Abstract
India has one of the highest incidences of oral squamous cell carcinoma (OSCC), with 75,000–80,000 new cases a year. The outcome in early oral cancer is better, but a significant proportion (12–14%) of these patients still relapses and dies due to locoregional or distant recurrences. Several clinicopathological and molecular factors have been used to prognosticate and predict outcomes in these patients of OSCC. The present study aims to validate Brandwein Gensler (BG) risk predictive model in surgically treated OSCC patients in a tertiary care centre in North India. All oral cavity cancer patients, treated in the Department of Surgical Oncology, King George’s Medical University, between 2013 and 2017, were reviewed. Patients with histologically diagnosed OSCC, aged > 18 years undergoing primary surgical resection were included in the study. The final histopathological evaluation was done by a dedicated pathologist to categorize patients according to BG model risk categories. This model comprises of three factors, lymphocytic host response, perineural invasion and worst pattern of invasion, scored by the method described by Brandwein Gensler et al. The sum of these scores is used to define low, moderate and high risk categories. The study, conducted during 2013–2017, included 149 patients. Median age was 45 years (range 25–75 years). Tobacco use was noted in 143 patients. Buccal mucosa was the most common site (51%). Surgical margins were clear (> 5 mm) in 97.9% cases. Postoperative radiotherapy was given in 47.7% patients. Locoregional recurrences (LRR) (primary site and neck) were documented in 17 of the 149 patients (11.4%). There was no synchronous or metachronous distant metastasis noted in any of the study patients. Six patients had disease specific mortality. Among the 17 patients with LRR, majority (11) belonged to the high risk category of the BG risk model. Adjuvant radiotherapy had been administered in 10 of these 11 recurrent patients belonging to the high risk category. The Brandwein Gensler risk model is predictive of locoregional recurrences (p = 0.02) for OSCC undergoing primary surgery. It can be used to devise strategies to prevent recurrences or identification of recurrences at an earlier point for salvage. The benefit of further escalation of adjuvant therapy in the high risk category needs further studies, as 90% patients in this group recurred despite complete adjuvant treatment.
Keywords: Oral cavity cancer, Risk model, Surgery, Recurrence, Adjuvant
Introduction
India has one of the highest incidences of oral squamous cell carcinoma (OSCC), with 75,000–80,000 new cases a year. Oral cancer has the highest mortality and is responsible for 22.9% of cancer deaths in Indian men. There are twice as many deaths annually from oral cancer as compared to lung cancer [1].
The outcome of treatment is related to several clinical and pathological factors. Majority of patients present with advanced disease which has a high morbidity and mortality. The outcome in early oral cancer is better but a significant proportion (12–14%) of patients still relapses and dies due to locoregional or distant recurrences.
Several clinicopathological and molecular factors have been used to prognosticate and predict outcomes in these patients of OSCC. A recently described prognostic model to predict outcome in OSCC is the Brandwein Gensler risk model [2]. The Brandwein Gensler risk model has been validated in western multicentre studies for advanced as well as early disease.
Our aim is to validate the Brandwein Gensler risk model in a cohort of OSCC patients undergoing primary surgery for early and operable advanced disease in a tertiary care teaching hospital in North India.
Materials and Methods
All oral cavity cancer patients who underwent primary surgery in the Department of Surgical Oncology, King George’s Medical University, between 2013 and 2017, were reviewed. Inclusion criteria were age > 18 years, patients with histologically diagnosed OSCC and subjected to surgical resection. Patients with recurrent or inoperable disease, prior systemic chemotherapy/radiotherapy, refusing to enroll in study, and those positive for HIV, HBsAg, and HCV were excluded. Patients were managed by standard treatment protocols for oral cavity cancers as per NCCN guidelines. All cases underwent diagnostic punch biopsy for histopathological confirmation of the diagnosis prior to surgery. All surgeries were primary treatment with curative intent. The final pathological evaluation was done by a dedicated pathologist. Resection margins were histologically assessed as follows: margins ≥ 5 mm from carcinoma were deemed negative; margins < 5 mm were deemed close.
