Abstract
To evaluate the performance characteristics of PD-L1 immunohistochemistry (IHC) combined positive scoring (CPS) in core biopsies and aspirate cell blocks from patients with head and neck squamous cell carcinoma (HNSqCCa). PD-L1 IHC using the SP263 antibody was performed on 20 paired cases which consisted of a small biopsy and an excisional specimen. The scores were compared at both the 1% and 20% cutpoints. Using the CPS result obtained from the resected specimen or excisional biopsy as the gold standard, PD-L1 IHC performed on the core biopsy or cell block identified 4 of 6 positive cases (66%) at the 20% cutpoint and 12 of 17 (70%) positive patients at the 1% cutpoint. False positive cases were uncommon at both cutpoints. CPS scoring should be used with caution in small biopsies from patients with HNSqCCa. A negative result should prompt consideration of an excisional biopsy and repeat testing.
Keywords: CPS, PD-L1, Head and neck, Squamous cell carcinoma
Introduction
In the last 5 years, immune checkpoint inhibitors targeting the PD-1/PD-L1 pathway have created a revolution in the field of oncology. At the current time, immune checkpoint inhibitors have been approved by the United States Federal Drug Administration (FDA) for the treatment of melanoma, small cell and non small cell lung carcinomas, renal cell carcinoma, and invasive urothelial carcinoma among others. The role of these drugs in the management of head and neck squamous cell carcinoma has been evolving rapidly. In 2016, two checkpoint inhibitors, pembrolizumab and nivolumab, were approved for use as second line therapy in patients with metastatic head and neck cancer whose tumors had progressed or recurred following standard therapy [1, 2]. In June 2019, pembrolizumab was approved by the FDA for use as first line therapy in selected patients, either alone or in combination with platinum based chemotherapy depending on the level of PD-L1 expression by immunohistochemistry (IHC) [3].
Despite the rapid adoption and widespread use of checkpoint inhibitors, a number of important questions remain unanswered in regards to optimal patient selection, both in head and neck cancer as well as in other tumor types. While a subset of patients treated with these drugs exhibit dramatic and durable clinical responses, many exhibit minimal or no response and are better managed with standard chemotherapy in the first line setting. In order to identify the population of patients most likely to benefit from treatment with checkpoint inhibitors, a number of predictive biomarkers have been explored including PD-L1 IHC, tumor mutational burden, and microsatellite instability.
In the KEYNOTE 048 trial, monotherapy with a PD-1 inhibitor, pembrolizumab, was found to be superior to standard chemotherapy in patients with a PD-L1 combined positive score (CPS) by IHC of > 20% and also to a lesser degree in the patient arm with CPS scores of > 1% [4] On the basis of these findings, pembrolizumab is currently approved for first-line use as monotherapy in patients with CPS scores of ≥ 1% and as part of a regimen that includes platinum based chemotherapy in all patients.
Of note, the study design for the KEYNOTE 048 trial specified that all enrolled patients must have an excisional or core biopsy available for CPS testing with aspirate material explicitly prohibited. As many patients with newly diagnosed head and neck squamous cell carcinoma will only have a small biopsy (core biopsy or fine needle aspirate (FNA)) available for testing, we were interested in comparing CPS scores obtained from FNA cell blocks and core biopsies with those derived from larger excisional biopsies or resection specimens.
Materials and Methods
Following institutional review board approval with waiver of consent, a retrospective review of deidentified pathology reports from 2009–2019 at our institution was performed. A total of 24 patients with head and neck squamous cell carcinoma were identified who had undergone FNA or core biopsy of a metastatic lesion followed by an excisional biopsy or definitive resection. The interval in all cases between the initial biopsy and excisional biopsy or resection was less than 8 weeks and no patient received chemotherapy or radiation in the interim. All specimens had at least 100 cells available for review on hematoxylin and eosin stained sections. After excluding patients with insufficient remaining material for testing, 20 patients remained (Table 1). Our study population included 14 men and 6 women ranging in age from 36 to 80.
Table 1.
