Abstract
The cancer stem cells deliver uncontrolled proliferative capacity within the tumor imparting to increasing size while epithelial mesenchymal transition adds to the invasive potential. Studies using specific markers elucidating the role of these phenomena may bring advancement in the targeted therapy of tumor. SOX2 and OCT4 are two among few stem cell markers indicative of proliferative potential and WNT5A is an epithelial mesenchymal transition marker indicative of invasive potential. We aimed to determine the association between expression of SOX2, OCT4 and WNT5A in oral epithelial dysplasia, oral squamous cell carcinoma and normal oral mucosa. 20 cases of oral squamous cell carcinoma, 20 cases of oral epithelial dysplasia (leukoplakia with dysplasia) and 25 normal oral mucosa tissues specimens were immunohistochemically stained to assess SOX2, OCT4 and WNT5A expression. SOX2 expression was higher in oral squamous cell carcinoma than in oral epithelial dysplasia and very low in normal oral mucosa. OCT4 was very low in oral squamous cell carcinoma and oral epithelial dysplasia when compared to SOX2, while negative in normal tissues. Co-expression of SOX2 and OCT4 showed statistically non-significant difference for tumor proliferation. WNT5A expression was found to be increasing from normal oral mucosa to oral epithelial dysplasia and oral squamous cell carcinoma. In conformity with present study, SOX2 itself can act as a potential marker for proliferation in tumor cells while OCT4 has non-significant role in regulation of tumor behavior in oral squamous cell carcinoma as well as in oral epithelial dysplasia. WNT5A can be a putative marker in studying invasive potential of oral squamous cell carcinoma.
Keywords: Oral squamous cell carcinoma, Oral epithelial dysplasia, OCT4, SOX2, WNT5A
Introduction
Cancer stem cells (CSC) are specialized population of cells in cancerous tissue with ability to initiate a tumor as well as to sustain their self-renewal property [1–3]. This property is usually shown by the cells of embryonic origin, known as embryonic stem cells (ESC) [1, 3]. These population of cells have high tumorigenic potential and are believed to be largely responsible for the biological characteristics of cancer, namely, rapid growth, invasion, and metastasis [1]. One among few accepted theories proposes that CSCs arise as a result of epigenetic or genetic alterations in the resident tissue stem cells [4]. Identification of these CSCs in tumor tissue is the new area of research anticipating common molecules might exist between CSCs and ESCs.
The expression profiles of many proteins markers like SOX2, OCT4, NANOG, CD44, CD133, CD24 and ALDH1 have been studied as putative CSC markers in oral squamous cell carcinoma (OSCC) samples and cell lines [4–9]. So far no single protein marker could unequivocally identify the CSCs [4, 5]. SOX2 (SRY-related HMG-box gene2) is shown to act as an important transcriptional factor to maintain the self-renewal capability of ESCs. The SOX2 gene is located on chromosome 3q26.3–q27, belongs to the SOXB1 group and encodes for a protein consisting of 317 amino acids. SOX2 is comprised of three main domains: N-terminal, HMG and transactivation domain. Studies on SOX2 proves its crucial role in stem cell maintenance and an important factor to reprogram somatic cells back towards pluripotency. SOX2 expression amplification has been found in several cancer like glioblastoma, small-cell lung cancer and many forms of squamous cell carcinoma. The role of SOX2 in behavior of cancer has been studies by many authors. In breast, prostate, pancreatic and cervical cancers SOX2 has been shown to promote cellular proliferation, evade apoptotic signals and promote invasion, migration and metastasis [10]. OCT4 (Octamer binding protein 4) is a member of the family of POU domain transcription factor known to bind in partnership with SOX2, and act as a key regulator essential for the pluripotency and self-renewal capacity of ESCs [11, 12]. OCT4 and SOX2 together are considered as master regulators for self-renewal and maintenance of the stem cell population in the undifferentiated tissue. The transcriptional factors, OCT4 and SOX2 are co-expressed in ESCs but double positive co-expression profile of these markers cannot be demonstrated in normal mucosa [13]. These markers are studied in ensuring the safety of surgical margins as the presence of stem cell markers SOX2 and OCT4, has the capacity to form spheroids and show chemo resistance [14]. Excessive cell proliferation and invasion are hallmarks of epithelial carcinomas. The invasiveness is thought to be attained by undergoing epithelial mesenchymal transition (EMT) [15]. EMT is an event that allows or converts an epithelial cell to undergo multiple changes to transform to a mesenchymal cell with additional ability to migrate and invade the extracellular matrix. The cells seen at the invasive front of tumor are considered to be the cells that gradually enter into subsequent steps of the invasion-metastasis cascade [16].