The scoring system followed was as described in the original papers [3, 4]. The Brandwein Gensler risk categories were based on the scores, which are derived from the sum of the specimen Lymphocytic Host Response (LHR), Worst Pattern of Invasion (WPOI) and Perineural invasion (PNI). The scoring system assigns points to various defined histopathological characteristics of the three variables viz. LHR, WPOI and PNI (Fig. 1). A total score of 3 or more is high risk, 1–2 is intermediate risk and 0 is low risk. Detailed description of this scoring system is available in the original publications [3, 4].
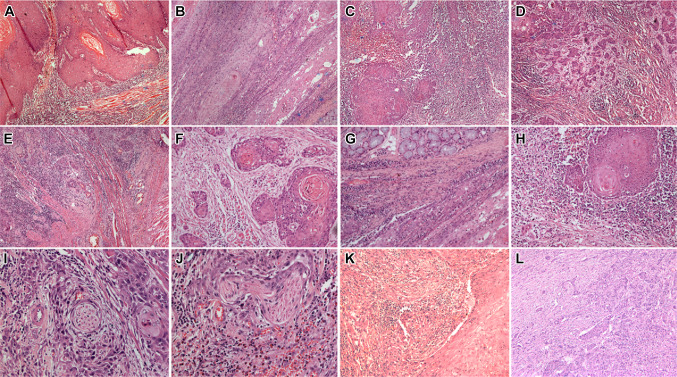
Fig. 1.
Microphotographs showing oral squamous cell carcinoma with A-E: worst pattern of invasion 1 (a), 2 (b), 3 (c), 4 (d), and 5 (e); f, g and h show intensity of lymphocytic infiltrates from 1 + , 2 + to 3 + ; i depicts PNI in small nerve and j in a large nerve; k shows large vessel invasion and l shows small vessel invasion (H&E × 100 × digital magnification)
After completion of treatment, patients were followed up at 2 monthly intervals. Recurrence and mortality was recorded. Clinicopathologic and demographic information (age, gender, date of surgery, T stage, N stage, and adjuvant therapy) was collected. Locoregional recurrence (LRR) and disease specific mortality were the two time-to-event outcomes recorded. Locoregional recurrence was defined as recurrence in the region of primary or in the cervical nodal region within a period of 2 years from the date of surgery. Recurrences at primary site and nodal sites were combined as they represent failure of initial definitive treatment directed against locoregional disease. Distant metastatic disease on the other hand is a manifestation of systemic spread of disease, usually not addressed by initial treatment. Disease specific mortality is defined as death due to the presence/recurrence of disease.
Statistical analysis was done using SPSS software. A multivariate analysis was done for the individual components of the score. Receiver Operator Characteristics (ROC) analyses were performed for the risk categories with respect to LRR and disease specific survival (DSS). All reported p values are two sided, and statistical significance was claimed at ≤ 0.05.
Results
The total number of patients in the study was 149. The clinicopathologic data is presented in Table 1.While the most common site in males was buccal mucosa, it was tongue in females. Males were more likely to present with T3, T4 tumors. The median follow up period was 18 months (range 1–45 months). There were seventeen (11.4%) recurrences, 12 in males and 5 in females. These were all locoregional recurrences. No distant metastasis was noted. Six patients had disease specific mortality.
Table 1.
Clinico-pathologic data of the study patients
| N = 149 | N | % |
|---|---|---|
| Median age | ||
| 45 years (25–75) | ||
| Gender | ||
| Male | 120 | 80.5 |
| Female | 29 | 19.5 |
| Tobacco use | 143 | 95.9 |
| Alcohol use | 7 | 4.6 |
| Site | ||
| Buccal mucosa | 76 | 51 |
| Tongue | 46 | 30.8 |
| Lip | 4 | 2.6 |
| Alveolus | 19 | 12.7 |
| Palate | 4 | 2.6 |
| T stage | ||
| T1 | 42 | 28.2 |
| T2 | 59 | 39.5 |
| T3 | 19 | 12.7 |
| T4 | 27 | 18.1 |
| N stage | ||
| N0 | 98 | 65.7 |
| N1 | 11 | 7.4 |
| N2 | 40 | 26.8 |
| Treatment | ||
| Surgery only | 78 | 52.3 |
| Surgery + PORT | 71 | 47.7 |
| Margin | ||
| Clear | 146 | 97.9 |
| Close | 3 | 2.1 |
| Recurrence | 17 | 11.4 |
T stage of the disease was significantly associated with nodal involvement. Higher the T stage of the disease more likely the presence of nodal disease (p = 0.0005). T1 stage was associated with lower risk of recurrence (p = 0.006). Despite the fact that recurrences were more frequent in patients with advanced T (p = 0.046) and N stage (p = 0.01) disease, they also occurred in patients with early disease. There were 4 recurrences in 72 patients of early disease (T1/T2, N0). The distribution of risk scores in these patients is shown in Table 2. There is no significant association of the recurrence risk with the risk category (p = 0.94, AUC = 0.511).
