Abstract
Clear cell odontogenic carcinoma (CCOC) is a rare and aggressive malignant epithelial neoplasm, which occurs most frequently in the mandible of elderly patients. Morphologically, CCOC shares similar characteristics with other clear cell tumors, especially hyalinizing clear cell carcinoma of the salivary glands (HCCC). Both CCOC and HCCC are known to harbor EWSR1 rearrangements, especially the EWSR1–ATF1 gene fusion, which indicates a possible link between the two lesions. So far, this fusion has been demonstrated in five cases of CCOC in the literature. Herein, we add another CCOC case to the literature, which arose in the mandible of an 82-year-old female patient and was proven to harbor the EWSR1–ATF1 gene fusion. Immunohistochemically, this case was focally positive for CK7, CK14, CK19 and p63. The patient was referred to surgical treatment; however, she died of disease 2 months after the diagnosis, thereby demonstrating the aggressive nature of this tumor.
Keywords: Odontogenic tumors, Clear cell odontogenic carcinoma, Salivary gland neoplasms, Hyalinizing clear cell carcinoma, Malignant odontogenic tumor, EWSR1–ATF1 gene fusion
Introduction
First described by Hansen et al. in 1985 [1], clear cell odontogenic carcinoma (CCOC) is considered to be a rare and aggressive malignant odontogenic tumor. Previously recognized by WHO under the denomination “clear cell odontogenic tumor”, this tumor was reclassified as a malignancy in 2005 [2] and it is still denominated CCOC in the 2017 classification [3].
CCOC is recognized as a locally aggressive tumor, with high recurrence rates and the capacity to metastasize to distant sites [4, 5]. Its clinical and radiological features are not specific, although this tumor often presents itself as osteolytic lesions with ill-defined limits [4, 6]. Morphologically, CCOC shares similar characteristics with other clear cell tumors, especially hyalinizing clear cell carcinoma of the salivary glands [5]. Due to the rarity of these lesions, they often pose a diagnostic dilemma for the pathologists. Immunohistochemistry and molecular biology techniques are typically important tools in the diagnostic process of CCOC, as well as complete clinical and radiographic information. In this work, we report a case of CCOC in the mandible of a female patient in which the EWSR1–ATF1 fusion gene was detected.
Case Report
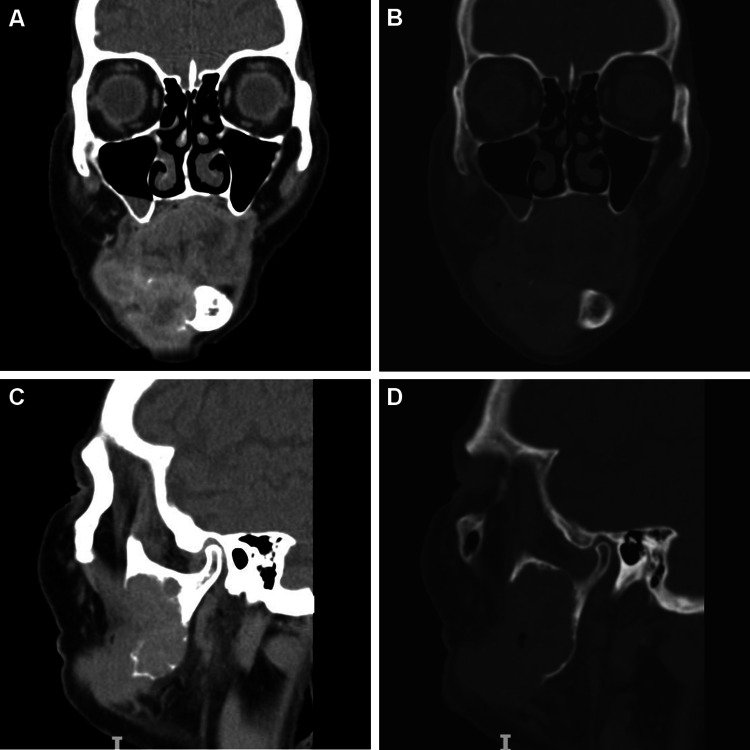
An 82-year-old woman was referred to a Maxillofacial Surgery service for facial asymmetry, right mandible swelling and pain. The symptoms were present for approximately 10 months prior to the consultation. Intraoral examination revealed that the swelling had a soft consistency and was covered by healthy-appearing mucosa. A computed tomography exam was performed and it revealed an ill-defined and destructive lesion extending from the mandible body to the ramus and coronoid process (Figs. 1, 2). At this point, diagnosis of ameloblastoma, myxoma or ameloblastic carcinoma were considered by the surgeons and an incisional biopsy was performed.