WNT5A is another secreted signaling protein that belongs to the WNT family of cysteine-rich proteins [17]. WNT pathway signals through activation of Jun N-terminal kinase (JNK), whereas the WNT/Ca2+ pathway involve intracellular Ca2+ signaling but also activation of protein kinase C (PKC). It has been reported that PKC and JNK activities play important roles in regulation of OSCC cell migration [18]. It is a crucial factor in the development of organs and to control cellular functions like proliferation, differentiation, apoptosis, survival, polarity, migration and invasion [18]. The involvement of WNT5A in many tumors like pancreatic cancer, malignant melanomas and breast cancer has been observed [19–21]. Upregulated levels of WNT5A may in turn regulate other molecules like matrix metalloproteinase, laminin and other proteins like E-Cadherin and β-catenins which are involved in degradation of basal lamina thus triggering the metastatic spread of tumor. Increased expression of WNT5A can downregulate the E-Cadherin expression which has a key role in EMT by decreasing cell adhesion and increasing migration [22].
Oral squamous cell carcinoma (OSCC) is often preceded by a pre malignant lesion in which molecular pathways involving various signaling proteins play a major role in transformation. In the present study we evaluated the immunohistochemical expression of SOX2, OCT4 and WNT5A in formalin fixed paraffin embedded (FFPE) tissues of oral epithelial dysplasia (OED) and OSCC and compared their expression with normal oral mucosa (NOM).
Materials and Method
The present study was conducted in the Department of Oral Maxillofacial Pathology, PGIDS Rohtak, Haryana, India after the approval by Institutional Ethical Committee (PGIDS/IEC/15/17 dated 30/11/17).
Study Sample
The study sample comprised a total of 65 cases in which 20 were of OSCC (12 well differentiated + 8 moderately differentiated), 20 cases of OED (cases of leukoplakia with dysplasia) (8 mild dysplasia + 12 moderate dysplasia) and 25 NOM. Samples of inflammation free gingival tissues obtained from cases of surgical removal of impacted molars of health individuals were taken as NOM. All specimens were procured from routine biopsy specimen in the department during study period.
Selection Criteria
Patients with primary lesions were taken for study. Patient history was assessed for absence of any systemic conditions and previous treatment history for inclusion in the study. Histologically proven cases were selected for the study. Unwilling patients and with systemic diseases or previously treated cases were excluded from the study.
Immunohistochemistry
Formalin fixed paraffin embedded (FFPE) blocks of each, 4 microm thick sections on poly l lysine coated slides were subjected to immunohistochemical analysis of SOX2 (RTU, Primary antihuman rabbit polyclonal antibody, Master Diagnostica, Spain), OCT4 (RTU, Primary antihuman rabbit monoclonal antibody OCT, Master Diagnostica, Spain) and WNT5A (Primary antihuman rabbit monoclonal antibody, 1:1000, Abexxa, UK). Tissue sections were deparaffinized in xylene (twice), treated with a graded series of alcohol (100%, 95%, 85% and 75% ethanol), and then incubated in phosphate buffered saline (PBS, pH 7.4) for 5 min. For SOX2 and OCT4 heat induced antigen retrieval was done by immersion in 10 mM Tris–EDTA with pH 9 at 600 W in pressure boiler while for WNT5A heat induced antigen retrieval was done in 10 mM citrate buffer with pH 6 at 600 W in pressure boiler until two whistles. Endogenous peroxidase was inactivated by 3% hydrogen peroxide for 10 min. For WNT5A sections were blocked again using non-fat dry milk in distilled water for 10 min. The tissue sections were incubated with primary antibodies against SOX2 and OCT4 for 40 min while for WNT5A (1:1000 dilution in antibody diluent), slides were incubated for 10 min in humidifying chamber followed by incubation with secondary polyclonal conjugate (Dako, Glostrup, Denmark) for 30 min. Lastly, tissue sections were treated with diaminobenzidine as a substrate chromogen and counterstained with haematoxylin. As negative controls, tissue sections were treated with phosphate buffered saline instead of the primary antibody. Tonsils, seminoma and breast carcinoma sections were taken as positive controls for SOX2, OCT4 and WNT5A respectively. The slides were then mounted, observed and evaluated using research microscope (Nikon Eclipse Ni-U) under 400X magnification using NIS Elements imaging software (version 5.01).
