Abstract
Mitogen-activated protein kinase (MAPK) signaling cascade is highly conserved across the species triggering the self-adjustment of the cells by transmitting the external signals to the nucleus. The cascade consists of MAPK kinase kinases (MAPKKKs), MAPK kinases (MAPKKs) and MAPKs. These kinases are functionally interrelated through activation by sequential phosphorylation. MAPK cascade is involved in modulating the tolerance and regulating the growth and developmental processes in plants through transcriptional programming. The cascade has been well characterized in Arabidopsis, Tobacco and rice, but limited information is available in wheat due to complexity of genome. MAPK-based sensors have been reported to be highly specific for the external or intracellular stimuli activating specific TF, stress-associated genes (SAGs) and stress-associated proteins (SAPs) linked with heat-stress tolerance and other biological functions especially size, number and quality of grains. Even, MAPKs have been reported to influence the activity of ATP-binding cassette (ABC) transporter superfamily involved in stabilizing the quality of the grains under adverse conditions. Wheat has also diverse network of MAPKs involved in transcriptional reprogramming upon sensing the terminal HS and in turn protect the plants. Current review mainly focuses on the role of MAPKs as signaling sensor and modulator of defense mechanism for mitigating the effect of heat on plants with focus on wheat. It also indirectly protects the nutrient depletion from the grains under heat stress. MAPKs, lying at pivotal positions, can be utilized for manipulating the heat-stress response (HSR) of wheat to develop plant for future (P4F).
Keywords: MAPK, Thermotolerance, Signaling cascade, Protein kinases, Terminal heat stress, Wheat
Introduction
Global climate change has severely affected the growth, development and yield of most of the important crops, worldwide. The impact has now been visible in terms of decrease in the production and depletion in the nutrients and quality of grains grown under aberrant environment. Cereals are highly sensitive to heat stress (HS), especially wheat, rice, etc. and even slight elevation in temperature during critical stages defunct the normal metabolism of the plant by aggregating/denaturating the key enzymes involved in various pathways. It also disrupts the pollen tube growth, normal fertilization process, and seed-setting of wheat grown under terminal HS. A plant has to respond in a coherent manner to cope with the environmental stresses affecting the growth and development. Wheat, being sensitive to HS, has developed their own in-built defense mechanism to cope with various stresses (Kumar et al. 2019a). The defense mechanism of any plants starts with the signaling cascade, which senses the stress and triggers the defense networks upon stress or sometimes before the onset of stress by acting as sensors. The activities of signaling molecules are regulated by the kinases and phosphatases involved in the phosphorylation/ dephosphorylation of specific proteins involved in the key pathways (Hardie 1999).
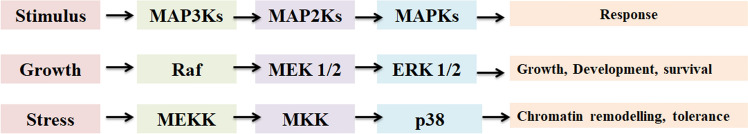
Plants have diverse types of kinases which showed differential expression in response to external stimuli. Mitogen-activated protein kinases (MAPKs) are involved in regulating growth and differentiation, other than activating the defense mechanism under stresses. The signal in response to stimuli transduces very fast with high accuracy due to the phosphorylation/dephosphorylation of proteins by kinases (Brown et al. 1997). MAPKs have been reported to be localized in the cytoplasm, nucleus and are involved in different signal transduction pathways (Gupta and Chakraborty 2013; Wang et al. 2007, 2015; Sheikh et al. 2013). MAPK signaling cascade plays very important role in protecting the plant from various biotic and abiotic stresses (Morrison 2012). A flow chart depicting different cellular responses triggered upon activation of MAPKs in the presence of different stimuli is shown in Fig. 1. The spatio-temporal expression of upstream receptors and abundance of ligands decide the specificity of MAPK signaling as mediator for the regulation and intracellular signal transduction.
Fig. 1.
Role of activated MAPK in triggering the cellular responses; External stimulus in the form of growth factors and stress responses trigger the activation of MAPK which in turn activates the transcription factors resulting in the expression of different cellular responses
MAPK cascade in plant
MAPK signaling cascades have been observed to be highly conserved across the species (Xu et al. 2017). Their specificity is mediated by the subcellular localization, assembly of protein complexes along with their coordinated expression (Mitula et al. 2015; Bigeard and Hirt 2018). Three specific kinases act in MAPK cascade in a sequential manner—MAPK, MAPKK and MAPKKK (Fig. 2). The activation of these kinases occurs in a sequential manner starting from the phosphorylation of MAPKK (MKK or MEK) by MAPKKK (MEKK) and further phosphorylation of MAPK by MAPKK. These kinases are functionally interrelated in terms of activation because they require sequential phosphorylation (Takahashi et al. 2020). MAP2K is activated by MAP3K by phosphorylating the serine/threonine residues present in the conserved motif S/T-X3-5-S/T (Lu et al. 2015). Further, activated MAP2K undergoes sequential phosphorylation for activating the MAPK (Zlobin et al. 2019). MAPKK kinase receives the signals from the external or intracellular stimuli in a direct or indirect way before activating the other kinases (Xu and Zhang 2015b; Fig. 2).
Fig. 2.