Fig. 1.
Computed tomography images in coronal (a, b) and sagittal (c, d) views evidenced an ill-defined and destructive lesion extending from the right mandible body and ramus to the coronoid process
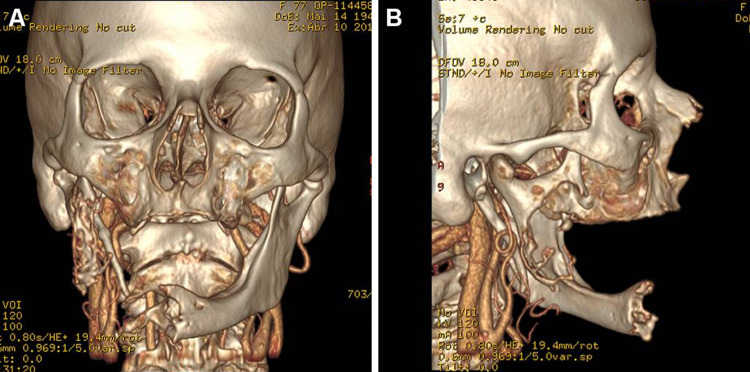
Fig. 2.
Computed tomography 3D reconstruction in coronal (a) and sagittal (b) views evidencing the destructive nature of the lesion
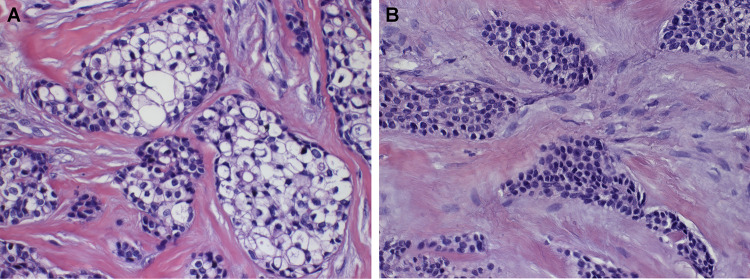
Histopathological examination revealed proliferation of neoplastic epithelial cells with predominantly clear cytoplasm, which were surrounded by hyalinized connective tissue. The cells had polygonal morphology, with round to ovoid nuclei, arranged in nests and islands. Cells with a lightly eosinophillic cytoplasm were seen admixed with the clear cells in some areas (Figs. 3, 4).
Fig. 3.
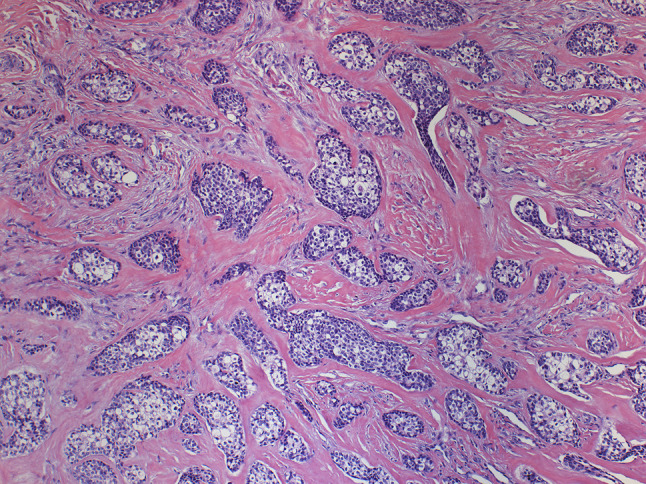
Clear cell odontogenic carcinoma showing proliferation of epithelial cells with predominantly clear cytoplasm embedded in a hyalinized stroma (Hematoxylin and eosin, ×100 in the original)
Fig. 4.