Case summary. Scoring was performed on tissue taken from primary tumors (PT) as well as metastatic lymph nodes (LN).
| Case# | Age/gender | Site of origin | Initial biopsy type | Biopsy CPS | Excision type | Excision CPS |
|---|---|---|---|---|---|---|
| 1 | 80/F | Oral tongue | Cell block (LN) | 20 | Excisional bx (PT) | 30 |
| 2 | 80/F | Unknown primary | Cell block (LN) | 100 | Resection (LN) | 30 |
| 3 | 49/M | Oropharynx, p16+ | Cell block (LN) | 0 | Excisional bx (PT) | 3 |
| 4 | 45/M | Oropharynx, p16+ | Cell block (LN) | 0 | Excisional bx (PT) | 10 |
| 5 | 41/F | Oral tongue | Cell block (LN) | 10 | Excisional bx (PT) | < 1 |
| 6 | 72/F | Hypopharynx | Cell block (LN) | < 1 | Resection (LN) | 2 |
| 7 | 70/F | Unknown primary | Cell block (LN) | 5 | Resection (LN) | 12 |
| 8 | 54/M | Oropharynx, p16+ | Cell block (LN) | 5 | Excisional bx (PT) | 7 |
| 9 | 49/M | Oropharynx, p16+ | Cell block (LN) | 0 | Excisional bx (PT) | < 1 |
| 10 | 53/M | Oropharynx, p16+ | Core biopsy (LN) | 15 | Resection (PT) | 5 |
| 11 | 61/M | Oropharynx, p16+ | Core biopsy (LN) | 2 | Resection (LN) | 15 |
| 12 | 87/M | Unknown primary | Core biopsy (LN) | 0 | Resection (LN) | 0 |
| 13 | 62/M | Pyriform sinus | Core biopsy (LN) | 2 | Excisional bx (PT) | 35 |
| 14 | 78/M | Unknown primary | Core biopsy (LN) | 40 | Resection (LN) | 75 |
| 15 | 47/F | Oropharynx, p16+ | Core biopsy (LN) | 7 | Resection (LN) | 5 |
| 16 | 44/M | Oropharynx, p16+ | Core biopsy (LN) | <1 | Resection (PT) | 5 |
| 17 | 36/M | Oropharynx, p16- | Core biopsy (LN) | 15 | Excisional bx (PT) | 25 |
| 18 | 70/M | Oral tongue | Core biopsy (LN) | 1 | Resection (LN) | 7 |
| 19 | 75/M | Unknown primary | Core biopsy (LN) | < 1 | Resection (LN) | 1 |
| 20 | 53/M | Oropharynx, p16+ | Cell block (LN) | 50 | Resection (PT) | 40 |
The initial biopsy was a core biopsy in 10 cases and FNA in 10. The subsequent specimen was an excisional biopsy in 9 cases and a resection specimen in 11. The primary tumor was an HPV driven oropharyngeal squamous cell carcinoma in 9 cases, head and neck squamous cell carcinoma of unknown primary site in 5 cases, squamous cell carcinoma of the oral tongue in 3 cases, hypopharyngeal squamous cell carcinoma 2 cases, and non HPV related oropharyngeal squamous cell carcinoma in 1 case. All core biopsies and FNA cell blocks were obtained from metastatic lymph nodes. A metastatic lymph node was used for PD-L1 IHC in the subsequent resected specimen if available (9 cases). In cases where a metastatic lymph node was not available (11 cases), PD-L1 IHC was performed on the primary tumor.
Per the standard procedure of our department, all core biopsies, excisional biopsies, and resection specimens were fixed in formalin and processed via Sakura Tissue-Tek VIP. After embedding in paraffin, 4 µm thick sections were cut and stained with hematoxylin and eosin using standard techniques.
Aspirates specimens were initially collected in saline. The material was subject to centrifugation and a clot was formed by the addition of thrombin and discarded plasma. The clot was then fixed in 10% neutral buffered formalin and processed via standard histologic techniques as outlined above.
PD-L1 immunohistochemistry was performed using the SP263 anti PD-L1 clone (Ventana Medical Systems, Tucson AZ) stained on the VENTANA benchmark ULTRA platform optimized with the OptiView DAB IHC Detection kit (Ventana Medical Systems) according to the manufacturer’s instructions. Sections of placenta were included as positive controls.