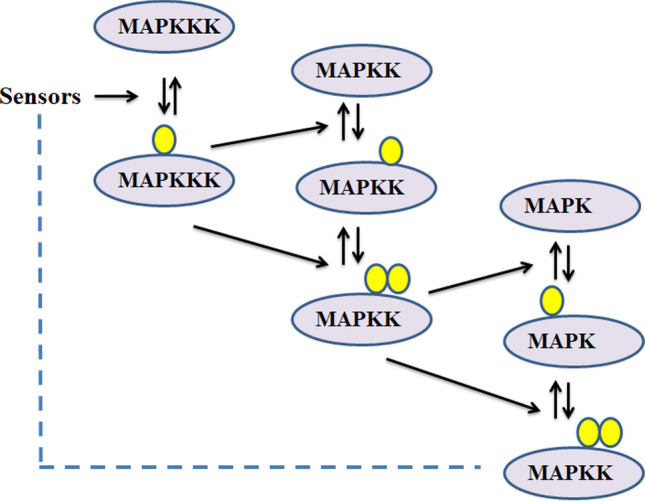
Activation of kinases involved in Mitogen Activated Protein Kinase (MAPK) cascade; Activation of MAPK is dependent upon phosphorylation from the topmost MAPKKK to MAP2K and from MAP2K to MAPK receiving signal; yellow circle designate the phosphorylating site
Very limited MAPK genes have been identified and reported from wheat, reason being the complexity of the genome. We also identified 21 putative MAPK genes from wheat using whole transcriptome sequencing and gel-free proteomics approaches (unpublished work from our laboratory). Few of the putative MAPK genes were cloned from wheat, characterized and submitted in NCBI GenBank [MAPK-6 (acc. no. MH429128), MAPK-11 (acc. no. MK854806), Putative MAPK-10824 (acc. no MN296518)]. Still, the different isoforms of MAPK genes, their enzymes and involvement in different pathways are still unknown in wheat. With the advent of technology, omic platform has been used to identify and characterize different members of MAPK cascade and first work has been reported in Arabidopsis, wherein about 110 genes were identified and linked with MAPK pathways (80 MAP3Ks, 10 MAP2Ks and 20 MAPKs) (de Zelicourt et al. 2016; Raja et al. 2017). Some of the MAPKKKs identified from Arabidopsis like MP3K6-7, YODA, ANP2/ANP3 were characterized to play important role in the growth and development (Cowan and Storey 2003; Chen et al. 2018). Similarly, MEKK1 and ANP1 were observed to modulate the tolerance against different environmental stresses (Nakagami 2006). Besides the presence of MEKK1 in nucleus and cytoplasm, it is also observed in endosomes and plasma membrane (Yang et al. 2010). Molecular characterization showed the involvement of copper transport protein (CTR1) in ethylene signaling (Meng et al. 2013). Some of the MAPKs like MPK3, MPK4 and MPK6 have been thoroughly characterized and reported to be activated by diverse stimuli like abiotic stresses, pathogens and oxidative stress (Jalmi and Sinha 2015). MPK4 showed negative impact, whereas MPK3 and MPK6 have positive signaling on defense mechanism against biotic and abiotic stresses (Wang et al. 2007). An elaborative description about the activation of different MAPKs under stress conditions and their associated TFs in activating the gene response is shown in Fig. 3 (adapted from Plotnikov et al. 2011).
Fig. 3.
Activation and expression of MAP kinases under stress response; The activation of MAP kinases in cytoplasm under stress response causes the further activation of various transcription factors resulting in expression and transcription of DNA (adapted from Plotnikov et al. 2011)
MAPK has two-lobed structures with T-E-Y/ T-D-Y phosphorylation motifs present in the activation loop or its active site present near domain interface (Rohm et al. 2020). The structural integrity of the specific MAPK depends upon the scaffold proteins, docked domains, and anchoring proteins (Gogl et al. 2019). MAPK cascade which is basically a linear system of phosphorylation/ dephosphorylation of kinases linked the external environmental factors with a series of intracellular cellular responses required for mitigating the effect of stresses. The phosphorylation/dephosphorylation of substrates includes TFs, splicing factors, regulators and other protein kinases (Wang et al. 2020a). They identified more than 5000 pairs of putative kinase–substrate and also mapped the targets during stress response of the plant.
The basic module of signaling is—signal perception, generation of secondary messengers, modulation of intracellular Ca2+ levels, initiation of phosphorylation cascades regulating the stress-associated genes (Abhinandan et al. 2018; Fig. 4). MAPK cascade which is highly conserved across plant species shares maximum similarities in the eleven domains predicted to be involved in catalytic function of the enzyme (Ichimura et al. 2002). The MAP3Ks lying upstream of the cascade have two major subgroups known as MEKK and Rapidly Accelerated Fibrosarcoma (Raf)—like kinases (Guo and Wang 2017). Further, the MAP2Ks lying upstream of the MAPKs are subdivided in A, B, C and D, out of which group A & C are involved in modulating the tolerance mechanisms against different abiotic stresses.
Fig. 4.
Activation of MAP kinases through generation of Reactive Oxygen Species (ROS) under biotic and abiotic stress response; NADPH oxidase produces ROS under stress condition which activates the MAPK and secondary messenger (Ca2+) in the subsequent steps leading to the modulation of gene expression
MAPKs, being at pivotal position, play very important role in transducing the signal in the cascade and have been further classified into 4 groups—MAPKs with T-E-Y motif (group A, B and C), and MAPKs with T-D-Y motif (group D) (Jagodzik et al. 2018). Most of the reports about the MAPKs and their functional roles have been validated in the modal plants like Arabidopsis, tobacco and rice. The member of MAPKs identified till date has been characterized to play very important role in signaling of biotic and abiotic stresses (Teige et al. 2004; Wang et al. 2020b).