Table 2.
Distribution of risk categories and recurrences
| Risk categories | Total | Recurrence | |
|---|---|---|---|
| T1/2 N0 | Total | ||
| Low | 29 | 1 | 1 (3.4%) |
| Intermediate | 64 | 2 | 5 (7.8%) |
| High | 56 | 1 | 11 (19.7%) |
| 149 | 4 | 17 | |
Margin was clear in 146 patients and close in three patients. There was no statistical association between margin status and recurrence (p = 0.55). Lymphovascular invasion (LVI) was seen in 42 patients, of which ten had recurrences (p = 0.003). Adjuvant radiation therapy was administered in 71 patients, of which 13 had recurrence after treatment completion.
Worst Pattern of Invasion (WPOI)
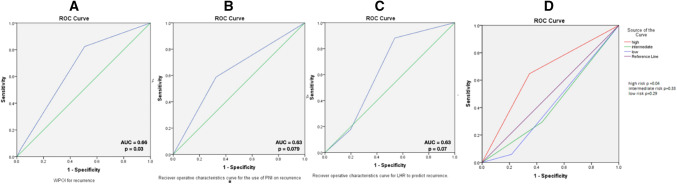
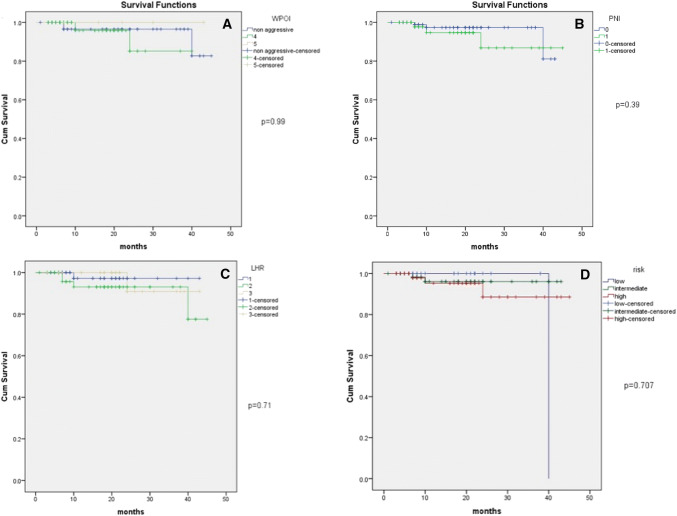
Majority of the study specimens had non aggressive pattern of invasion (45.6%). The aggressive type WPOI was significantly associated with locoregional recurrence (p = 0.016). The receiver operator characteristics (ROC) curve showed that WPOI is predictive of recurrence (Fig. 2a). On Kaplan–Meier survival plots, disease specific survival was not significantly different in the aggressive and non aggressive types of WPOI with a p value of 0.99. (Figure 3a).
Fig. 2.
Reciever operator characteristics curve with respect to for a WPOI, b PNI, c LHR and d risk categories
Fig. 3.
Survival functions in relation to a WPOI, b PNI, c LHR, and d risk categories
Perineural Invasion (PNI)
Perineural invasion was seen in 53 specimens of the 149 included in the study (35.5%). It was seen in 10 of the seventeen patients with recurrence (58.8%). Perineural invasion had a statistically significant association with recurrence (p = 0.03), but is not specific or sensitive to predict a recurrence (Fig. 2b). Perineural invasion was not associated with the disease specific survival in the present study (p = 0.39) (Fig. 3b).