All slides were reviewed by a single pathologist with subspecialty interest in head and neck pathology and cytology and extensive experience in PD-L1 interpretation. CPS scoring of PD-L1 IHC was performed as widely reported elsewhere [5]. Briefly, tumor cells were counted as positive if they displayed any degree of perceptible membranous staining. Tumor associated immune cells were identified by their location within the tumor or tumor associated stroma and deemed positive if they showed any degree of cytoplasmic or membranous staining. Inflammatory cells determined to be associated with necrosis or normal lymphoid tissue were excluded. In cell block specimens, only inflammatory cells that were present in the same tissue fragment as tumor cells were counted. The CPS score was calculated via the formula ((PD-L1 positive tumor cells + PD-L1 positive mononuclear inflammatory cells)/Total tumor cells) × 100.
Results
For each of the paired cases, the CPS score obtained from the excisional biopsy or resection specimen was regarded as the “gold standard.” CPS scores from the paired aspirate cell blocks/core biopsies and excisional specimens are summarized in Table 1. Examples of PD-L1 CPS scoring in paired aspirate cell blocks/core biopsies and excisional specimens or resections are provided in Figs. 1, 2, 3, 4.
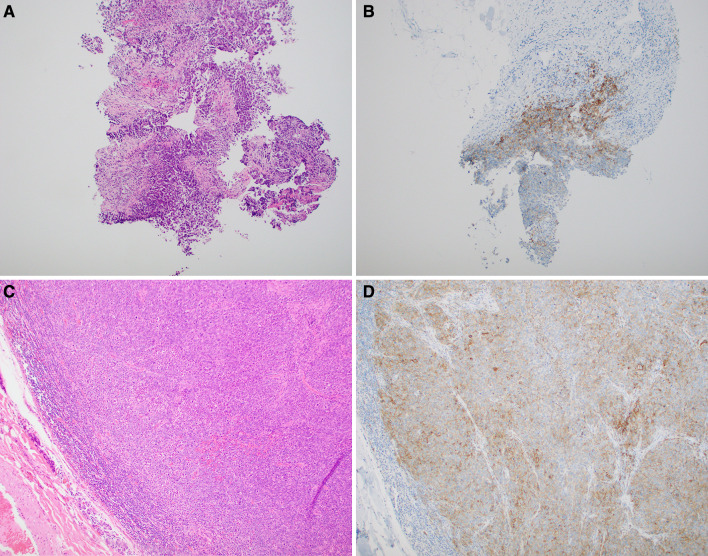
Fig. 1.
Case 14: a, b Core biopsy showing brisk PD-L1 IHC staining of peritumoral immune cells as well as membranous staining of tumor cells at the tumor-stromal interface. The overall CPS score for this biopsy was 40%. c, d The resected lymph node shows abundant membranous staining of tumor cells as well as labeling of peritumoral immune cells. The overall CPS score of this specimen was 75%
Fig. 2.
Case 9: a, b Cell block showing absent PD-L1 IHC staining of tumor cells with only rare immunoreactive tumor associated immune cells. The overall CPS score for this biopsy was less than 1%. c, d The resected oropharyngeal tumor demonstrated a similar lack of labeling in the tumor and immune cells. The overall CPS score of this specimen was less than 1%
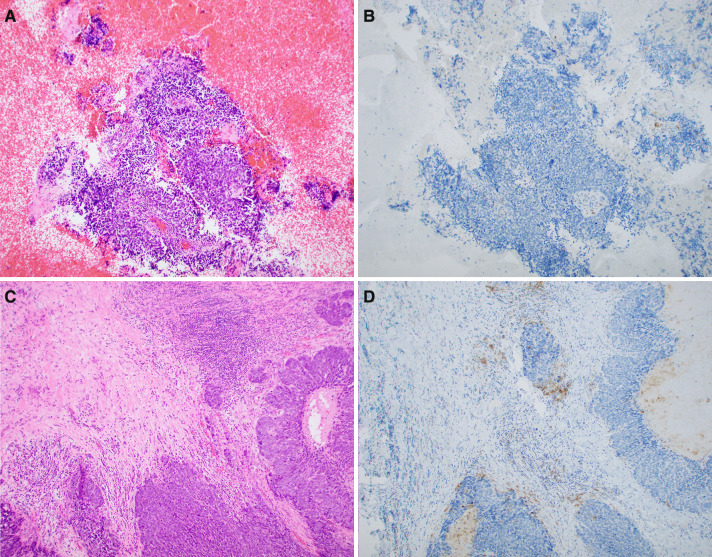
Fig. 3.