Upon oxidative stress, the relative fold expression of MAPK and CDPK has been reported to be in the range of 2.5–5.5-fold in diverse genotypes of wheat (Kumar et al. 2019a). Similar pattern of MAPK and CDPK activities was observed in wheat under HS. Both the kinases act as secondary signaling molecules in triggering various TFs especially HSFs. Induction of different TFs under HS leads to the modulation of various traits linked with HS tolerance like—increase in expression of HSPs, high activities of antioxidant enzymes and transporters, accumulation of osmolyte, etc. (Fig. 4). MAPK also protects the carbon assimilatory pathway under HS through activation of SAGs and helps in efficient transportation of photosynthates from source to sink through increase in the expression of transporters.
MAPK signal transduction: mechanism of action
The signal transduction linked with MAPK cascade has not much been characterized in agriculturally important crops. Though, MAPK signal transduction pathways have been characterized to limited extent in yeast, insects, fungi, nematodes and mammals (Jagodzik et al. 2018), the kinases involved in MAPK cascade act in a sequential fashion for the activation of the enzymes, result in the regulation of the genes/TFs in response to external stimuli (Widmann et al. 1999; Rohm et al. 2020). Even a kinase by name MAP4K (MAP3K kinase) has been identified to play important role in linking signaling with the cascade of MAPK (Taj et al. 2010; Flores et al. 2019).
MAPK cascade has been well characterized in Arabidopsis and a module consisting of MEKK, MKK, and MPK4—6 was observed to be involved in modulating the tolerance against abiotic stresses (Meng et al. 2013; Jagodzik et al. 2018). Muhammad et al. (2019) reported the differential accumulation of SlMAPK3 in different tissues of plant in response to different abiotic stresses and hormones. On the contrary, Yu et al. (2019) observed enhancement in the ROS homeostasis and thermotolerance on knock-out of SlMAPK3 gene in tomato. Different variants of MAPK are involved in diverse biological functions inside the tissues and plants, though the structural mapping of these genes is highly conserved.
ROS comes first for the activation of MAPK cascade
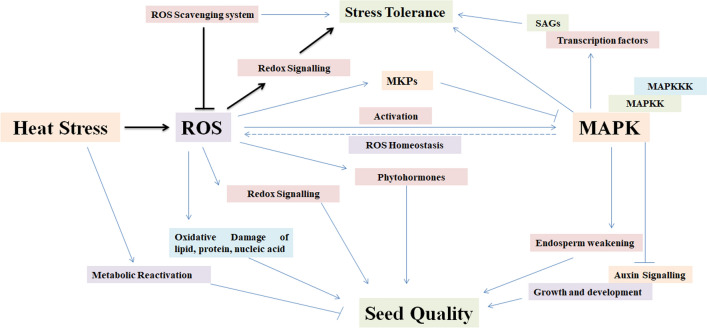
ROS plays very important role in regulating the activation of various signaling molecules like MAPKs, transcriptional factors, protein tyrosine kinases, protein tyrosine phosphatases, etc. ROS consists of superoxide anion radical (·O2 −), hydroxyl radicals (·OH), and hydrogen peroxide (H2O2) (Kumar et al. 2018). The production of ROS inside the cell is basically neutralized by the network of antioxidants and antioxidant proteins. ROS accumulation above safe limit causes impairment of various physiological processes other than damaging the macromolecules and micromolecules inside the cells (Kohli et al. 2019). ROS also plays very important role in redox homeostasis and activation of proteins induced by redox and regulating their activity under adverse conditions (Farooq et al. 2019; Fig. 5).
Fig. 5.
Role of reactive oxygen species (ROS) in activating the MAPK; ROS influences the expression of stress-associated genes through MAPK and regulates the quality of the seeds, MAPKs maintain the ROS homeostasis inside the cells and regulate the shape and size of the seeds by weakening the endospermic tissue
The source of ROS inside the cell is aerobic respiration which operates constantly under normal and adverse conditions, and the accumulation is countered by network of antioxidant enzymes (Kumar et al., 2019b). The situation of ROS production overpassing the scavenging activities of antioxidant enzymes leads to “oxidative stress” causing imbalance in the oxidant production and antioxidant potential of the cells (Wang et al. 2019). The regulation of accumulation of ROS inside the cells has direct effect on the activation and sequential phosphorylation of MAPKs and the effect is more visible on different metabolic pathways. Few laboratories have also reported the activation of MAPKs in different pathways through exogenous application of H2O2 under normal and adverse conditions (Farooq et al. 2019). The mechanism underlying the regulation of MAPK pathway by ROS is not known. ROS has been reported to modify the critical amino acids present in the active site and other important motifs of proteins and may be one of the possible mechanisms behind the activation of MAPK pathway (Yan 2014; Fig. 5). Lee and Back (2017) observed that MAP3Ks and oxidative signal-inducible kinases are involved in activating melatonin-induced defense signaling pathway in Arabidopsis. Similarly, Lu and He (2017) reported that ROS plays important role in activation of MAPK cascade and vice versa. Even the ROS has been reported to activate few of the members of MAPK cascade like MPK1, MPK2 (Arabidopsis), MPK3, MPK6, MPK4, etc. (Kovtun et al. 2000; Nakagami et al. 2006; Liu and He 2017).
Moreover, Guyton et al. (1996) reported that H2O2 activates the growth factor receptors inside the cells and in turn regulate the activation of MAPKs. Some of the intracellular kinases are also oxidatively modified by ROS to activate the MAPK cascade (Son et al. 2011). Similarly, c-Jun N-terminal kinases (JNKs) and p38 undergo phosphorylation through oxidation of thioredoxin in stressed cell by Apoptosis signal-regulating kinase 1 (ASK-1—a member of the MAP3K superfamily) (Zeke et al. 2016). MKPs have been reported to be the negative activator of MAPKs and undergo aggregation/degradation upon signaling from ROS. Son et al. (2011) reported that intracellular H2O2 degrade the MKPs by oxidizing the amino acid residue present at the critical site and is the probable reason behind the continuous activation of JNK pathway. MAPKs in turn maintain the redox homeostasis of the cell under HS. MAPKs trigger the TFs leading to the over-expression of SAGs like HSPs, SOD, CAT, GPX, etc. Antioxidant defense network also scavenges on the ROS and maintains the optimum level inside the cells under HS. Heat stress causes metabolic reactivation inside the endospermic tissue and decreases the quantity and quality of the grains (Fig. 5). Specific MAPKs have been reported to play important role in endosperm weakening and regulation of ABC transporters leading to increase in the shape and size of the grains without compromising with the quality of the grains.