High-power view of clear cell odontogenic carcinoma showing a biphasic pattern. a Cells with clear cytoplasm. b Cells with light eosinophilic cytoplasm. (Hematoxylin and eosin, ×400)
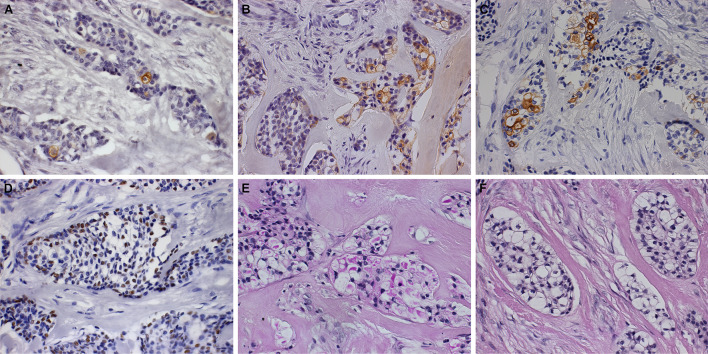
Immunohistochemistry was performed for cytokeratins (CK) 7, 14 and 19 and p63. All antibodies were focally positive in the neoplastic cells. Additionally, the histochemistry revealed clear cells to be positive for Periodic acid-Schiff (PAS) and sensitive to diastase (PAS-D) (Fig. 5).
Fig. 5.
Immunohistochemical and histochemical aspects. Clear cell odontogenic carcinoma exhibiting focal immunopositivity for CK7 (a), CK14 (b), CK19 (c) and p63 (d) (immunohistochemistry, ×400). The lesion was positive for PAS (e) and negative for PAS-diastase (f) (histochemistry, ×400)
Real-time polymerase chain reaction (PCR) confirmed the presence of the EWSR1–ATF1 fusion gene in this tissue sample. For this analysis, total RNA was isolated from formalin-fixed paraffin-embedded tissue using the MagMAX FFPE DNA/RNA Ultra Kit (A31881 Applied Biosystems, Carlsbad, CA, USA), and first-strand cDNA was synthesized using the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher, Waltham, Massachusetts, USA) according to the manufacturer’s instructions. In order to detect EWSR1–ATF1 fusion gene transcripts, real-time PCR was performed using the thermocycler 7500 real-time PCR System (Applied Biosystems, Carlsbad, CA, USA) and the fluorophore Sybr Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA). The used primers were 5′-CAA GGA TTA AAT GAC AGT GTG ACT C-3′ (forward) and 5′-CTT TCT GTG AGG AGC CTA TG-3′ (reverse) (Integrated DNA Technologies, Coralville, Iowa, USA).
The clinical, radiographic, histopathological, immunohistochemical and molecular features together supported the diagnosis of clear cell odontogenic carcinoma.
The patient was indicated for radical surgical treatment with a Head and Neck surgical team; however, she died of the disease 2 months after the diagnosis, before receiving treatment.
Discussion
The presence of a significant clear cell component in odontogenic neoplasms is very rare. These cells possibly originate from the dental lamina and are often a result of cytoplasmic accumulation of water, glycogen, mucopolysaccharides, mucin and lipids [7, 8]. Clear cells may also be a result of fixation artifacts or paucity of cellular organelles, intermediate filaments and immature zymogen granules [7]. In odontogenic lesions, cells with cytoplasmic clearing are found to contain PAS positive diastase labile material, which is indicative of glycogen accumulation [9].