Case 6: a, b Cell block showing absent PD-L1 IHC staining of tumor cells and rare immunoreactive peritumoral immune cells. Incorporating findings in other microscopic fields, the overall CPS score was less than 1% (c, d). The corresponding hypopharyngeal tumor showed focal labeling of tumor cells as well as scattered clusters of immunoreactve peritumoral and intratumoral immune cells. The overall CPS score of this specimen was 2%
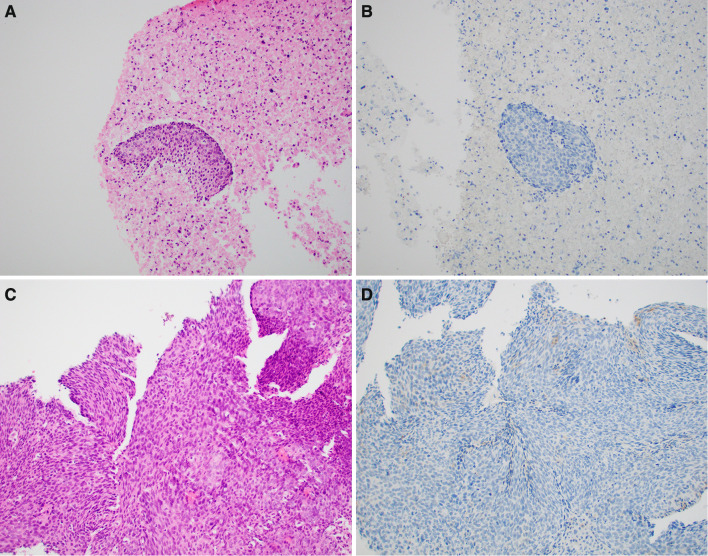
Fig. 4.
Case 16 a, b Core biopsy showing absent PD-L1 IHC staining of immune cells with focal nonspecific tumor cell staining and scattered immunoreactive stromal cells. The CPS score was 0%. c, d The corresponding resected oropharyngeal tumor shows scattered clusters of immunoreactive peritumoral immune cells as well as scattered intratumoral immune cells. The overall CPS score of this specimen was 5%
In 18 of 20 cases (90%), the result derived from the core biopsy or aspirate cell block agreed with that obtained from the excisional biopsy or resection specimen in regards to the 20% cutpoint. These data are summarized in Table 2.
Table 2.
Performance characteristics of PD-L1 CPS scoring at the 20% cutpoint
| Negative initial bx | Positive initial biopsy | |
|---|---|---|
| Negative excision | 14 | 0 |
| Positive excision | 2 | 4 |
Sensitivity: 67%
Specificity: 100%
Positive predictive value: 100%
Negative predictive value: 88%
Accuracy 90%
In one discrepant case (Patient 13), the result obtained from a core biopsy of a cervical metastasis was 2% while that derived from an excisional biopsy of the pyriform sinus mass was 35%. Review of the core biopsy demonstrated a uniform lack of staining in the tumor cells with occasional immune cell positivity. The pyriform sinus excisional biopsy specimen demonstrated occasional immunoreactivity in tumor cells and relatively brisk reactivity in the tumor infiltrating immune cells. Of note, immune staining for PD-L1 in this case was evenly distributed throughout the tumor.
In 14 of the 20 cases (70%), the CPS score obtained from the core biopsy or cell block agreed with that derived from the excisional biopsy or resection specimen in regards to the 1% cutpoint. Notably, in the 7 cases with a CPS score of less than 1% in the aspirate cell block or excisional biopsy, the result obtained from the excisional biopsy or resection was concordant in only 2 yielding an NPV of 28% at the 1% cutpoint. These data and are summarized in Table 3.
Table 3.
Performance characteristics of PD-L1 CPS scoring at the 1% cutpoint
| Negative initial bx | Positive initial biopsy | |
|---|---|---|
| Negative excision | 2 | 1 |
| Positive excision | 5 | 12 |
Sensitivity: 71%
Specificity: 67%
Positive predictive value: 92%
Negative predictive value: 28%
Accuracy 70%
In 5 of the 6 discrepant cases, the score derived from the cell block (3 patients) or core biopsy (2 patients) was less than 1% while that observed in the excisional biopsy or resection was above the 1% threshold. In 4 of these discrepant cases (Patients 3, 6, 16, 17), a similar pattern was observed in that a prominent tumor associated immune response was seen in the resection specimen or excisional biopsy that was not seen in the core biopsy or aspirate cell block. In these cases the tumor cells failed to label with PD-L1 in both of the paired specimens while the immune cells exhibited immunoreactivity in the excisional biopsy or resection which was not seen in the aspirate cell block or core biopsy presumably owing to an absence of a significant inflammatory component.