MAPKs — role in heat-stress tolerance of wheat
Plants are not mobile and have to bear many adverse biotic and abiotic stresses on day-to-day basis. Drought and heat stress have been reported to be the most important limiting factors in the normal growth and development of most of the plants especially cereals like wheat and rice (Wahid et al. 2007). To survive, wheat has developed very complex and inherited defense mechanisms to cope with the extremeness. Wheat has evolved over the period with changes in the extent of the environmental stresses to adapt itself to the changing climate (Ahanger et al. 2017). It has diverse sensors mounted in the membrane wall and is specific to perceive signal of different stresses and to transduce the information to kinase cascades like MAPK and CDPK for triggering the in-built defense mechanisms (Cristina et al. 2010). MAPK cascade plays very important role in signal transduction in response to external stimuli. Few literatures have reported the connection between the ROS and the activation of plant MAPK cascade under HS (Joshi et al. 2011; Tena et al. 2001). Wheat has developed different tolerance mechanisms like increase in the expression and activities of stress-associated genes (SAGs) and stress-associated proteins (SAPs), accumulation of metabolites, etc. (Kumar et al. 2013; 2019b; Fig. 6). H2O2 plays dual role as signaling molecule and in triggering the programmed cell death against various external and internal stimulants and in turn regulates the tolerance, growth and differentiation of the cells (Rohm et al. 2020). The signaling cascade in wheat against any stresses starts with the oxidative burst, followed by modulation of intracellular calcium (Ca2+) level (Xiong et al. 2002). Secondary messengers also influence the calcium level which in turn regulates the protein phosphorylation cascade (Tena et al. 2001). MAPK cascade is activated by ROS and Ca2+-dependent signals which further interact with the promoter regions of TF to regulate the expression of genes. A detailed understanding on ROS as a signaling molecule for MAPK activation and modulation of gene expression under HS in different tissues of wheat has been presented in the Fig. 6.
Fig. 6.
Role of MAPK in mitigating the effect of heat stress on different tissues of wheat through regulation of various molecular and biochemical traits linked with thermotolerance; ROS activates the MAPK which further influences the expression of SAGs/ SAPs, CDPKs and ABC transporters; MAPKs indirectly protect the cells from the HS by triggering various biological mechanisms involved in thermotolerance
Sensors have been characterized to have very high specificity for the external or intracellular stimuli, which are further transduced and amplified to activate specific TF. Heat stress causes destabilization of cell membrane leading to aggregation/denaturation of various membrane-bound sensors in different tissues of wheat. Wheat exposed to HS immediately triggered its signaling mechanism by activating the protein kinases which in turn regulate the expressions and activities of SAGs and SAPs involved in tolerance (Zhu et al. 2016). Heat stress triggers the signaling mechanism in three ways—osmotic/oxidative stress signaling, Ca2+-dependent signaling, and Ca2+-dependent salt overlay sensitive (SOS) signaling (Christina et al. 2010). Oxidative stress signaling or accumulation of osmolyte is considered as kinases based on mechanism for activating the proteins associated with abiotic stress tolerance in wheat (Choudhury et al. 2017; Sunkar et al. 2003). It has developed a series of network involved in scavenging the ROS produced inside the system in response to stresses. Increase in the expression and activities of antioxidant enzymes and accumulation of antioxidants modulates the tolerance level of wheat under stresses (Xiong et al. 2002). The oxidative stress signaling enhances the accumulation of osmolyte through activating the MAPK cascade especially protein tyrosine kinases (PTKs), histidine kinases (HKs), and G-protein receptors (Rohm et al. 2020; Ara and Sinha 2014). The secondary messenger-based signaling especially Ca2+-dependent has been well characterized in different intracellular processes. Abiotic stresses induce the transient Ca2+ influx inside the cell cytoplasm with the help of carrier protein like calcium-dependent protein kinases (CDPKs) (Tuteja and Mahajan 2007). Ca2+-dependent signaling has been characterized to play important role in regulating the expression of late embryogenesis abundant (LEA)-type genes, and SAPs (Nagaraju et al. 2019). Ca2+-dependent SOS signaling regulates the redox of the cell under salt stress (Rohm et al. 2020). Wide diversity has been reported with reference to the machinery involved in stress perception, and signaling in wheat (Novakovic et al. 2018). MAPKs play central role in regulating these sensors associated with perception and stabilize the integrity of the membrane under HS by regulating the expression of ABC transporters (Sinha et al. 2011).
The MAPK cascade characterized in wheat is involved in signaling against different stresses, hormonal treatment and other intracellular perception (Hirt 2000; Hettenhausen et al. 2015). MAPKs showed increase in the expression at transcript and protein levels in response to abiotic stresses, as observed in different plants (Sinha et al. 2011; Hasegawa et al. 2000). The intensity and duration of activation decide the extent of triggering of MAPK cascade (Cargnello and Roux 2011). The scaffolding and attenuation through phosphatases have been observed as main deciding factors for the activation and extent of activity of kinases involved in MAPK cascade (Sinha et al. 2011). Different stresses have been reported to activate specific type of MAPK for different durations and the activation also varies with the intensity of the perception (Rohm et al. 2020). The above findings clearly showed the importance of MAPKs in stress signaling in different tissues of wheat. To conclude, MAPKs regulate the oxidative stress-based signaling caused due to HS and influence various pathways involved in modulating the thermotolerance of wheat, like accumulation and activities of antioxidant enzymes, osmolytes, chaperones, SAGs, SAPs, etc. MAPKs trigger the expression and activities of network of SAGs/ SAPs in different tissues of wheat, like root, stem, leaves and spikes, which in turn enhances the HS tolerance of wheat.
