Scholar]
- 8.Cheung CC, Boerner SL, MacMillan CM, Ramyar L, Asa SL. Hyalinizing trabecular tumor of the thyroid: a variant of papillary carcinoma proved by molecular genetics. Am J Surg Pathol. 2000;24:1622–1626. doi: 10.1097/00000478-200012000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bishop JA, Ali S. Hyalinizing trabecular adenoma of the thyroid gland. Diagn Cytopathol. 2010;39:306–310. doi: 10.1002/dc.21413. [DOI] [PubMed] [Google Scholar]
- 10.Saglietti C, Piana S, La Rosa S, Bongiovanni M. Hyalinizing trabecular tumor of the thyroid: Fine needle aspiration cytological diagnosis and correlation with histology. J Clin Pathol. 2017;70:641–647. doi: 10.1136/jclinpath-2017-204360. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov YE. Hyalinizing trabecular tumor. In Diagnostic pathology and molecular genetics of the thyroid. A comprehensive guide for practicing thyroid pathology. 3rd ed. Philadephia: Wolters Kluwer
- 12.Jang H, Park CK, Son EJ, Kim EK, Kwak JY, Moon HJ, Yoon JH. Hyalinizing trabecular tumor of the thyroid: diagnosis of a rare tumor using ultrasonography, cytology and intraoperative frozen sections. Ultrasonography. 2016;35:131–139. doi: 10.14366/usg.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Han BK, Ko EY, Oh YL, Choe JH, Shin JH. The ultrasonography features of hyalinizing trabecular tumor of the thyroid are more consistent with its benign behavior than cytology or frozen section readings. Thyroid. 2011;21(3):253–259. doi: 10.1089/thy.2010.0202. [DOI] [PubMed] [Google Scholar]
- 14.Choi WJ, Baek JH, Ha EJ, Choi YJ, Hong MJ, Song DE, Sung JY, Yoo H, Jung SL, Lee HY, Lee JH. The ultrasonography features of hyalinizing trabecular tumor of the thyroid gland and the role of fine needle aspiration cytology and core needle biopsy in its diagnosis. Acta Radiol. 2015;56(9):1113–1118. doi: 10.1177/0284185114549225. [DOI] [PubMed] [Google Scholar]
- 15.Goellner JR, Carney JA. Cytologic features of fine needle aspirates of hyalinizing trabecular adenoma of the thyroid. Am J Clin Pathol. 1989;91:115–119. doi: 10.1093/ajcp/91.2.115. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan A, Lin X, Nayar R. Thyroid Bethesda reporting category, ‘suspicious for papillary thyroid carcinoma’, pitfalls and clues to optimize the use of this category. Cytopathology. 2013;24(2):85–91. doi: 10.1111/j.1365-2303.2012.00966.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim T, Oh YL, Kim KM, Shin JH. Diagnostic dilemmas of hyalinizing trabecular tumours on fine needle aspiration cytology: a study of seven cases with BRAF mutation analysis. Cytopathology. 2011;22(6):407–413. doi: 10.1111/j.1365-2303.2011.00886.x. [DOI] [PubMed] [Google Scholar]
- 18.Ali S, Cibas ES. The Bethesda system for reporting thyroid cytopathology. 2. Berlin: Springer; 2018. [DOI] [PubMed] [Google Scholar]
- 19.British Thyroid Association, Royal College of Physicians. Guidelines for the management of thyroid cancer. In: Perros P editor. Report of the Thyroid Cancer Guidelines Update Group. 2nd ed. London: Royal College of Physicians; 2007
- 20.Nikiforov YE, Seethala RR, Tallini G, Baloch ZW, Basolo F, Thompson LD, et al. Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Oncol. 2016;2:1023–1029. doi: 10.1001/jamaoncol.2016.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maletta F, Massa F, Torregorssa L, et al. Cytological features of “non-invasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol. 2016;54:134–142. doi: 10.1016/j.humpath.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Faquin W, Wong L, Afrogheh A, et al. The impact of reclassifying non invasive FVPC on the risk of malignancy in the Bethesda system for reporting thyroid cytopathology. Cancer Cytopathol. 2016;124:181–187. doi: 10.1002/cncy.21631. [DOI] [PubMed] [Google Scholar]
