Abstract
In 2015, Lewis et al. first described low-grade papillary Scheneiderian carcinoma (LGPSC) of the sinonasal tract. Their case resembled a sinonasal papilloma clinically and histopathologically; however, invasion and metastasis resulted in the death of the patient despite absence of malignant cytologic features. Additional reports established LGPSC as a distinct entity and characterized its immunohistochemical profile. Diffuse expression of low molecular weight cytokeratins, positivity for p16 and p53 in at least 50% of cells, a high Ki-67 index, and absence of human papillomavirus (HPV)-DNA was observed across all reported cases. We report an additional case of LGPSC and describe the clinical, histologic, and immunohistochemical features. In contrast to sinonasal papillomas, the case was negative for HPV-DNA and showed no mutations in the EGFR and KRAS hotspot regions.
Keywords: Sinonasal papillary carcinoma, Schneiderian papilloma, Inverted papilloma, Sinonasal epithelium
Introduction
The sinonasal tract, comprising the nasal cavity and paranasal sinuses, is lined by a distinctive transitional, ciliated epithelium of ectodermal origin exclusive to this site. The most common benign neoplasms to arise from this epithelium are sinonasal papillomas (SPs). SPs are associated with either EGFR somatic mutations or HPV infection [1]. The World Health Organization (WHO) classification traditionally distinguishes SPs into inverted, exophytic, and oncocytic subtypes [2]. Inverted and oncocytic SPs have a low risk of malignant progression to conventional squamous cell carcinoma (SCC), estimated in literature between 5 and 10% [3], commonly associated with EGFR activating mutations [4].
Lewis et al. first described low-grade papillary Scheneiderian carcinoma (LGPSC) in 2015 with a case that resembled a SP clinically and histopathologically [5]. Despite the absence of frankly malignant morphological features, invasion and metastasis resulted in the patient’s death [5]. The invasive potential and morphological features of LGPSC were confirmed in recent reports [6–8]. We describe the clinical, histologic, and molecular features of an additional case of LGPSC.
Case Report
A 68 year-old male with chronic nasal obstruction and discharge underwent endoscopic nasal biopsy to confirm a clinical suspicion of SP. Endoscopy showed a soft, pink-gray, papillary growth obstructing the upper airways. The resulting biopsy diagnosis was suggestive of malignancy. A computed tomography (CT) scan revealed a 1.2 cm mass of the right inferior nasal cavity extending from the septum to the nasal floor. Positron emission tomography (PET) scanning showed high metabolic activity. A transnasal resection with complete surgical removal of the mass was performed. The patient was free of disease at 16 months post-surgery.
Materials and Methods
Hematoxylin and eosin stained sections were obtained from formalin fixed paraffin embedded (FFPE) tissue. Immunohistochemical (IHC) reactions with antibodies against cytokeratin (CK) 7, CK19, 34BetaE12, Ki-67, p53, p40, and p16 were performed on a OMNIS autostainer (Dako Agilent, Santa Clara, CA). HPV-DNA and specific genotypes were investigated with the INNO-Lipa HPV-genotyping Extra II test (Fujirebio Europe, Ghent, Belgium) as previously described [9]. Mutations in exons 18–21 of EGFR and exons 2 and 3 of KRAS were assessed with DNA-PCR amplification and direct sequencing with an Applied Biosystems 3130 Genetic Analyzer (Thermo Fisher Scientific). The patient’s clinical records were reviewed to identify possible risk factors or comorbidities.