Role of MAPKs in regulating the grain quality
MAPKs have been reported to play important role in regulating the grain growth, size, and stability under adverse conditions in different crops and modal plants. The combinatorial approach of MAPK along with other members of cascade has been reported in regulating various biological process associated with grain-filling, quality and stability (Fig. 6). Different combinations of MAP3ks, MAP2Ks and MAPKs have been observed to regulate diverse biological functions in crops especially cereals, like wheat and rice. Alomari et al. (2018) located MAPK gene in specific genomic region of wheat involved in enhancing the grain zinc concentration. Similarly, MKK3 has been characterized to regulate the seed dormancy in wheat. The grain size and weight of rice have been observed to be positively regulated by OsM3Ks signaling pathways in rice. Similarly, OsMKP1 has been observed to limit the growth of grains by deactivating the OsMAPK6. OsMAP3K5 influences the height and yield of rice, and a positive regulation has been established in different crop plants (Liu et al. 2019). MAPK6 has been characterized to regulate the grain size, and involved in activating the different signaling pathways in rice. MAP2K-3 and MKK3-A were found to regulate seed dormancy in wheat and barley (Nakamura et al. 2016). Similarly, Ali et al., (2019) identified a gene for seed dormancy lying on Chr 4A and reported the gene to be MAPKK. It was also observed to play very important role in post-harvest sprouting. Alomari et al. (2018) performed genome-wide association study (GWAS) followed by genotyping for characterizing the accumulation of Zinc in diverse genotype of European wheat and identified a set of candidate genes, mainly basic leucine zipper (bZIP) and MAPK, responsible for the accumulation of zinc in grains and their nutritional stability. SMG1/OsMKK4 and SMG2/OsMKKK10 have been reported to regulate the quality of rice grains and provide stability for some of the traits linked with grain chalkiness (Zhou et al. 2020). Mitsui et al. (2016) identified a putative manganese superoxide dismutase 1 (MSD1) gene and reported its role in enhancing the quality of rice grain through the activation of MAPK gene. Much research work has not been done in this particular area, especially in wheat to establish correlation between the traits linked with grain quality with that of expression and activities of MAPKs. MAPKs regulate the transportation and accumulation of photosynthates from leaves to the endospermic tissue by regulating the expression and activity of few of the ATP-binding cassette (ABC) transporter superfamily like TaABCC3 genes, which in turn influence the size and shape of the grains (Walter et al. 2015). Different isoforms of MAPKs have been identified and characterized to be involved in regulating different transporters involved in metabolic pathways associated with nutritional quality. Sinha et al. (2011) reported the role of ABC transporters in regulating the grain size and quality in diverse crops. It also regulates the transportation and accumulation of beneficial minerals, like iron, zinc, etc. in endospermic tissue of seeds. To conclude, MAPKs are indirectly involved in stabilizing the grain quality under adverse environmental conditions in diverse crop species, as evident from the increase in the expression and activities of candidate genes/proteins involved in maintaining the nutritional quality of grains.
Conclusion
MAPK research has explored different complex mechanisms in plant sciences and will continue to do so. MAPK-phosphorylated proteins, being the crucial regulator of environmental responses, act as excellent candidates for improving stress tolerance in plants and maintaining the quality of the grains under HS. It has also enabled us to predict and characterize novel targets. The external or intracellular signal is transduced to the nucleus via MAPK cascade, which activates the expression of sets of SAGs under HS. MAPK cascade has been observed to be highly conserved across the species and has not much been characterized in different crops species especially wheat. MAPKs work in a systemic way through a series of phosphorylation/dephosphorylation to transduce the perceptive signal in highly specific manner. MAPK cascade has been implicated in abiotic and biotic stress tolerance, other than the hormonal, redox perception inside the cells and regulation of grain shape and size. Few MAPK genes have been identified from wheat and characterized to be involved in beneficial mineral accumulation in endosperm as well as enhancing the grain number and size under HS. The MAPK cascade is regulated by transcriptional, translational and post-transcriptional regulations. MAPKs, lying at pivotal position, can be further manipulated using the advance tools of genetic engineering for enhancing the tolerance level against different biotic and abiotic stresses and stabilizing the quality of the grains. An in-depth knowledge about the regulation of MAPK cascades in wheat using the recent tools of integrated omics might help to develop novel strategies to improve stress tolerance without compromising with the quality of the grains under HS. There is a need to focus more on the kinase protein interaction, motif–motif interaction, kinase–substrate recognition, etc. in wheat to use it as potential heat sensors for mitigating the effect of stress well ahead of time.
Future prospects
The MAPK cascade has not been explored to the extent that it can elucidate the relationship between other signaling pathways, like Auxin, gibberellin, brassinosteroids, etc. (Jagodzik et al. 2018). The autophosphorylation mechanisms of kinases during different signaling network under stresses and other biological functions need to be further studied (Huang et al. 2000). Research should focus towards development of wheat MAPK with high phosphorylation efficiency through protein engineering. The functional characterization of MAPK diversity and redundancy must also be taken into account. Besides these probable areas for research, there remains some unanswered questions, like homologs of MAPKs in wheat, role of MAPKs in growth and development, whether MAPK can act as sensor for the stress, whether MAPK can be used to regulate the activity of key enzymes associated with different pathways, like tolerance, quality of the grains, effectiveness of MAPK as molecular marker in wheat breeding program, etc. The story of MAPK cascade will help us to unravel the mechanisms behind the complexity of thermotolerance in wheat and the information generated can be diligently utilized to develop ‘climate-smart’ crop.