Evaluation of Immunohistochemistry
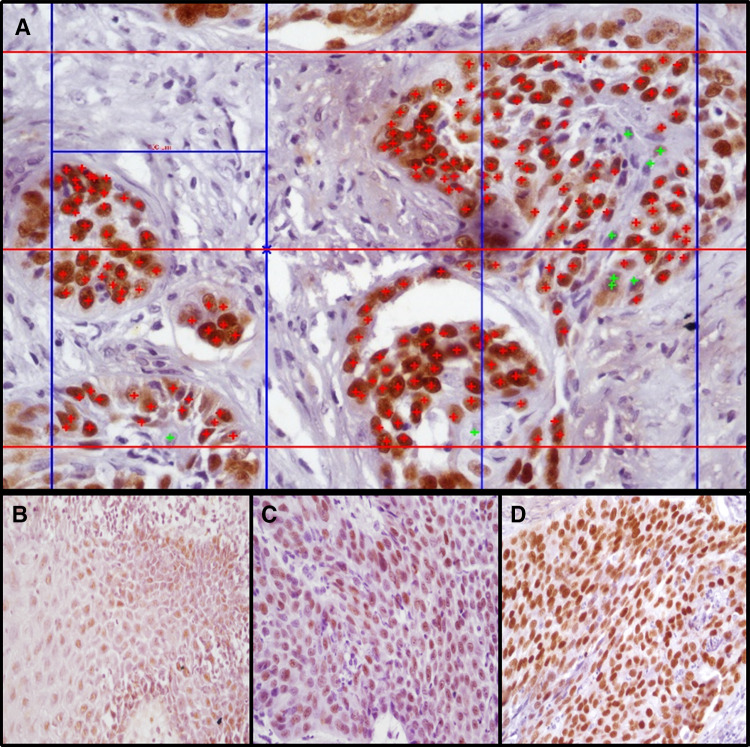
Expression of SOX2 and OCT4 was observed in the nuclei of epithelial cells. The whole slide was scanned and 1000 cells per specimen were counted under 400X. Percentage expression of SOX2 and OCT4 protein was evaluated (number of positive cells/total number of cells (minimum of 1000 cells) in chosen high power fields using grid aided image analysis in research software (Nikon) (Fig. 1a). SOX2 and OCT4 grading was done considering percentage of positivity (P) (number of positive cells to total cells in a section) and intensity (I) of staining (weak, moderate, strong). Percentage positivity (< 5% positivity = 0, 5–24% = 1,25–49% = 2, 50–74% = 3, > 75% = 4) and intensity was scored (No cells positive = 0, Weak = 1, Moderate = 2, Strong = 3) (Fig. 1b–d) and final score for each section was obtained by adding the individual scores (P + I) (0–3 = low expression, 4–7 = high expression).
Fig. 1.
a Photomicrograph depicting grid aided image analysis for calculation of percentage expression of SOX2 and OCT4 upon immunohistochemistry (400X, positive cells tagged red+ , negative cells tagged green+). b–d Photomicrographs showing scoring grades of intensity (b-weak, c-moderate, d-strong)
WNT5A showed positivity in membrane as well as cytoplasm of epithelial cells. The whole slide was scanned and total percentage of expression was noted. (Positive if > 50% of epithelial cells showed staining, Negative if < 50% of epithelial cells showed staining).
Statistical Analysis
Results were statistically analyzed by entering the findings into Microsoft excel worksheet and compared for statistical significance using SPSS version 25(IBM, US). Mean and standard deviation of percentage expression of SOX2 and OCT4 were derived and its significance was determined by Independent t–test. Intragroup and intergroup data significance for WNT5A expression was determined using Pearson chi square test. The co expression of SOX2 and OCT4 were statistically compared by using Spearman’s Rho coefficient. The p value ≤ 0.05 was considered significant and < 0.001 as highly significant.
Results
The present study consisted histologically proven 20 cases of OSCC, 20 cases of OED and 25 NOM. The demographic data of cases in study are shown in Table 1.
Table 1.