Histologic Findings
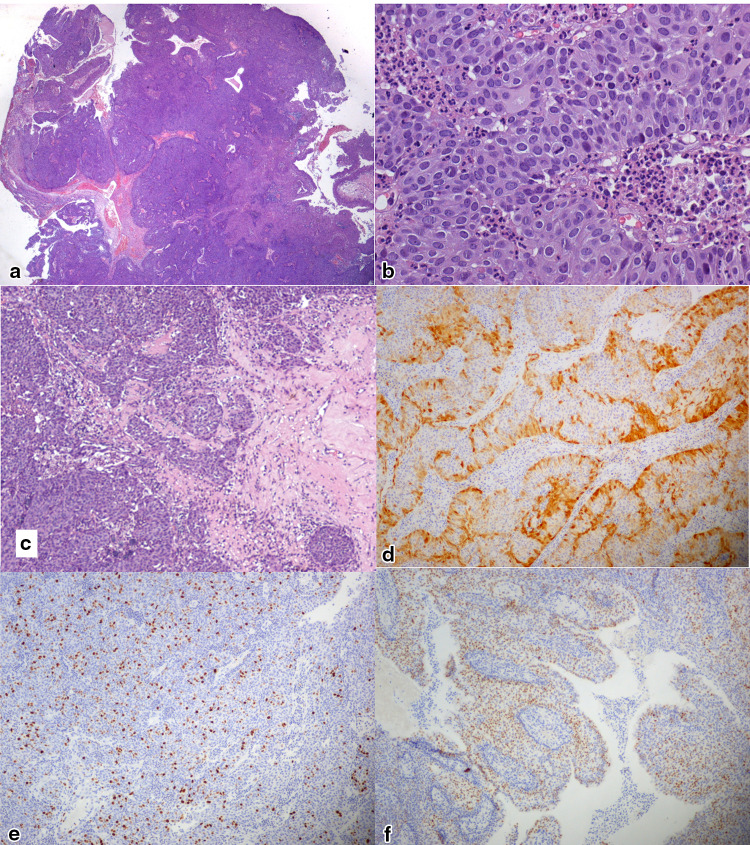
The histological and immunohistochemical characters of the lesion are documented in Fig. 1. Histologically, the lesion showed complex architecture with exophytic papillary projections and endophytic, pushing borders invading into the underlying connective tissues. The projections and invaginations comprised a neoplastic, multilayered epithelial proliferation that demonstrated focal surface keratinization and increased mitotic activity (up to 5 mitotic figures per 10 high powered fields). The cells had abundant, eosinophilic cytoplasm and round nuclei with fine chromatin and occasional small nucleoli. No stromal reaction to the neoplasm was observed. The residual adjacent mucosa was typical sinonasal transitional epithelium with focal squamous metaplasia.
Fig. 1.
Photomicrographs documenting the morphologic and immunohistochemical features of LGPSC observed in Case 1: a low-power view (5 ×) of H&E-stained sections shows a complex papillary growth of epithelial neoplastic cells with broad, pushing nests; b high-power view (40 ×) of H&E-stained sections showing basaloid non-keratinizing cells with abundant cytoplasm, mitoses, and prominent granulocytic infiltrate; c neoplastic nests show deeply infiltrative behavior (10 ×); d P16 staining at the periphery of neoplastic cell cords and nests (10 ×); e MIB1-Ki67 was expressed in a variable proportion of neoplastic cells averaging 20% (10 ×); f P53 was expressed in 50% of neoplastic cells (10 ×)
Immunohistochemical expression of p40 and high molecular weight cytokeratins proved squamous differentiation of the neoplastic cells. CK19 was diffusely positive while CK7 expression was more heterogeneous, ranging from negative to full-thickness positivity in different areas. Strong p16 expression was noted at the periphery of the cell sheets while central areas were negative (70% positive cells overall). This finding prompted HPV analysis which was negative for HPV-DNA. Fifty percent of neoplastic cells expressed p53. The Ki-67 index showed wide variability and ranged from 2 to 50% in different areas. No EGFR or KRAS activating mutations were detected by PCR (Table 1).
Table 1.
Summary of relevant clinical and molecular features of reported LGPSC cases
| Sex/age | p16 | p53 | Ki67 | HPV-DNA | Smoke | Initial pathologic diagnosis | Recurrence | Nodal metastasis | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|---|
| Lewis 2015 [5] | F/47 | 50% | 50% | 5–60% | Negative | NA | Schneiderian papillomas | Multiple | Yes | 18 years (DOD) |
| Jeong 2017 [6] | F/42 | Peripheral | 50% | 10% | NA | NA | Schneiderian oncocytic papilloma | No | No | 21 months |
| Brown 2018 [7] | F/65 | NA | NA | NA | NA | NA | Schneiderian papilloma and nasal polyps | 1 month | No | 3 months |
| Williamson 2019 [8] | F/56 | negative | NA | 15% | Negative | NA | Transitional proliferation with inverted growth | 13 months | No | >12 months |
| Our case | M/68 | 70% (peripheral) | 50% | 50% | Negative | Former heavy | None | No | No | 16 months |
Discussion
Recently characterized, reports of nasal LGPSC are limited to five cases including this one. All share the morphologic and clinical features observed in the original paper [5–8]. This new entity raises questions regarding its origin from the sinonasal epithelium and relationship with the more common SP. It is worth noting that all LGPSCs were diagnosed upon recurrence of what was originally thought to be a SP. Although this might suggest a possible evolution from SP, revision of the original samples, when available, documented peculiar histopathological features, distinct from conventional SP, and increasing morphological atypia with disease progression [5, 7].