Acknowledgements
The authors acknowledged the financial assistance received from Council of Scientific and Industrial Research (CSIR) under Extra Mural Grant (Sanction no 38(1436)/17/EMR-II).
Abbreviations
- MAPK
Mitogen-activated protein kinase
- JNKs
C-Jun N-terminal kinases
- ASK-1
Apoptosis signal-regulating kinase 1
- SAGs
Stress-associated genes
- SAPs
Stress-associated proteins
- HS
Heat stress
- MSD1
Manganese superoxide dismutase 1
- bZIP
Basic leucine zipper
- CDPKs
Calcium-dependent protein kinases
- LEA
Late embryogenesis abundant
- SOS
Salt overlay sensitive
- ROS
Reactive oxygen species
- Raf
Rapidly Accelerated Fibrosarcoma
Funding
The financial assistance was received from Council of Scientific and Industrial Research (CSIR) under Extra Mural Grant (Sanction no 38(1436)/17/EMR-II).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
Ranjeet R. Kumar, Email: ranjeetranjaniari@gmail.com
Kirti Arora, Email: chugh.kirti29@gmail.com.
Suneha Goswami, Email: suneha08@gmail.com.
Akshay Sakhare, Email: sakhare.akshaya@gmail.com.
Bhupinder Singh, Email: bhupindersinghiari@yahoo.com.
Viswanathan Chinnusamy, Email: viswa.chinnusamy@gmail.com.
Shelly Praveen, Email: shellypraveen@hotmail.com.
References
- Abhinandan K, Skori L, Stanic M, et al. Abiotic stress signalling in wheat: an inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahanger MA, Akram NA, Ashraf M, et al. Plant responses to environmental stresses-from gene to biotechnology. AoB Plants. 2017 doi: 10.1093/aobpla/plx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Cao J, Jiang H, et al. Unravelling molecular and genetic studies of wheat (Triticum aestivum L.) resistance against factors causing pre-harvest sprouting. Agronomy. 2019;9(3):117. doi: 10.3390/agronomy9030117. [DOI] [Google Scholar]
- Alomari DZ, Eggert K, von Wirén N, et al. Identifying candidate genes for enhancing grain Zn concentration in wheat. Front Plant Sci. 2018;9:1313. doi: 10.3389/fpls.2018.01313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara H, Sinha AK (2014) Conscientiousness of mitogen activated protein kinases in acquiring tolerance for abiotic stresses in plants. Proc Indian Natl Sci Acad 10.16943/ptinsa/2014/v80i2/1
- Bigeard J, Hirt H. Nuclear signaling of plant MAPKs. Front Plant Sci. 2018;9:469. doi: 10.3389/fpls.2018.00469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Hoek JB, Kholodenko BN. Why do protein kinase cascades have more than one level? Trends. Biochem Sci. 1997;22:8. doi: 10.1016/S0968-0004(97)82216-5. [DOI] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and Function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011 doi: 10.1128/mmbr.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang D, Zhang Y, et al. Evidence for a specific and critical role of mitogen-activated protein kinase 20 in uni-to-binucleate transition of microgametogenesis in tomato. New Phytol. 2018;219(1):176–194. doi: 10.1111/nph.15150. [DOI] [PubMed] [Google Scholar]
- Choudhury FK, Rivero RM, Blumwald E, Mittler R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017 doi: 10.1111/tpj.13299. [DOI] [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Mitogen-activated protein kinases: new signalling pathways functioning in cellular responses to environmental stress. J Exp Biol. 2003;206(7):1107–1115. doi: 10.1242/jeb.00220. [DOI] [PubMed] [Google Scholar]
- Cristina M, Petersen M, Mundy J. Mitogen-activated protein kinase signalling in plants. Annu Rev Plant Biol. 2010 doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- de Zelicourt A, Colcombet J, Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016;21(8):677–685. doi: 10.1016/j.tplants.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Farooq MA, Niazi AK, Akhtar J, et al. Acquiring control: the evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem. 2019;141:353–369. doi: 10.1016/j.plaphy.2019.04.039. [DOI] [PubMed] [Google Scholar]
- Flores K, Yadav SS, Katz AA, Seger R. The nuclear translocation of mitogen-activated protein kinases: molecular mechanisms and use as novel therapeutic target. Neuroendocrinol. 2019;108(2):121–131. doi: 10.1159/000494085. [DOI] [PubMed] [Google Scholar]
- Gógl G, Kornev AP, Reményi A, Taylor SS. Disordered protein kinase regions in regulation of kinase domain cores. Trends Biochem Sci. 2019 doi: 10.1016/j.tibs.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo XJ, Wang JR. Global identification, structural analysis and expression characterization of bHLH transcription factors in wheat. BMC Plant Biol. 2017 doi: 10.1186/s12870-017-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Chakrabarty SK. Gibberellic acid in plant: still a mystery unresolved. Plant Signal Behav. 2013;8(9):e25504. doi: 10.4161/psb.25504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton KZ, Liu Y, Gorospe M, et al. Activation of mitogen-activated protein kinase by H2O2: role in cell survival following oxidant injury. J Biol Chem. 1996 doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- Hardie DG. Plant protein serine/threonine kinases: classification and functions. Annu Rev Plant Physiol Plant Mol Biol. 1999 doi: 10.1146/annurev.arplant.50.1.97. [DOI] [PubMed] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu J-K, Bohnert HJ. Plant cellular and molecular responses to high salinity. Ann Rev Plant Biol. 2000;51(1):463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Hettenhausen C, Schuman MC, Wu J. MAPK signaling: a key element in plant defense response to insects. Insect Sci. 2015;22(2):157–164. doi: 10.1111/1744-7917.12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt H. MAP kinases in plant signal transduction. In: Hirt H, editor. MAP kinases in plant signal transduction. Results and problems in cell differentiation. Berlin, Heidelberg: Springer; 2000. pp. 1–9. [DOI] [PubMed] [Google Scholar]