Showing demographic data and percentage expression of SOX2 and OCT4 in oral squamous cell carcinoma (OSCC), oral epithelial dysplasia (OED) and normal oral mucosa (NOM)
| Study group | Sample size (no) | Mean age (years) | Sex (M/F)a |
Percentage expression of SOX2 ( mean ± SD) | Percentage expression of OCT4 ( mean ± SD) |
|---|---|---|---|---|---|
| OSCC | 20 | 50.20 | 18/2 | 63.30 ± 22.35 | 3.97 ± 17.75 |
| Well differentiated | 12 | 68.26 ± 17.02 | 6.61 ± 22.92 | ||
| Moderate differentiated | 8 | 55.87 ± 28.20 | 0.00 ± 0.00 | ||
| OED | 20 | 43.95 | 20/0 | 58.67 ± 35.91 | 8.66 ± 26.78 |
| Mild dysplasia | 8 | 59.00 ± 37.93 | 9.81 ± 22.75 | ||
| Moderate dysplasia | 12 | 58.67 ± 36.23 | 7.89 ± 27.33 | ||
| NOM | 25 | 40.10 | 17/8 | 4.32 ± 14.96 | 0.00 ± 0.00 |
The percentage expression values of the main study groups are given in bold, while values obtained within the study group are not in bold
SD standard deviation
aMale/female
Pattern of Expression of SOX2, OCT4 and WNT5A in OSCC, OED and NOM
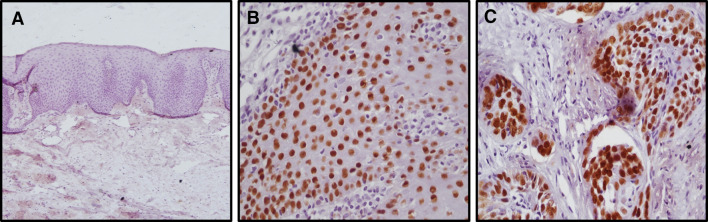
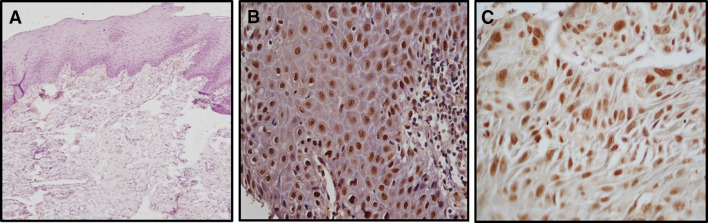
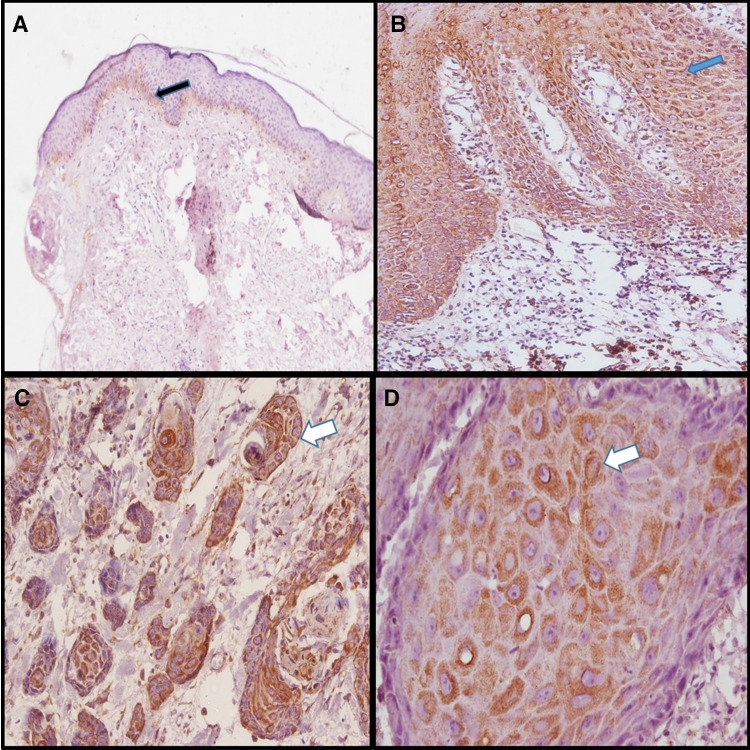
Expression was limited to the nucleus of basal as well as few parabasal layers in SOX2 positive NOM (Fig. 2a). The SOX2 expression was observed in parabasal to the superficial layers in OED (Fig. 2b) and in OSCC all the dysplastic cells in superficial epithelium as well as tumor islands showed positivity (Fig. 2c). OCT4 was found to be negative for NOM (Fig. 3a) while expression pattern was similar to SOX2 in OED (Fig. 3b) and OSCC (Fig. 3c). The expression of WNT5A was negative in NOM (Fig. 4a) whereas cytoplasmic and membranous expression in dysplastic cells were seen in OED (Fig. 4b). In OSCC, the dysplastic tumor cell islands invading into the connective tissue were strongly positive for WNT5A (Fig. 4c, d).
Fig. 2.
SOX2 expression by IHC in NOM,OED and OSCC. a Showing negative expresion of SOX2 in NOM (100X). b Showing positive nuclear expresion of SOX2 in dysplastic cells of OED (400X). c Showing positive nuclear expresion of SOX2 in dysplastic islands of OSCC (400X)
Fig. 3.
OCT4 expression by IHC in NOM,OED and OSCC. a Showing negative expresion of OCT4 in NOM (100X). b Showing positive nuclear expresion of OCT4 in dysplastic cells of OED (400X). c Showing positive nuclear expresion of OCT4 in dysplastic cells of OSCC (400X)
Fig. 4.