Lymphocytic Host Response (LHR)
Lymphocytic host response type 1 was the most common type (42.3%) of LHR seen. Lymphocytic host response showed significant association with recurrence (p = 0.009). However, LHR could not be used for prediction of recurrence (Fig. 2c) or survival (Fig. 3c).
Table 2 shows the distribution of risk categories in the study cases. Adjuvant treatment was given in 71 patients, of which 3, 32 and 36 patients belonged to the low, intermediate and high risk category, respectively. Among the 17 patients with recurrence, there were 4 patients who did not receive adjuvant treatment post surgery, one belonging to the high risk category. Thirteen patients had a recurrence despite receiving adjuvant therapy.
ROC curve of the risk model showed that the risk model was able to predict LRR in the high risk category (p = 0.04) (Fig. 2d and Table 3). Disease specific survival did not show significant difference in the various risk categories (p = 0.7) (Fig. 3d).
Table 3.
Receiver operator characteristics of the risk categories for LRR
| Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95% CI) | p Value | |
|---|---|---|---|---|---|
| High | 64.7 (38.3–85.7) | 65.4 (57.1–73.9) | 93.5 (88.3–96.5) | 19.6 (13.8–27.2) | 0.04 |
| Intermediate | 29.4 (10.3–55.9) | 56.2 (46.4–63.9) | 85.88 (81.2–89.2) | 7.8 (3.8–15.4) | 0.33 |
| Low | 100 | 0 | – | 96.6 | 0.29 |
Multivariate analysis by logistic regression, including factors aggressive WPOI, LVI, LHR, PNI, T stage, N stage, adjuvant treatment, and high risk category, showed only T (p = 0.02) and LVI (p = 0.019) had a significant association with LRR. None of the factors were independently associated with DSS. A subset analysis of T1, T2 cases revealed no association of any of the above factors with recurrence.
Discussion
Oral cavity squamous cell carcinoma is the most common cancer in India amongst men (11.28%) and fifth amongst women (4.3%) [1, 2]. Loco-regional recurrence is the most common cause of treatment failure, with 11.6% of the study patients having LRR. The search for definitive predictors of outcome has been historically centered on clinical and pathological factors, which have not been satisfactory in stratifying disease biology. In the ongoing search for predictive and prognostic markers, several molecular markers and genetic signatures have been captured [5, 6]. Given the complexity and cost of these new markers, their practical application is limited. Among the various risk models investigated, Brandwein-Gensler (BG) Risk Model is promising and simple to use.
In this study, we have evaluated the Brandwein Gensler Risk Model [3, 4], which is a combination of three important pathological factors, in predicting the outcome in surgically treated OSCC. These pathological factors are—worst pattern of invasion, perineural invasion and lymphocytic host response.
We have attempted to validate the Brandwein Gensler Risk Model for predicting recurrence and adverse survival outcomes in OCSCC in North India. In the present study, the high risk category was significantly predictive of recurrences (p = 0.02), with 11 out of the 17 recurrences falling in the high risk category. The sensitivity of the high risk category was 64.7% and specificity was 65.4%. The negative predictive value and positive predictive value were 93.5% and 19.6%, respectively. In the study done in May 2010, Brandwein Gensler et al. [7] demonstrated similar findings with high risk status independently predicting decreased time to progression (p = 0.015). However, this study included all sites of head and neck carcinoma. Vered et al. [8] have demonstrated that young patients with high risk scores had a higher risk of recurrence (p = 0.02). Li et al. [3] have shown high risk category to be 58.9% sensitive and 70.8% specific, respectively, for LRR. They found a NPV and PPV of 88.2% and 31.7%, respectively.
Tumor pattern of invasion has been consistent component of most multiparameter histological predictive models that have been advocated [9–11]. In the present study, WPOI was found to be an important predictive factor of LRR with a p value of 0.03, sensitivity of 82.4% and specificity of 49.2%. Li et al. [3] have reported sensitivity of 89.3% and specificity of 38.3% in WPOI 4 to predict LRR. The present study could not demonstrate the correlation of WPOI with DSS, unlike the study by Li et al. [3] which showed WPOI to be significant predictor of LRR and DSS.