In one case (Patient 4), the resection specimen exhibited a CPS score of 35% owing primarily to strong but patchy tumor cell staining while no tumor cell staining was seen in the cell block despite abundant well preserved cellularity A single case (Patient 5) whose excisional biopsy of a tongue squamous cell carcinoma displayed a CPS score of < 1% was found to have a CPS score of 10% in the paired aspirate cell block. On review, the aspirate contained abundant necrotic debris as well as a brisk mixed inflammatory infiltrate that involved the few viable fragments of keratinizing tumor. While tumor cell staining was not seen in either specimen, the presence of PD-L1 immunoreactivity in a mixed inflammatory cell infiltrate associated with tumor fragments in the cell block warranted a score of 10%.
In 9 of the paired cases, the initial biopsy and subsequent excisional specimen were both derived from a metastatic lymph node. In all 9 cases the CPS score at the 20% cutpoint was concordant while 7 of 9 were concordant at the 1% cutpoint.
In 11 of the paired cases we evaluated, the initial biopsy was obtained from a metastatic lymph node while the subsequent excisional specimen was derived from the primary tumor site. In 2 of these paired cases, the CPS scores were discordant at the 20% cutpoint while 4 cases were discordant at the 1% cutpoint.
The overall rates of agreement at both cutpoints between the initial small biopsy and subsequent excisional specimen were similar in cases where the initial biopsy specimen was an FNA cell block (5/9, 55%) and core biopsy (7/11, 63%).
Generally speaking in regards to our cases, we noticed a significant degree of intratumoral heterogeneity in regards to immunoreactivity for PD-L1 in resected specimens. Notably, positive immune cells and tumor cells tended to be located at the periphery of the tumor at the junction between the tumor and the peritumoral stroma.
Discussion
PD-L1 immunohistochemistry is increasingly employed as a predictive biomarker to select patients for treatment with checkpoint inhibitors in a number of different tumor types. PD-L1 IHC was first adopted as the standard of care to select patients with metastatic non small cell lung carcinoma whose tumors lack an actionable mutation for single agent treatment with a checkpoint inhibitor [6]. In this context, the current cutpoints in the first line setting have been tumor proportion scores (TPS) of 1% and 50%. TPS scoring differs from the CPS scoring system used in head and neck squamous cell carcinoma and other tumor types in that only tumor cell staining is counted while immune cell staining is ignored.
The bulk of the prior work comparing the suitability of various specimen types for PD-L1 IHC has focused on TPS scoring and non small cell lung carcinoma. Most studies have demonstrated a moderate degree of concordance between cell blocks and small biopsies on the one hand and resected specimens on the other with levels of concordance between the two ranging from 67–95% around the clinically relevant cutpoints [7–12]. In most discrepant cases, the TPS scores derived from the cell block or small biopsy were lower than those obtained from the larger specimens. The lack of correlation in discrepant cases has largely been attributed to intratumoral heterogeneity in expression of PD-L1 by the tumor cells. In our series of resected head and neck squamous cell carcinoma cases, we similarly observed a significant degree of intratumoral heterogeneity in regards to tumor cell staining with positive cells tending to be located at the periphery of the tumor adjacent to the peritumoral stroma.
Immune cell staining for PD-L1, which is incorporated into the CPS score, has been noted to be similarly heterogeneous. We observed that in resected specimens of head and neck squamous cell carcinoma, immune cell staining tended to be primarily localized to the peritumoral stroma with few tumor infiltrating lymphocytes in most cases. This pattern of cuffing of tumor by a PD-L1 positive immune cell infiltrate has been noted to be particularly characteristic of HPV driven oropharyngeal carcinoma [13]. One possible explanation for the underscoring of aspirate cell blocks and core biopsies relative to resected specimens and excisional biopsies is a failure to sample the peritumoral stroma in small biopsies. In four of our discrepant cases around the 1% cutpoint, we noted that while tumor cell staining was similarly negative in both paired specimens, the degree of immune cell staining was considerably less in the core biopsy or aspirate cell block relative to the excisional biopsy or resection.