WNT5A expression by IHC in NOM, OED and OSCC. a Showing WNT5A in NOM (100X), weak focal positivity seen in membrane basal cell layers in few cases (black arrow). b Showing positive membraneous and cytoplasmic expression of WNT5A in dysplastic cell layers of OED (200X) (blue arrow). c Showing positive membraneous and cytoplasmic expression of WNT5A in dysplastic cells of tumor islands in OSCC (200X) (white arrow). d Positive membraneous and cytoplasmic expression of WNT5A in dysplastic cells within tumor islands in OSCC (400X)(white arrow)
Percentage Expression of SOX2, OCT4 and WNT5A in OSCC, OED and NOM
Percentage expression of SOX2 and OCT4 in study groups has been tabulated in Table 1 and WNT5A in Table 2. SOX2 expression percentage showed statistically significant difference among study groups (Independent Sample t-Test, p value 0.024 = OSCC/OED, 0.028 = OSCC/NOM and 0.000 = OED/NOM). Statistical comparison of percentage expression of OCT4 between OSCC and OED was non-significant while OSCC/NOM as well as OED/NOM were significant (Independent Sample t-Test, p value 0.020 and 0.001 respectively). The statistical comparison of expression of WNT5A among groups showed significant difference (Pearson chi square, p value of 0.000). Post hoc analysis showed that NOM has significantly higher negative results implying that expression of WNT5A is very less when compared to expression in OSCC and OED.
Table 2.
Showing result of WNT5A expression (No. of samples) in oral squamous cell carcinoma (OSCC), oral epithelial dysplasia (OED) and normal oral mucosa (NOM)
| Study group | Sample size (no) | Percentage expression of WNT5A ( number of cases and percentage) |
|---|---|---|
| OSCC | 20 | Positive = 18(90%) |
| Negative = 2(10%) | ||
| Well differentiated | 12 | Positive = 11(91.7%) |
| Negative = 1(8.3%) | ||
| Moderate differentiated | 8 | Positive = 7(87.5%) |
| Negative = 1(12.5%) | ||
| OED | 20 | Positive = 9(45%) |
| Negative = 11(55%) | ||
| Mild dysplasia | 8 | Positive = 2(25%) |
| Negative = 6(75%) | ||
| Moderate dysplasia | 12 | Positive = 7(58.3%) |
| Negative = 5(41.7%) | ||
| NOM | 25 | All negative |
The percentage expression values of the main study groups are given in bold, while values obtained within the study group are not in bold
Comparison of Final Score (P + I) of Expression of SOX2 and OCT4
The final score of expression was calculated by sum of percentage of expression and intensity of staining. The high expression score (4–7) for SOX2 was 80.0% and 75% in OSCC and OED respectively. This score was found to be 83.30% for well differentiated and 75% for moderately differentiated OSCC while it was 87.5% for mild dysplasia and 75.0% for moderate dysplasia. For OCT4 the same was 5% and 10% in OSCC and OED respectively and was found to be 8.3% for well differentiated and 0.00 for moderately differentiated OSCC whereas 12.5% for mild dysplasia and 8.3% for moderate dysplasia.
Co Expression of SOX2, OCT4 and WNT5A
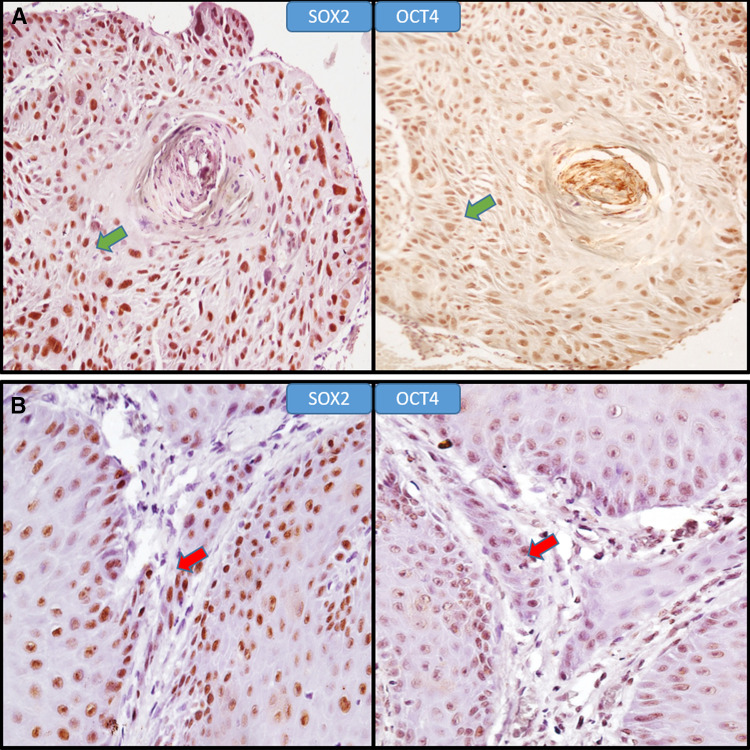
The co-expression of SOX2 and OCT4 proteins was assessed in same sample by evaluating positivity of expression within same areas of tissue specimen. The only OSCC sample with OCT4 positivity showed co-expression with SOX2 (Fig. 5a) whereas for OED two samples showed co-expression (Fig. 5b). The statistical correlation for the co-expression was found to be non-significant in both study groups (Spearman’s Rho coefficient).