The risk model was not able to predict DSS in the present study (p = 0.7). None of the risk category or the score variables were predictive of the DSS. In their study in 2013, Li et al. demonstrated a significant correlation of risk categories with DSS (p = 0.0005) [3].
We evaluated some other factors, in addition to BG risk model for, locoregional recurrence—age, gender, T and N stage, margin status, LVI, histologic differentiation and adjuvant therapy. The maximum number of patients in this study were < 65 years of age, including all those with recurrences. The correlation of prognosis with age is controversial in the literature reviewed [5, 12]. However, the present study did not demonstrate any influence of age on prognosis. In the present study, although the disease itself was more common in the males, we found that gender of a patient did not have a significant effect on the locoregional recurrence. On multivariate analyses, gender was not a significant factor to LRR or DSS. A review by Massano et al. [5] found that apparently, there are no prognostic differences between males and females.
T and N stage were found to be prognostically significant on univariate analysis (p = 0.046 and 0.01, respectively), however, on multivariate analyses, N stage did not demonstrate an influence on LRR. Gonzales et al. [13] have found categorical influence of T and N staging on prognosis. In the present study only T stage was close to significance on a multivariate regression analyses, proving its importance in prognosis.
Margin status of the surgical specimen was not predictive of locoregional recurrences with no recurrences in the close (< 5 mm) group (p = 0.55). A strong correlation between disease free margin and higher survival rates, with delayed time to recurrence has been demonstrated by Woolgar et al. and Guerra et al. [14, 15] The findings of the present study were similar to findings of Brandwein Gensler et al. [4] where they found no association of margin status with the LRR (p = 0.2) and OS (p = 0.8). Lymphovascular invasion has been found by various studies to be highly prognostic, we found LVI to be significantly associated with recurrent cases (p = 0.003). However, the present study found no association with disease specific mortality. Jones et al. [16] found a significant association between LVI and survival (p = 0.015). Liu et al. [17] found LVI to be an independent predictor of DSS. Histiological differentiation has been controversial in terms of prognosis [11]. The present study found that differentiation had no influence on either risk of recurrence or DSS.
Adjuvant therapy is an integral part of the curative treatment of oral cavity carcinomas. Adjuvant therapy has a significant influence on reducing recurrences in those with primaries more than T2 and nodal involvement (p = 0.012). However, 11 patients belonging to the high risk category had recurrences despite the administration of adjuvant radiation therapy.
Molecular markers such as p53, p16, EGFR, VEGF, cyclins, Ki 67/MIBI, HPV, c-myc, and uridine phosphorylase have been tried with varied results as described in the review by Massano et al. [5] Genetic expression analyses in OCSCC cases has been used to divide molecular subtypes to predict cervical nodal metastases [6]. To use these on a large scale with affordable means is still not possible.
The present study is not without its own drawbacks. The first limitation of this study was that the majority of OCSCC patients presenting to our centre, who are borderline resectable are subjected to neoadjuvant chemotherapy. They were not included in this study and thus there is a selection bias. On a subset analysis, patients with smaller lesions did not show the model predicting recurrence risk unlike the assessment by Li et al. [3] The duration of the study is short and a longer follow up study may reveal valuable information regarding long term outcomes and differences in recurrence, mortality and survival.
Conclusion
The Brandwein Gensler risk model is predictive of locoregional recurrences (p = 0.02) for oral cavity squamous cell carcinoma (OCSCC), but not disease specific survival. It can be used to apply strategies to prevent recurrences or identification of recurrences at an earlier point for salvage. A surgical pathologist can easily score the specimens into the three different categories of the Risk Model.
The benefit of further escalation of adjuvant therapy in the high risk category needs further studies, as 90% patients in this group recurred despite curative intent surgery followed by complete adjuvant treatment. The use of the score in small lesions is highly limited. The role of the risk model in borderline operable OCSCC is an uncharted area where further studies may prove to be useful.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with Ethical Standards
Conflict of interest
All the authors declare that they have no conflict of interest.
Informed Consent
Informed valid consent obtained from patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dikshit R, et al. Cancer mortality in India: a nationally representative survey. Lancet. 2012;379(9828):1807–1816. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 2.B.W. Stewart, C.P. Wild, ed. World Cancer Report 2014 (International Agency for Research on Cancer, Lyon, 2014).