- 23.Bizzarro T, Martini M, Capodimonti S, et al. The morphologic analysis of non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) on liquid based cytology: some insights of their identification in our institutional experience. Cancer. 2016;124:699–710. doi: 10.1002/cncy.21777. [DOI] [PubMed] [Google Scholar]
- 24.Rhee YY, Jung HK, Kim SH, Kim SH. Hyalinizing trabecular tumor of the thyroid gland, a diagnostic challenge in fine needle aspiration cytology: case report. J Pathol Transl Med. 2018;52:252–256. doi: 10.4132/jptm.2018.04.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirokawa M, Suzuki A, Kuma S. Hyalinizing trabecular tumor. In: Kakudo K, editor. Thyroid FNA cytology: differential diagnoses and pitfalls. 2. Springer: New York; 2019. [Google Scholar]
- 26.Llyod RV, Osamura RY, Kloppel G, Rosai J. WHO classifications of tumors of the endocrine organs. 4. Lyon: International Agency for Research on Cancer; 2017. Tumors of the thyroid gland. [Google Scholar]
- 27.Gaffney RL, Carney JA, Sebo TJ, Erickson LA, Volante M, Papotti M, Lloyd RV. Galectin-3 expression in hyalinizing trabecular tumors of the thyroid gland. Am J Surg Pathol. 2003;27(4):494–498. doi: 10.1097/00000478-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Galgano MT, Mills SE, Stelow EB. Hyalinizing trabecular adenoma of the thyroid revisited: a histologic and immunohistochemical study of thyroid lesions with prominent trabecular architecture and sclerosis. Am J Surg Pathol. 2006;30(10):1269–1273. doi: 10.1097/01.pas.0000209858.13035.0a. [DOI] [PubMed] [Google Scholar]
- 29.Hirokawa M, Carney JA. Cell membrane and cytoplasmic staining for MIB-1 in hyalinizing trabecular adenoma of the thyroid gland. Am J Surg Pathol. 2000;24(4):575–578. doi: 10.1097/00000478-200004000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Leonardo E, Volante M, Barbareschi M, Cavazza A, Dei Tos AP, Bussolati G, Papotti M. Cell membrane reactivity of MIB-1 antibody to Ki67 in human tumors: fact or artifact? Appl Immunohistochem Mol Morphol. 2007;15(2):220–223. doi: 10.1097/01.pai.0000213122.66096.f0. [DOI] [PubMed] [Google Scholar]
- 31.Ohtsuki Y, Kimura M, Murao S, Okada Y, Teratani Y, Matsumoto M, Kurabayashi A, Iguchi M, Lee GH, Furihata M. Immunohistochemical and electron microscopy studies of a case of hyalinizing trabecular tumor of the thyroid gland, with special consideration of the hyalinizing mass associated with it. Med Mol Morphol. 2009;42(3):189–194. doi: 10.1007/s00795-008-0415-x. [DOI] [PubMed] [Google Scholar]
- 32.Galgano MT, Mills SE, Stelow EB. Hyalinizing trabecular adenoma of the thyroid revisisted: a histologic and immunohistochemical study of thyroid lesions with prominent trabecular architecture and sclerosis. Am J Surg Pathol. 2006;30:1269–1273. doi: 10.1097/01.pas.0000209858.13035.0a. [DOI] [PubMed] [Google Scholar]
- 33.Lenggenhager D, Maggio EM, Moch H, Rössle M. HBME-1 expression in hyalinizing trabecular tumours of the thyroid gland. Histopathology. 2013;62(7):1092–1097. doi: 10.1111/his.12123. [DOI] [PubMed] [Google Scholar]
- 34.Dell’Aquila M, Gravina C, Cocomazzi A, Capodimonti S, Musarra T, Sfregola S, Fiorentino V, Revelli L, Martini M, Fadda G, Pantanowitz L, Larocca LM, Rossi ED. A large series of hyalinizing trabecular tumors: cytomorphology and ancillary techniques on fine needle aspiration. Cancer Cytopathol. 2019 doi: 10.1002/cncy.22139. [DOI] [PubMed] [Google Scholar]
- 35.Gowrishankar S, Pai SA, Carney JA. Hyalinizing trabecular carcinoma of the thyroid gland. Histopathology. 2008;52(4):529–531. doi: 10.1111/j.1365-2559.2008.02945.x. [DOI] [PubMed] [Google Scholar]