To our knowledge, ours is the first study to address the issue of CPS scoring in core biopsies and aspirate cell blocks in patients with head and neck squamous cell carcinoma obtained in a clinical context. This issue is of significant relevance as many patients with head and neck cancer and in particular HPV driven oropharyngeal carcinoma will initially come to clinical attention due to an enlarged cervical lymph node which will then be biopsied by these modalities.
As a checkpoint inhibitor, pembrolizumab, has now been approved by the FDA as first line therapy in this disease, accurate assessment of the CPS in patients with head and neck cancer is of paramount importance in determining initial therapy. Based on the results of the KEYNOTE 048 trial, patients who present with metastatic or unresectable disease are eligible for single agent therapy with pembrolizumab if their tumor demonstrates a CPS ≥ 1%. As was demonstrated in this trial, the clinical benefit was most pronounced in the patient population with a CPS > 20% and thus accurately assessing the CPS around both cutpoints is of value in triaging patients appropriately.
In the emerging therapeutic paradigm, patients with metastatic or recurrent disease with CPS > 20% would likely receive single agent pembrolizumab while patients with CPS = 1–20% would be eligible for either single agent pembrolizumab or a regimen combining platinum based chemotherapy and pembrolizumab. Patients with CPS < 1% would receive either platinum based chemotherapy alone or a combined regimen.
While overall concordance between small biopsies and larger excisional biopsies in our series was good at the 20% cutpoint (90%), it should be noted that PD-L1 IHC on the aspirate cell block or core biopsy specimen failed to identify 2 of the 6 positive cases at this cutpoint. Notably, NPV was good as 14 of 16 cases with a CPS < 20% in the small biopsy also had a CPS < 20% in the larger excision specimen. While sensitivity was modest (67%), specificity was 100% as there were no small biopsies with CPS > 20% in which the subsequent larger biopsy or excision had a CPS < 20%.
At the 1% cutpoint, the concordance rate was 70%. Sensitivity (71%) and specificity (67%) were both modest. In our study group, the negative predictive value at the 1% cutpoint was very low (28%).
Given these findings, CPS scoring in core biopsies and aspirate cell blocks should be interpreted with caution. While a positive result at the 20% breakpoint in a small biopsy was found to be accurate in all of our tested cases, false negative results were common. At the 1% cutpoint, false negative cases were frequent and outnumbered true negative cases.
The performance characteristics of CPS scoring in our series are likely to be similar to those seen in other clinical practices as the proportion of patients whose excisional biopsies or resected specimens were scored as positive at the 20% (6/20, 30%) and 1% (17/20, 85%) cutpoints are similar to those seen in the KEYNOTE 048 trial (41% and 85% respectively) which included 601 patients with head and neck squamous cell carcinoma.
An interesting observation in our series is the relatively greater proportion of discrepant cases at the 1% and 20% cutpoints in cases where the initial biopsy was from a metastatic lymph node and the tissue evaluated in the subsequent excision was from the primary tumor. The issue of concordance between PD-L1 CPS scores in primary tumors and metastatic lymph nodes in head and neck squamous cell carcinoma has not yet been examined in the literature to our knowledge. In the case of non small cell lung carcinoma, it has been noted that PD-L1 TPS scores will differ between the primary tumor and synchronous metastatic lymph nodes at the clinically relevant cutpoints in 30–38% of cases [14, 15].
Our study utilized the SP263 PD-L1 antibody clone as opposed to the 22c3 clone as was used in the KEYNOTE 048 trial and which was approved as a companion diagnostic assay for use with pembrolizumab. Results obtained with the 22c3 clone are likely to be similar to those seen in our study as a comparison studies in head and neck squamous cell carcinoma have documented excellent concordance between the two antibodies in regards to both tumor cell as well as immune cell scoring [16].
While PD-L1 IHC using CPS scoring is currently the standard of care in selecting patients for checkpoint inhibitor therapy in the settings of gastric adenocarcinoma, cervical carcinoma, and urothelial carcinoma, variability between results obtained from small biopsies and resected specimens remains an unresolved issue with very few published studies directly addressing this question. In the context of gastric adenocarcinoma, a series published by Yamashita et al. comparing single mucosal biopsies and resected gastrectomy specimens only showed concordance between these two specimen types in 48.8% of cases at the clinically relevant 1% cutpoint [17]. Similar to our findings, the authors noted that the majority of the discordant cases were due to false negative results obtained from the mucosal biopsy specimen.