Fig. 5.
Coexpression of SOX2 and OCT4 in OED and OSCC. a Positive co-expression of SOX2 and OCT4 in same location of OSCC tissue specimen (400X) (green arrows). b Positive co-expression of SOX2 and OCT4 in same location of OED tissue specimen (400X) (red arrows)
Discussion
Recent studies on OSCC and OED are focusing on the molecular pathways which lead to their initiation and progression. CSC proliferation and EMT are two such alleyways for tumor progression which have gained attention in the present time and numerous researches have been targeting markers for these pathways namely the WNT, JAK-STAT, PI3K-AKT etc. OCT4 and SOX2 have demonstrated to be good indicators of stem cell capacity while WNT5A, belonging to WNT family is of interest for assessing the migration potential of tumor cells [23].
Studies recording the SOX2/OCT4 expression have shown varying results in literature. In the present study, {SOX2 – OED/OSCC = 58.80%/63.30%} increasing percentage of SOX2 from OED to OSCC were similar to the expression studied by Bin Qiao et al. [13] {SOX2 positivity = 90% in (Potentially malignant disorders) PMD and 100% in OSCC} and Lutao Du et al. [24] which showed 62.2% SOX2 positivity in OSCC. The study results were in concordance with study by Cong-Fa Huang et al. in which the tongue SCC showed 62% positivity for SOX2 [25]. The increase in mean expression from NOM to OED and to OSCC showed statistically significant difference thereby identifying the proliferative potential and transformation of OED into OSCC.
The expression of OCT4 in OSCC and OED even though less but on comparison with NOM showed significant difference {OCT4 – OED/OSCC 8.66%/3.97%}. The present study results were similar to the observation by Nan Ge et al. on hypopharyngeal SCC in which they observed a low expression percentage of 9.4% for OCT4 [26]. In a study by Bin Qiao et al. the percentage positivity of OCT4 was 70% in PMD and 60% in OSCC tissues [13]. This higher expression may be due to the cases selected in their study, as they included lichen planus along with leukoplakia for PMD and primary OSCC with nodal metastasis. Similar to our study, individual expression of SOX2 and OCT4 was seen in basal layer of NOM but their double positivity was absent i.e. they did not show co-expression in any normal samples.
In another study by Ting-Ying Fu et al. on OSCC and NOM, the increase in expression pattern of SOX2 was found to be higher in OSCC as compared to NOM whereas it was reverse for OCT4 [11]. Qi Wang et al. reported that 17.90% and 22.84% of esophageal carcinomas had a strong positive expression for OCT3/4 and SOX2, respectively with a positive mutual coordination with co-localization of OCT3/4 and SOX2 in same areas of the tumor [27]. They observed a mean positive expression of 64.20% of SOX2 and 30.86% of OCT4. Nan Ge et al. observed SOX2 expression of 71.8% and OCT4 expression of 9.4% in cases of hypopharyngeal SCC [26]. Weiren Luo et al. recorded 55.7% high molecular expression of SOX2 and 35.2% expression of OCT4 in his study on nasopharyngeal carcinoma. Expression of OCT4 in non-cancerous epithelial cells was negative [12].
The co-expression of these markers were defined as its positive expression on same location within a tumor mass. In present study we found such co expression only in one case of OSCC and two cases of OED. Bin Qiao et al. found similar co-expression of SOX2 and OCT4 in 12/20 cases of PMDs and 38/116 cases of OSCC [13]. Co expression of SOX2 and OCT4 along with other stem cell markers like CD44 and NANOG were observed in 6 cases of moderately differentiated buccal squamous cell carcinoma by Yu HH et al. [28]. They observed different subsets of CSC, all of which expressed both SOX2 and OCT4 along with other stem cell markers [28]. Hence it is opined that some subsets of stem cells may show both SOX2 and OCT4 but some with SOX2 alone. Our study results were similar on confirming the possibility of such subsets of stem cells with expression of both SOX2 and OCT4 or either SOX2 without OCT4 positivity.