A recent systematic review found 107 well-documented cases of CCOC in the English literature [10]. This tumor is most often reported in female patients in the sixth decade of life and posterior mandible is the most affected site, with no ethnic predilection. Local symptoms of CCOC are frequently described as swelling, with or without pain, tooth mobility and ulceration/bleeding. Radiological aspects are unspecific, but X-ray images are almost always radiolucent and poorly defined [4, 10]. The duration of symptoms prior to diagnosis ranges from a few months to many years, which is uncommonly long for a malignancy [10]. CCOC shows high rates of recurrence; therefore, early and aggressive surgical treatment with clear margins are recommend for these patients [4, 6, 10].
Microscopically, CCOC is characterized by the proliferation of neoplastic epithelial cells with predominantly clear cytoplasm arranged in islands and strands. This lesion may present itself in three different variants: (1) monophasic: composed almost entirely of clear cells with well-defined borders and centrally located nuclei; (2) biphasic: composed of one population of clear cells and another population of hyperchromatic polygonal cells with eosinophilic cytoplasm; (3) ameloblastic: characterized by predominantly columnar cells with ameloblastic differentiation at the periphery of tumor islands [4–6, 10]. In the reported case, we observed a biphasic histology.
Differential diagnosis of CCOC includes benign odontogenic tumors, such as ameloblastoma and the clear cell variant of calcifying epithelial odontogenic tumor; metastatic tumors, as renal clear cell carcinoma and melanoma; tumors of salivary origin, as mucoepidermoid carcinoma and, especially, hyalinizing clear cell carcinoma (HCCC) [5, 11]. Immunohistochemistry is a valuable aid to differentiate some of those lesions (i.e. metastatic tumors). However, findings indicate that both CCOC and HCCC show a high degree of morphologic and immunophenotypic overlap, thereby making it impractical and challenging to distinguish them [5]. Facing this, tumor location (in the jaws versus in oral mucosa/salivary glands) is the most important differentiating criterion between CCOC and HCCC [5].
In 2011, Antonescu et al. [12] demonstrated a specific gene fusion in a group of HCCC, which was not present in other salivary gland tumors: the EWSR1–ATF1. 2 years later, the same research group [13] observed that CCOC also harbors EWSR1 rearrangements and they even showed a CCOC case that harbored the EWSR1–ATF1 gene fusion, thus establishing a link between HCCC and CCOC despite different tumor origins. Since then, four cases of CCOC harboring EWSR1–ATF1 fusion and one case presenting EWSR1–CREB1 fusion have been reported in the literature [14–16]. Now, we add a new case of CCOC to this group, where EWSR1–ATF1 was confirmed. The findings strengthen the relationship between CCOC and HCCC and reinforce the question whether both lesions represent the same entity or whether at least a subgroup of CCOC are actually a central variant of HCCC [13].
As previously reported, the identification of EWSR1–ATF1 is useful for differentiating between CCOC and other tumors with clear cell changes, especially in small biopsy specimens, i.e., clear cell variants of calcifying epithelial odontogenic tumor or central squamous cell carcinoma with clear cell features [13]. However, in our experience, molecular analysis is not mandatory for diagnosing CCOC. This diagnosis may be achieved with adequate material for histopathological and radiographical analysis, patient’s medical history and the aid of immunohistochemistry.
In summary, we have presented a case of CCOC that shows an aggressive clinical course and poor prognosis, which was proven to harbor the EWSR1–ATF1 fusion gene. Further studies would be required to unveil the frequency of this fusion in CCOC and its relation to salivary HCCC. The identification of these gene rearrangements can be helpful in determining targeted therapies for these lesions in the future.
Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001. T. Santana received a scholarship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) during the period in which this study was carried out.
Funding
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001. During the preparation of this manuscript, T. Santana received a scholarship from from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Compliance with Ethical Standards
Conflict of interest
The authors state that there is no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thalita Santana, Email: thalitasc@usp.br.
Felipe Ledo de Andrade, Email: ledofelipe@hotmail.com.
Maria Carolina de Sousa Melo, Email: mariacarolinasmelo@gmail.com.
Glauber Bareia Liberato da Rocha, Email: glauber.rocha@hc.fm.usp.br.
Marília Trierveiler, Email: trierveiler@usp.br.