In the setting of urothelial carcinoma, de Joong et al. compared scoring in transurethral bladder resection specimens and the paired subsequent cystectomy specimen and found concordance in 67 of 82 (81.7%) cases [18]. These results are not as applicable to our findings in that they examined immune cell area staining as opposed to CPS. Also of note, the amount of material typically present in a transurethral resection specimen is considerably more than that present in a core needle biopsy or aspirate cell block as was examined in our series. Application of the findings in this study in bladder cancer are also confounded by the use of neoadjuvant chemotherapy in some of their cases in the interval between the initial transurethral resection and subsequent cystectomy.
To our knowledge, the only study addressing the issue of CPS scoring in small biopsies in head and neck cancer is a series of 33 resected head and neck squamous cell carcinoma cases and accompanying tissue microarrays derived from 6 ex vivo core biopsies from each these specimens published by Rasmussen et al. [19]. In this series, CPS expression at the 1% cutpoint was concordant amongst all 6 biopsies in 52% of cases and was concordant in 54% of cases at the 50% cutpoint. In contrast to our study, true biopsies obtained in a clinical context were not used and no comparison was made to whole histologic sections from the resection. Similar to the findings in our series, very few cases with CPS < 1% were found and as such the NPV at the 1% cutpoint was 0%.
We find that CPS scoring for head and neck squamous cell carcinoma should be performed and interpreted with caution in core biopsies and aspirate cell blocks. While the positive predictive value of a positive result in an aspirate cell block or core biopsy at the 1% and 20% cutpoints was good (100% and 92% respectively), negative predictive values at both cutpoints was lower, particularly at the 1% cutpoint (28%). An assessment of the performance of CPS scoring at the 1% cutpoint was hampered by the small number of true negative cases. Based on these findings, we conclude that while a positive CPS score at either clinically relevant cutpoint is relatively reliable, a negative result should be interpreted with caution and should trigger consideration of an excisional biopsy or procurement of a larger specimen if the use of a checkpoint inhibitor without chemotherapy is being considered clinically.
In light of our findings and those of Yamashita et al., CPS scoring in small biopsies of other tumor types should be used and interpreted with caution at the current time until further data emerge to validate this practice. Furthermore, concordance between CPS scores derived from the primary tumor and those obtained from metastatic lymph nodes remains an open question.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn MJ, Soria A, Machiels JP, Mach N, Mehra R, Burtness B, Zhang P, Cheng J, Swaby RF, Harrington KJ. KEYNOTE-040 investigators. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167. doi: 10.1016/S0140-6736(18)31999-8. [DOI] [PubMed] [Google Scholar]
- 2.Ferris RL, Blumenschein G, Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, Worden F, Saba NF, Iglesias Docampo LC, Haddad R, Rordorf T, Kiyota N, Tahara M, Monga M, Lynch M, Geese WJ, Kopit J, Shaw JW, Gillison ML. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-first-line-treatment-head-and-neck-squamous-cell-carcinoma Accessed 19 July 2019.