- Huang Y, Li H, Gupta R, Morris PC, Luan S, Kieber JJ. ATMPK4, an Arabidopsis homolog of mitogen-activated protein kinase, is activated in vitro by AtMEK1 through threonine phosphorylation. Plant Physiol. 2000;122(4):1301–1310. doi: 10.1104/pp.122.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Shinozaki K, Tena G, et al. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci. 2002;7(7):301–308. doi: 10.1016/s1360-1385(02)02302-6. [DOI] [PubMed] [Google Scholar]
- Jagodzik P, Tajdel-Zielinska M, Ciesla A, et al. Mitogen-activated protein kinase cascades in plant hormone signaling. Front Plant Sci. 2018 doi: 10.3389/fpls.2018.01387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalmi SK, Sinha AK. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front Plant Sci. 2015 doi: 10.3389/fpls.2015.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi RK, Kar B, Nayak S. Characterization of mitogen activated protein kinases (MAPKs) in the Curcuma longa expressed sequence tag database. Bioinf. 2011 doi: 10.6026/97320630007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli SK, Khanna K, Bhardwaj R, et al. Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants. 2019;8(12):641. doi: 10.3390/antiox8120641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000 doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Wankhede DP, Sinha AK. Signal convergence through the lenses of MAP kinases: paradigms of stress and hormone signaling in plants. Front Biol. 2013;8(1):109–118. doi: 10.1007/s11515-012-1207-1. [DOI] [Google Scholar]
- Kumar RR, Hasija S, Goswami S, et al. Gamma irradiation protect the developing wheat endosperm from oxidative damage by balancing the trade-off between the defence network and grains quality. Ecotoxicol Environ Saf. 2019 doi: 10.1016/j.ecoenv.2019.03.020. [DOI] [PubMed] [Google Scholar]
- Kumar RR, Singh K, Ahuja S, et al. Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct Integr Genomics. 2018 doi: 10.1007/s10142-018-0648-2. [DOI] [PubMed] [Google Scholar]
- Kumar RR, Tasleem M, Jain M, et al. Nitric oxide triggered defense network in wheat: augmenting tolerance and grain-quality related traits under heat-induced oxidative damage. Environ Exp Bot. 2019 doi: 10.1016/j.envexpbot.2018.11.016. [DOI] [Google Scholar]
- Lee HY, Back K. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J Pineal Res. 2017 doi: 10.1111/jpi.12379. [DOI] [PubMed] [Google Scholar]
- Liu Y, He C. A review of redox signaling and the control of MAP kinase pathway in plants. Redox Biol. 2017;11:192–204. doi: 10.1016/j.redox.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhu Y, Xu X, et al. OstMAPKKK5, a truncated mitogen-activated protein kinase kinase kinase 5, positively regulates plant height and yield in rice. Crop J. 2019 doi: 10.1016/j.cj.2019.03.001. [DOI] [Google Scholar]
- Lu K, Guo W, Lu J, et al. Genome-wide survey and expression profile analysis of the mitogen-activated protein kinase (MAPK) gene family in Brassica rapa. PLoS ONE. 2015 doi: 10.1371/journal.pone.0132051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang H, He Y, et al. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell. 2013 doi: 10.1105/tpc.112.104695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui T, Yamakawa H, Kobata T. Molecular physiological aspects of chalking mechanism in rice grains under high-temperature stress. Plant Prod Sci. 2016 doi: 10.1080/1343943X.2015.1128112. [DOI] [Google Scholar]
- Mitula F, TajdelCies̈la MA, et al. Arabidopsis ABA-activated kinase MAPKKK18 is regulated by protein phosphatase 2C ABI1 and the ubiquitin-proteasome pathway. Plant Cell Physiol. 2015 doi: 10.1093/pcp/pcv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK. MAP kinase pathways. Cold Spring Harb Perspect Biol. 2012 doi: 10.1101/cshperspect.a011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad T, Zhang J, Ma Y, et al. Overexpression of a mitogen-activated protein kinase SlMAPK3 positively regulates tomato tolerance to cadmium and drought stress. Molecules. 2019 doi: 10.3390/molecules24030556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraju M, Kumar SA, Reddy PS, et al. Genome-scale identification, classification, and tissue specific expression analysis of late embryogenesis abundant (LEA) genes under abiotic stress conditions in Sorghum bicolor L. PLoS ONE. 2019 doi: 10.1371/journal.pone.0209980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami H, Soukupová H, Schikora A, et al. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem. 2006 doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Pourkheirandish M, Morishige H, et al. Mitogen-activated protein kinase kinase 3 regulates seed dormancy in barley. Curr Biol. 2016;26:775–781. doi: 10.1016/j.cub.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Novaković L, Guo T, Bacic A, et al. Hitting the wall—sensing and signaling pathways involved in plant cell wall remodelling in response to abiotic stress. Plants. 2018;7(4):89. doi: 10.3390/plants7040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A, Zehorai E, Procaccia S. Seger R (2011) The MAPK cascades: signaling components, nuclear roles and mechanisms of nuclear translocation. Biochim Biophysic Acta (BBA)-Mol Cell Res. 1813;9:1619–1633. doi: 10.1016/j.bbamcr.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Raja V, Majeed U, Kang H, et al. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ Exp Bot. 2017 doi: 10.1016/j.envexpbot.2017.02.010. [DOI] [Google Scholar]