In a study by Jinghu Cai et al. [29] on three cancer cell lines, they observed that at the protein level SOX2 may suppress further OCT4 expression via a positive loop, while downregulation of OCT4 could upregulate SOX2 expression via negative feedback in OCT4 low cancer cells. This was similar to the observation in present study as OCT4 was negative in most cases with high SOX2 expression. OCT4 plays the role of derivation while SOX2 plays the role of stem cell property. In the absence of OCT4 expression, neoplasms could not be initiated from normal tissues. Without SOX2 expression, the neoplastic cells could not be self-renewed to maintain tumor growth [29].
In our study the mean percentage of expression (P) (0–4) and intensity of staining (I) (0–3) were scored and summed for the final score (P + I) (0–7). The final score was categorized as low expression (0–3) and high expression (4–7) which was found to be more relative to the actual expression in the tissue sample. The high expression score (4–7) for SOX2 was 80.0% and 75% in cases of OSCC and OED respectively. Higher intensity of staining was mostly seen in cases of OSCC than OED. Similarly the high expression score for OCT4 was 5% and 10% in cases of OSCC and OED respectively.
WNT5A expression (45% in OED and 90% in OSCC) were in concordance with study by Progmet et al. on NOM, dysplasia and OSCC in which 38.1% positivity was observed in dysplasia and 81% in OSCC [22]. The WNT5A protein expression in OSCC was cytoplasmic especially in the cells towards the periphery of tumor islands and cells towards the invasive front of tumor. In another study by Haji Bo et al. in pancreatic cancer using immunohistochemistry, found an overall WNT5A expression of 81.3% in tumor cells and adjacent normal tissues had only 16.4% expression. They also observed WNT5A positive tumors expressed a high expression of Vimentin, beta catenin and low E-Cadherin levels favoring EMT [19].
Our study conclude that SOX2 is significantly associated with CSC like behavior of tumor cells in OSCC increasing its expression from OED towards malignancy while OCT4 expression was sparse and insignificant. Thus SOX2 itself can act as a potential marker for proliferation in tumor cells. The WNT5A positivity significantly increased from OED towards OSCC with higher expression in the tumor cells on periphery of tumor islands aiding in invasion. Hence it may be used as a putative marker in regulating the tumor invasion potential denoting the transformation from dysplasia to malignancy.
Compliance with Ethical Standards
Conflict of interest
All authors have indicated they have no potential conflicts of interest and no financial relationships relevant to this article to disclose.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Guo W, Lasky JL, Wu H. Cancer stem cells. Pediatr Res. 2006;59(S4):59R. doi: 10.1203/01.pdr.0000203592.04530.06. [DOI] [PubMed] [Google Scholar]
- 3.Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma. 2005;52(6):435. [PubMed] [Google Scholar]
- 4.Baillie R, Tan ST, Itinteang T. Cancer stem cells in oral cavity squamous cell carcinoma: a review. Front Oncol. 2017;7:112. doi: 10.3389/fonc.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Major AG, Pitty LP, Farah CS. Cancer stem cell markers in head and neck squamous cell carcinoma. Stem Cells Int. 2013 doi: 10.1155/2013/319489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monroe MM, Anderson EC, Clayburgh DR, Wong MH. Cancer stem cells in head and neck squamous cell carcinoma. J Oncol. 2011;2011:762780. doi: 10.1155/2011/762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufhold S, Garbán H, Bonavida B. Yin Yang 1 is associated with cancer stem cell transcription factors (SOX2, OCT4, BMI1) and clinical implication. J Exp Clin Cancer Res. 2016;35(1):84. doi: 10.1186/s13046-016-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J Pathol. 2011;223(2):148–162. doi: 10.1002/path.2793. [DOI] [PubMed] [Google Scholar]
- 9.Costea DE, Tsinkalovsky O, Vintermyr OK, Johannessen AC, Mackenzie IC. Cancer stem cells–new and potentially important targets for the therapy of oral squamous cell carcinoma. Oral Dis. 2006;12(5):443–454. doi: 10.1111/j.1601-0825.2006.01264.x. [DOI] [PubMed] [Google Scholar]
- 10.Weina K, Utikal J. SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med. 2014;3(1):19. doi: 10.1186/2001-1326-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu TY, Hsieh IC, Cheng JT, Tsai MH, Hou YY, Lee JH, Liou HH, Huang SF, Chen HC, Yen LM, Tseng HH. Association of OCT 4, SOX 2, and NANOG expression with oral squamous cell carcinoma progression. J Oral Pathol Med. 2016;45(2):89–95. doi: 10.1111/jop.12335. [DOI] [PubMed] [Google Scholar]