- 4.Rischin D, Harrington G, Grell R, et al. Protocol-specified final analysis of the phase 3 KEYNOTE-048 trial of pembrolizumab (pembro) as first-line therapy for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) J Clin Oncol. 2019;37(15_suppl):6000. doi: 10.1200/JCO.2019.37.15_suppl.6000. [DOI] [Google Scholar]
- 5.Kulangara K, Zhang N, Corigliano E, Guerrero L, Waldroup S, Jaiswal D, Ms MJ, Shah S, Hanks D, Wang J, Lunceford J, Savage MJ, Juco J, Emancipator K. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143(3):330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR, KEYNOTE-024 Investigators Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 7.Pak MG, Roh MS. Cell-blocks are suitable materials for PD-L1 immunohistochemistry: comparison of cell-blocks and matched surgical resection specimens in lung cancer. Cytopathology. 2019;30(6):578–585. doi: 10.1111/cyt.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez A, Brandler TC, Zhou F, Moreira AL, Schatz-Siemers N, Simsir A. Assessment of programmed death-ligand 1 (PD-L1) immunohistochemical expression on cytology specimens in non-small cell lung carcinoma. Am J Clin Pathol. 2019;151(4):403–415. doi: 10.1093/ajcp/aqy164. [DOI] [PubMed] [Google Scholar]
- 9.Noll B, Wang WL, Gong Y, Zhao J, Kalhor N, Prieto V, Staerkel G, Roy-Chowdhuri S. Programmed death ligand 1 testing in non-small cell lung carcinoma cytology cell block and aspirate smear preparations. Cancer Cytopathol. 2018;126(5):342–352. doi: 10.1002/cncy.21987. [DOI] [PubMed] [Google Scholar]
- 10.Munari E, Zamboni G, Sighele G, Marconi M, Sommaggio M, Lunardi G, Rossi G, Cavazza A, Moretta F, Gilioli E, Caliò A, Netto GJ, Hoque MO, Martignoni G, Brunelli M, Vacca P, Moretta L, Bogina G. Expression of programmed cell death ligand 1 in non-small cell lung cancer: comparison between cytologic smears, core biopsies, and whole sections using the SP263 assay. Cancer Cytopathol. 2019;127(1):52–61. doi: 10.1002/cncy.22083. [DOI] [PubMed] [Google Scholar]
- 11.Sakata KK, Midthun DE, Mullon JJ, Kern RM, Nelson DR, Edell ES, Schiavo DN, Jett JR, Aubry MC. Comparison of programmed death ligand-1 immunohistochemical staining between endobronchial ultrasound transbronchial needle aspiration and resected lung cancer specimens. Chest. 2018;154(4):827–837. doi: 10.1016/j.chest.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvée S, Washetine K, Mouroux J, Vénissac N, Poudenx M, Otto J, Sabourin JC, Marquette CH, Hofman V, Hofman P. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27(1):147–153. doi: 10.1093/annonc/mdv489. [DOI] [PubMed] [Google Scholar]
- 13.Poropatich K, Hernandez D, Fontanarosa J, Brown K, Woloschak G, Paintal A, Raparia K, Samant S. Peritumoral cuffing by T-cell tumor-infiltrating lymphocytes distinguishes HPV-related oropharyngeal squamous cell carcinoma from oral cavity squamous cell carcinoma. J Oral Pathol Med. 2017;46(10):972–978. doi: 10.1111/jop.12605. [DOI] [PubMed] [Google Scholar]
- 14.Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88(1):24–33. doi: 10.1016/j.lungcan.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 15.Uruga H, Bozkurtlar E, Huynh TG, Muzikansky A, Goto Y, Gomez-Caraballo M, Hata AN, Gainor JF, Mark EJ, Engelman JA, Lanuti MD, Mino-Kenudson M. Programmed cell death ligand (PD-L1) expression in stage II and III lung adenocarcinomas and nodal metastases. J Thorac Oncol. 2017;12(3):458–466. doi: 10.1016/j.jtho.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson A, Slodkowska E, Jungbluth A, Liu SK, Vesprini D, Enepekides D, Higgins K, Katabi N, Xu B, Downes MR. PD-L1 immunohistochemistry assay concordance in urothelial carcinoma of the bladder and hypopharyngeal squamous cell carcinoma. Am J Surg Pathol. 2018;42(8):1059–1066. doi: 10.1097/PAS.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamashita K, Iwatsuki M, Harada K, Koga Y, Kiyozumi Y, Eto K, Hiyoshi Y, Ishimoto T, Iwagami S, Baba Y, Miyamoto Y, Yoshida N, Komohara Y, Ajani JA, Baba H. Can PD-L1 expression evaluated by biopsy sample accurately reflect its expression in the whole tumour in gastric cancer? Br J Cancer. 2019;121(3):278–280. doi: 10.1038/s41416-019-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Jong JJ, Stoop H, Nieboer D, Boormans JL, van Leenders GJLH. Concordance of PD-L1 expression in matched urothelial bladder cancer specimens. Histopathology. 2018;73(6):983–989. doi: 10.1111/his.13710. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen JH, Lelkaitis G, Håkansson K, Vogelius IR, Johannesen HH, Fischer BM, Bentzen SM, Specht L, Kristensen CA, von Buchwald C, Wessel I, Friborg J. Intratumor heterogeneity of PD-L1 expression in head and neck squamous cell carcinoma. Br J Cancer. 2019;120(10):1003–1006. doi: 10.1038/s41416-019-0449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]