Thus far, two mutually exclusive oncogenic pathways leading to the development of SP and associated SCC, namely HPV infection and MAPK pathway activation, have been identified [1, 10, 11]. Our observation that, besides HPV DNA [5, 8], also EGFR or KRAS mutation are not found in LGSPC suggests that development of LGPSC is independent from benign papillomas of the sinonasal epithelium [5, 8]. A progressive spectrum of epithelial squamous metaplasia with increasing dysplasia to frank invasion is often observed with malignant transformation of SP into SCC, suggesting a multi-step pathogenesis [5]. In support of an independent process, dysplastic features have not been described in the mucosa surrounding the reported LGSPC [5–7], leaving open the question of their evolution from normal sinonasal epithelium.
Expression of p16 in SP and derived carcinomas, as well as in LGSPC, suggests that its role is relevant to neoplastic transformation of the sinonasal epithelium through a pathway of activation independent from HPV infection [11].
Follow-up of this patient is necessary to determine whether radical surgical treatment is appropriate to cure early stage disease. Previous reports show that incomplete resection leads to repeated recurrence and the possibility of regional metastasis and death, thus correct initial diagnosis is important for proper management in early stages [5, 7, 8]. Chemotherapy was insufficient for disease control [5] while intensity-modulated radiotherapy was associated with 21-months disease-free follow-up in a case with indeterminate resection margins [6]. It is important that head and neck pathologists distinguish this new entity from conventional benign SP to recommend appropriate treatment, radical resection when feasible, and close follow-up.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Udager AM, McHugh JB, Goudsmit CM, et al. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann Oncol. 2018;29:466–471. doi: 10.1093/annonc/mdx736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop JA, Bell D, Westra WH. Carcinomas of the nasal cavity, paranasal sinuses and skull base. In: El-Naggar AK, editor. World Health Organization classification of head and neck tumours. Lyon: IARC Press; 2017. pp. 14–26. [Google Scholar]
- 3.Nudell J, Chiosea S, Thompson LD. Carcinoma ex-schneiderian papilloma (malignant transformation): a clinicopathologic and immunophenotypic study of 20 cases combined with a comprehensive review of the literature. Head Neck Pathol. 2014;8:269–286. doi: 10.1007/s12105-014-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Udager AM, Rolland DCM, McHugh JB, et al. High-frequency targetable EGFR mutations in sinonasal squamous cell carcinomas arising from inverted sinonasal papilloma. Cancer Res. 2015;75:2600–2606. doi: 10.1158/0008-5472.CAN-15-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JS, Chernock RD, Haynes W, et al. Low-grade papillary schneiderian carcinoma, a unique and deceptively bland malignant neoplasm. Am J Surg Pathol. 2015;39–5:714–721. doi: 10.1097/PAS.0000000000000390. [DOI] [PubMed] [Google Scholar]
- 6.Jeong HJ, Roh J, Lee BJ, Cho KJ. Low-grade papillary schneiderian carcinoma: a case report. Head Neck Pathol. 2018;12:131–135. doi: 10.1007/s12105-017-0832-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CS, Abi Hachem R, Pendse A, Madden JF, Francis HW. Low-Grade papillary schneiderian carcinoma of the sinonasal cavity and temporal bone. Ann Otol Rhinol Laryngol. 2018;127:974–977. doi: 10.1177/0003489418803391. [DOI] [PubMed] [Google Scholar]
- 8.Williamson A, Sharma R, Cooper L, McGarry G. Low-grade schneiderian carcinoma: rare or underecognised? Otolaryngol Case Rep. 2019;11:100107. doi: 10.1016/j.xocr.2019.02.001. [DOI] [Google Scholar]
- 9.Morbini P, Dal Bello B, Alberizzi P, et al. Oral HPV infection and persistence in patients with head and neck cancer. Oral Surg Oral Med. 2013;116:474–484. doi: 10.1016/j.oooo.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Udager AM, McHugh JB, Betz BL, et al. Activating KRAS mutations are characteristic of oncocytic sinonasal papilloma and associated sinonasal squamous cell carcinoma. J Pathol. 2016;239:394–398. doi: 10.1002/path.4750. [DOI] [PubMed] [Google Scholar]
- 11.Shah AA, Evans MF, Adamson CS, Peng Z, Rajendran V, Cooper K. HPV DNA is associated with a subset of Schneiderian papillomas but does not correlate with p16(INK4a) immunoreactivity. Head Neck Pathol. 2010;4:106–112. doi: 10.1007/s12105-010-0176-4. [DOI] [PMC free article] [PubMed] [Google Scholar]