- Röhm S, Krämer A, Knapp S (2020) Function, structure and topology of protein kinases. In: Topics in medicinal chemistry. Springer, Berlin, Heidelberg. 10.1007/7355_2020_97
- Sheikh AH, Raghuram B, Jalmi SK, et al. Interaction between two rice mitogen activated protein kinases and its possible role in plant defense. BMC Plant Biol. 2013 doi: 10.1186/1471-2229-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK, Jaggi M, Raghuram B, Tuteja N. Mitogen-activated protein kinase signaling in plants under abiotic stress. Plant Signal Behav. 2011;6(2):196–203. doi: 10.4161/psb.6.2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y, Cheong Y-K, Kim N-H, et al. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J Signal Transduct. 2011 doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R, Bartels D, Kirch HH. Overexpression of a stress-inducible aldehyde dehydrogenase gene from arabidopsis thaliana in transgenic plants improves stress tolerance. Plant J. 2003;35(4):452–464. doi: 10.1046/j.1365-313X.2003.01819.x. [DOI] [PubMed] [Google Scholar]
- Taj G, Agarwal P, Grant M, Kumar A. MAPK machinery in plants. Plant Signal Behav. 2010 doi: 10.4161/psb.5.11.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Zhang J, Hsu PK, et al. MAP3Kinase-dependent SnRK2-kinase activation is required for abscisic acid signal transduction and rapid osmotic stress response. Nat Commun. 2020 doi: 10.1038/s41467-019-13875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, Dangl JL, Hirt H. The MKK2 pathway mediates cold and salt stress signaling in arabidopsis. Mol Cell. 2004;15(1):141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Tena G, Asai T, Chiu W-L, Sheen J. Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol. 2001;4(5):392–400. doi: 10.1016/S1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- Tuteja N, Mahajan S. Calcium signaling network in plants: an overview. Plant Signal Behav. 2007;2(2):79–85. doi: 10.4161/psb.2.2.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat tolerance in plants: an overview. Environ Exp Bot. 2007 doi: 10.1016/j.envexpbot.2007.05.011. [DOI] [Google Scholar]
- Walter S, Kahla A, Arunachalam C, Perochon A, Khan MR, Scofield SR, Doohan FM. A wheat ABC transporter contributes to both grain formation and mycotoxin tolerance. J Exp Bot. 2015;66(9):2583–2593. doi: 10.1093/jxb/erv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Xiao K, Jiang S, et al. Mechanisms of reactive oxygen species in plants under drought stress. Kexue Tongbao/Chinese Sci Bull. 2019 doi: 10.1360/N972018-01116. [DOI] [Google Scholar]
- Wang G, Liang YH, Zhang JY, Cheng ZM. Cloning, molecular and functional characterization by overexpression in arabidopsis of MAPKK genes from grapevine (Vitis vinifera) BMC Plant Biol. 2020 doi: 10.1186/s12870-020-02378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chevalier D, Larue C, Cho SK, Walker JC. The protein phosphatases and protein kinases of Arabidopsis thaliana. Arab B. 2007 doi: 10.1199/tab.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Hsu CC, Du Y, et al. Mapping proteome-wide targets of protein kinases in plant stress responses. Proc Natl Acad Sci USA. 2020 doi: 10.1073/pnas.1919901117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jia C, Li J, et al. Identification of six mitogen-activated protein kinase (MAPK) genes in banana (Musa acuminata L. AAA group, cv. Cavendish) under infection of Fusarium Oxysporum f. sp cubense tropical race 4. Acta Physiol Plant. 2015 doi: 10.1007/s11738-015-1868-x. [DOI] [Google Scholar]
- Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu J-K. Cell signaling during cold, drought, and salt stress. Plant Cell. 2002;14:S165–S183. doi: 10.1105/tpc.000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Liu R, Zhang Q, et al. The diversification of evolutionarily conserved MAPK cascades correlates with the evolution of fungal species and development of lifestyles. Genome Biol Evol. 2017 doi: 10.1093/gbe/evw051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 2015;20(1):56–64. doi: 10.1016/j.tplants.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Yan LJ. Protein redox modification as a cellular defense mechanism against tissue ischemic injury. Oxid Med Cell Longev. 2014 doi: 10.1155/2014/343154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Ali GS, Yang L, et al. Calcium/calmodulin-regulated receptor-like kinase CRLK1 interacts with MEKK1 in plants. Plant Signal Behav. 2010 doi: 10.4161/psb.5.8.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Wang L, Zhao R, et al. Knockout of SlMAPK3 enhances tolerance to heat stress involving ROS homeostasis in tomato plants. BMC Plant Biol. 2019 doi: 10.1186/s12870-019-1939-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeke A, Misheva M, Reményi A, Bogoyevitch MA. JNK signaling: regulation and functions based on complex protein-protein partnerships. Microbiol Mol Biol Rev. 2016 doi: 10.1128/mmbr.00043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xia D, He Y. Rice grain quality—traditional traits for high quality rice and health-plus substances. Mol Breed. 2020 doi: 10.1007/s11032-019-1080-6. [DOI] [Google Scholar]
- Zhu JK. Abiotic Stress Signaling and Responses in Plants. Cell. 2016 doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlobin A, Bloodworth JC, Osipo C (2019) Mitogen-Activated Protein Kinase (MAPK) Signaling. In: Badve S., Kumar G. (eds) predictive biomarkers in oncology. Springer, Cham. 10.1007/978-3-319-95228-4_16