References
- 1.Hansen LS, Eversole LR, Green TL, Powell NB. Clear cell odontogenic tumor—a new histologic variant with aggressive potential. Head Neck Surg. 1985;8:115–123. doi: 10.1002/hed.2890080208. [DOI] [PubMed] [Google Scholar]
- 2.Barnes L, Eveson JW, Reichart P, Sidransky D. WHO classification of head and neck tumors. Lyon: IARC Press; 2005. [Google Scholar]
- 3.Wright JM, Vered M. Update from 4th edition the of the World Health Organization classification of head and neck tumours: odontogenic and maxillofacial bone tumors. Head Neck Pathol. 2017;11:68–77. doi: 10.1007/s12105-017-0794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadj Saïd M, Ordioni U, Benat G, Gomez-Brouchet A, Chossegros C, Catherine JH. Clear cell odontogenic carcinoma: a review. J Stomatol Oral Maxillofac Surg. 2017;118:363–370. doi: 10.1016/j.jormas.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Bilodeau EA, Hoschar AP, Barnes LL, Hunt JL, Seethala RR. Clear cell carcinoma and clear cell odontogenic carcinoma: a comparative clinicopathologic and immunohistochemical study. Head Neck Pathol. 2011;5:101–107. doi: 10.1007/s12105-011-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loyola AM, Cardoso SV, De Faria PR, Servato JPS, De Paulo LF, Eisenberg ALA, et al. Clear cell odontogenic carcinoma: report of 7 new cases and systematic review of the current knowledge. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:483–496. doi: 10.1016/j.oooo.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Jain A, Shetty DC, Juneja S, Narwal N. Molecular characterization of clear cell lesions of head and neck. J Clin Diagnostic Res. 2016;10:18–23. doi: 10.7860/JCDR/2016/14394.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Said-Al-Naief N, Klein MJ. Clear cell entities of the head and neck: a selective review of clear cell tumors of the salivary glands. Head Neck Pathol. 2008;2:111–115. doi: 10.1007/s12105-008-0052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilodeau EA, Collins BM. Odontogenic cysts and neoplasms. Surg Pathol Clin. 2017;10:177–222. doi: 10.1016/j.path.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Guastaldi FPS, Faquin WC, Gootkind F, Hashemi S, August M, Iafrate AJ, et al. Clear cell odontogenic carcinoma: a rare jaw tumor. A summary of 107 reported cases. Int J Oral Maxillofac Surg. 2019 doi: 10.1016/j.ijom.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilodeau EA, Prasad JL, Alawi F, Seethala RR. Molecular and genetic aspects of odontogenic lesions. Head Neck Pathol. 2014;8:400–410. doi: 10.1007/s12105-014-0588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1–ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom Cancer. 2011;570:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 13.Bilodeau EA, Weinreb I, Antonescu CR, Zhang L, Dacic S, Muller S, et al. Clear cell odontogenic carcinomas show EWSR1 rearrangements. Am J Surg Pathol. 2013;37:1001–1005. doi: 10.1097/PAS.0b013e31828a6727. [DOI] [PubMed] [Google Scholar]
- 14.Ginat DT, Villaflor V, Cipriani NA. Oral cavity clear cell odontogenic carcinoma. Head Neck Pathol. 2016;10:217–220. doi: 10.1007/s12105-015-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancoskie AE, Sreekantaiah C, Jacob J, Rosenberg A, Edelman M, Antonescu CR, et al. EWSR1 and ATF1 rearrangements in clear cell odontogenic carcinoma: presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:e115–e118. doi: 10.1016/j.oooo.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Vogels R, Baumhoer D, van Gorp J, Eijkelenboom A, Verdijk M, van Cleef P, et al. Clear cell odontogenic carcinoma: occurrence of EWSR1-CREB1 as alternative fusion gene to EWSR1-ATF1. Head Neck Pathol. 2019;13:225–230. doi: 10.1007/s12105-018-0953-z. [DOI] [PMC free article] [PubMed] [Google Scholar]