- 12.Luo W, Li S, Peng B, Ye Y, Deng X, Yao K. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS ONE. 2013;8(2):e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiao B, He B, Cai J, Yang W. The expression profile of Oct4 and Sox2 in the carcinogenesis of oral mucosa. Int J Clin Exp Pathol. 2014;7(1):28. [PMC free article] [PubMed] [Google Scholar]
- 14.Lazarevic M, Milosevic M, Trisic D, Toljic B, Simonovic J, Nikolic N, Mikovic N, Jelovac D, Petrovic M, Vukadinovic M, Milasin J. Putative cancer stem cells are present in surgical margins of oral squamous cell carcinoma. J BU ON. 2018;23(6):1686–1692. [PubMed] [Google Scholar]
- 15.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Investig. 2009;119(6):1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katoh M. WNT/PCP signaling pathway and human cancer. Oncol Rep. 2005;14(6):1583–1588. [PubMed] [Google Scholar]
- 18.Prgomet Z, Axelsson L, Lindberg P, Andersson T. Migration and invasion of oral squamous carcinoma cells is promoted by WNT 5A, a regulator of cancer progression. J Oral Pathol Med. 2015;44(10):776–784. doi: 10.1111/jop.12292. [DOI] [PubMed] [Google Scholar]
- 19.Bo H, Zhang S, Gao L, Chen Y, Zhang J, Chang X, Zhu M. Upregulation of Wnt5a promotes epithelial-to-mesenchymal transition and metastasis of pancreatic cancer cells. BMC Cancer. 2013;13(1):496. doi: 10.1186/1471-2407-13-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medrek C, Landberg G, Andersson T, Leandersson K. Wnt-5a-CKIα signaling promotes β-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells. J Biol Chem. 2009;284(16):10968–10979. doi: 10.1074/jbc.M804923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dissanayake SK, Wade M, Johnson CE, O'Connell MP, Leotlela PD, French AD, Shah KV, Hewitt KJ, Rosenthal DT, Indig FE, Jiang Y. The Wnt5A/protein kinase C pathway mediates motility in melanoma cells via the inhibition of metastasis suppressors and initiation of an epithelial to mesenchymal transition. J Biol Chem. 2007;282(23):17259–17271. doi: 10.1074/jbc.M700075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prgomet Z, Andersson T, Lindberg P. Higher expression of WNT5A protein in oral squamous cell carcinoma compared with dysplasia and oral mucosa with a normal appearance. Eur J Oral Sci. 2017;125(4):237–246. doi: 10.1111/eos.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SH, Oh SY, Do SI, Lee HJ, Kang HJ, Rho YS, Bae WJ, Lim YC. SOX2 regulates self-renewal and tumorigenicity of stem-like cells of head and neck squamous cell carcinoma. Br J Cancer. 2014;111(11):2122. doi: 10.1038/bjc.2014.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Du L, Yang Y, Xiao X, Wang C, Zhang X, Wang L, Zhang X, Li W, Zheng G, Wang S, Dong Z. Sox2 nuclear expression is closely associated with poor prognosis in patients with histologically node-negative oral tongue squamous cell carcinoma. Oral Oncol. 2011;47(8):709–713. doi: 10.1016/j.oraloncology.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Huang CF, Xu XR, Wu TF, Sun ZJ, Zhang WF. Correlation of ALDH 1, CD 44, OCT 4 and SOX 2 in tongue squamous cell carcinoma and their association with disease progression and prognosis. J Oral Pathol Med. 2014;43(7):492–498. doi: 10.1111/jop.12159. [DOI] [PubMed] [Google Scholar]
- 26.Ge N, Lin HX, Xiao XS, Guo L, Xu HM, Wang X, Jin T, Cai XY, Liang Y, Hu WH, Kang T. Prognostic significance of Oct4 and Sox2 expression in hypopharyngeal squamous cell carcinoma. J Transl Med. 2010;8(1):94. doi: 10.1186/1479-5876-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang QI, He W, Lu C, Wang Z, Wang J, Giercksky KE, Nesland JM, Suo Z. Oct3/4 and Sox2 are significantly associated with an unfavorable clinical outcome in human esophageal squamous cell carcinoma. Anticancer Res. 2009;29(4):1233–1241. [PubMed] [Google Scholar]
- 28.Yu HH, Featherston T, Tan ST, Chibnall AM, Brasch HD, Davis PF, Itinteang T. Characterization of cancer stem cells in moderately differentiated buccal mucosal squamous cell carcinoma. Front Surg. 2016;3:46. doi: 10.3389/fsurg.2016.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cai J, He B, Li X, Sun M, Lam AK, Qiao B, Qiu W. Regulation of tumorigenesis in oral epithelial cells by defined reprogramming factors Oct4 and Sox2. Oncol Rep. 2016;36(2):651–658. doi: 10.3892/or.2016.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]