- 3.Li Y, Bai S, Carroll W, Dayan D, et al. Validation of risk model: high risk classification and tumor pattern of invasion predict outcome for patients with low-stage oral cavity squamous cell carcinoma. Head Neck Pathol. 2013;7:211–223. doi: 10.1007/s12105-012-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandwein-Gensler M, Teixeira MS, Lewis CM, et al. Oral squamous cell carcinoma: histologic risk assessment, but not margin status, is strongly predictive of local disease free and overall survival. Am J Surg Pathol. 2005;29(2):167–178. doi: 10.1097/01.pas.0000149687.90710.21. [DOI] [PubMed] [Google Scholar]
- 5.Massano J, Regateiro FS, Januario G, Ferreira A. Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102(1):67–76. doi: 10.1016/j.tripleo.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 6.Zevallos JP, Mazul AL, Walter V, Hayes DN. Gene expression subtype predicts nodal metastasis and survival in human papillomavirus-negative head and neck cancer. Laryngoscope. 2019;129:154–161. doi: 10.1002/lary.27340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandwein-Gensler M, Smith RV, Wang B, et al. Validation of the histological risk model in a new patient cohort with primary head and neck squamous cell carcinoma. Am J Surg Pathol. 2010;34:676–688. doi: 10.1097/PAS.0b013e3181d95c37. [DOI] [PubMed] [Google Scholar]
- 8.Vered M, Dayan D, Dobriyan A, et al. Oral tongue squamous cell carcinoma: recurrent disease is associated with histopathologic risk score and young age. J Cancer Res Clin Oncol. 2010;136:1039. doi: 10.1007/s00432-009-0749-3. [DOI] [PubMed] [Google Scholar]
- 9.Jakobsson PA, Eneeroth CM, Killander D, Moberger G, Martensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Physiol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- 10.Bryne M, Boysen M, Alfsen CG, et al. The invasive front of carcinomas: the most important area for tumor prognosis. Anticancer Res. 1998;18:4757–4764. [PubMed] [Google Scholar]
- 11.Anneroth G, Batsakis J, Luna M. Review of the literature and recommended system of malignancy grading in oral squamous cell carcinoma. Scand J Dent Res. 1987;95:229–249. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 12.Lo WL, Ko S-Y, Chi LY, Wong YK, Chang RC-S. Outcomes of oral cancer in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac Surg. 2003;61:751–758. doi: 10.1016/S0278-2391(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 13.Gonzales-Moles MA, Esteban F, Rodriguez-Archilla A, Ruiz-Avila I, Gonzales-Moles S. Importance of tumour thickness measurement in prognosis of tongue cancer. Oral Oncol. 2002;38:394–397. doi: 10.1016/S1368-8375(01)00081-1. [DOI] [PubMed] [Google Scholar]
- 14.Guerra MFM, Gias LN, Campo FR, Perez JS. Marginal and segmental mandibulectomy in patients with oral cancer: a statistical analysis of 106 cases. J Oral Maxillofac Surg. 2003;61:1289–1296. doi: 10.1016/S0278-2391(03)00730-4. [DOI] [PubMed] [Google Scholar]
- 15.Woolgar JA, Rogers SN, Lowe D, Brown JS, Vaughan ED. Cervical lymph node metastasis in oral cancer: the importance of even microscopic extracapsular spread. Oral Oncol. 2003;39:130–137. doi: 10.1016/S1368-8375(02)00030-1. [DOI] [PubMed] [Google Scholar]
- 16.Jones HB, Sykes A, Bayman N, Sloan P, Swindell R, Patel M, Musgrove B. The impact of lymphovascular invasion on survival in oral carcinoma. Oral Oncol. 2009;45(1):10–15. doi: 10.1016/j.oraloncology.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu SA, Wang CC, Jiang RS, Lee FY, Lin WJ, Lin JC. Pathological features and their prognostic impacts on oral cavity cancer patients among different sub sites—a single institute’s experience in Taiwan. Sci Rep. 2017;7:7451. doi: 10.1038/s41598-017-08022-w. [DOI] [PMC free article] [PubMed] [Google Scholar]