- 36.Li M, Carcangiu ML, Rosai J. Abnormal intracellular and extracellular distribution of basement membrane material in papillary carcinoma and hyalinizing trabecular tumors of the thyroid: implication for deregulation of secretory pathways. Hum Pathol. 1997;28(12):1366–1372. doi: 10.1016/s0046-8177(97)90225-2. [DOI] [PubMed] [Google Scholar]
- 37.McCluggage WG, Sloan JM. Hyalinizing trabecular carcinoma of thyroid gland. Histopathology. 1996;28(4):357–362. doi: 10.1046/j.1365-2559.1996.d01-432.x. [DOI] [PubMed] [Google Scholar]
- 38.Molberg K, Albores-Saavedra J. Hyalinizing trabecular carcinoma of the thyroid gland. Hum Pathol. 1994;25(2):192–197. doi: 10.1016/0046-8177(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 39.Rothenberg HJ, Goellner JR, Carney JA. Hyalinizing trabecular adenoma of the thyroid gland: recognition and characterization of its cytoplasmic yellow body. Am J Surg Pathol. 1999;23(1):118–125. doi: 10.1097/00000478-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Papotti M, Riella P, Montemurro F, Pietribiasi F, Bussolati G. Immunophenotypic heterogeneity of hyalinizing trabecular tumours of the thyroid. Histopathology. 1997;31(6):525–533. doi: 10.1046/j.1365-2559.1997.3070893.x. [DOI] [PubMed] [Google Scholar]
- 41.Papotti M, Volante M, Giuliano A, Fassina A, Fusco A, Bussolati G, Santoro M, Chiappetta G. RET/PTC activation in hyalinizing trabecular tumors of the thyroid. Am J Surg Pathol. 2000;24(12):1615–1621. doi: 10.1097/00000478-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 42.Salvatore G, Chiappetta G, Nikiforov YE, Decaussin-Petrucci M, Fusco A, Carney JA, Santoro M. Molecular profile of hyalinizing trabecular tumours of the thyroid: high prevalence of RET/PTC rearrangements and absence of B-RAF and N-RAS point mutations. Eur J Cancer. 2005;41(5):816–821. doi: 10.1016/j.ejca.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Sheu SY, Vogel E, Worm K, Grabellus F, Schwertheim S, Schmid KW. Hyalinizing trabecular tumour of the thyroid-differential expression of distinct miRNAs compared with papillary thyroid carcinoma. Histopathology. 2010;56(5):632–640. doi: 10.1111/j.1365-2559.2010.03526.x. [DOI] [PubMed] [Google Scholar]
- 44.Nikiforov YE, Carty SE, Chiosea SL, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120:3627–3634. doi: 10.1002/cncr.29038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nikiforova MN, Nikitski AV, Panebianco F, et al. GLIS rearrangement is a genomic hallmark of hyalinizing trabecular tumor of the thyroid gland. Thyroid. 2019;29:161–173. doi: 10.1089/thy.2018.0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchiò C, Da Cruz Paula A, Gularte-Merida R, Brandes TBA, Da Silva EM, Silveira C, et al. PAX8-GLIS3 gene fusion is a pathognomonic genetic alteration of hyalinizing trabecular tumors of the thyroid. Mod Pathol. 2019 doi: 10.1038/s41379-019-0313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sung SY, Shen HY, Hsieh CB, Duh QY, Su TF, Chan DC, Shih ML. Hyalinizing trabecular tumor of thyroid: does frozen section prevent unnecessarily aggressive operation? Six new cases and a literature review. J Chin Med Assoc. 2014;77(11):573–577. doi: 10.1016/j.jcma.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Nose V, Volante M, Papotti M. Hyalinizing trabecular tumor of the thyroid: an update. Endocr Pathol. 2008;19:1–8. doi: 10.1007/s12022-007-9002-2. [DOI] [PubMed] [Google Scholar]
- 49.Jones D, Kieliszak C, Patel SS, Selinsky C. Hyalinizing trabecular tumor of the thyroid gland and its significant diagnostic issues. Thyr Res. 2017;10:7–11. doi: 10.1186/s13044-017-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikiforova MN, Nikiforov YE, Ohori NP. GLIS rearrangements in thyroid nodules: a key to preoperative diagnosis of hyalinizing trabecular tumor. Cancer Cytopathol. 2019 doi: 10.1002/cncy.22163. [DOI] [PubMed] [Google Scholar]