Abstract
In this work, we isolated and selected oleaginous yeasts from rock field soils from two National Parks in Brazil (Caparaó and Serra dos Órgãos) with the potential to accumulate oil from xylose, the main pentose sugar found in lignocellulosic biomass. From the 126 isolates, two were selected based on their lipid contents. They were taxonomically identified as Papiliotrema laurentii (UFV-1 and UFV-2). Of the two, P. laurentii UFV-1 was selected as the best lipid producer. Under unoptimized conditions, lipid production by P. laurentii UFV-1 was higher in glucose than in xylose. To improve its lipid production from xylose, we applied response surface methodology (RSM) with a face-centered central composite design (CCF). We evaluated the effects of agitation rate, initial cell biomass (OD600), carbon/nitrogen ratio (C/N ratio) and pH on lipid production. P. laurentii UFV-1 recorded the highest lipid content, 63.5% (w/w) of the cell dry mass, under the following conditions: C/N ratio = 100:1, pH value = 7.0, initial OD600 = 0.8 and agitation = 300 rpm. Under these optimized conditions, biomass, lipid titer and volumetric lipid productivity were 9.31 g/L, 5.90 g/L and 0.082 g/L.h, respectively. Additionally, we determined the fatty acid composition of P. laurentii UFV-1 as follows: C14:0 (0.5%), C16:0 (28.4–29.4%), C16:1 (0.2%), C18:0 (9.5–11%), C18:1 (58.6–60.5%), and C20:0 (0.7–0.8%). Based on this composition, the predicted properties of biodiesel showed that P. laurentii UFV-1 oil is suitable for use as feedstock in biodiesel production.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02373-4) contains supplementary material, which is available to authorized users.
Keywords: Biodiesel, Lignocellulosic sugars, Oleaginous yeast, Response surface methodology, Screening
Introduction
Concern about global warming has grown over recent years due to increases in the emission of greenhouse gases produced by the burning of fossil fuels. The report presented in 2016 by Bloomberg New Energy Finance (BNEF), an organization that provides services to assist in the evaluation of investments in the renewable energy market, pointed out that the peak of fossil fuel consumption will be reached in 2025 (MacDonald 2016). Based on this proposition, the majority of nations have committed to reducing the emission of greenhouse gases at the Conference of the Parties, Paris, France (COP21). This has given encouragement to the idea of producing biofuels as an alternative to fossil fuels.
As one of the biofuels on the market, ethanol has been produced on a large scale and can be used as an alternative to gasoline or added to it as an additive. Biodiesel is another important biofuel that stands out as an alternative to diesel fuel. Currently, its production is based on a transesterification reaction between vegetable oils and methanol derived from natural gas. Nevertheless, it presents two main drawbacks: the need for large areas of arable land and water resources for edible plant cultivation (Li et al. 2007; Leung et al. 2010; Christophe et al. 2012).
On the other hand, microbial oil can be produced rapidly by cultivating microorganisms in bioreactors, thereby avoiding the need for arable land. Moreover, considering the entire biodiesel process including plant cultivation to yield oil, the use of microbial oil has two further advantages over edible oil: lower emission of greenhouse gases and a more favorable energy balance for biodiesel production (Caspeta and Nielsen 2013).
Microorganisms considered oleaginous can accumulate over 20% of their cell mass as lipids. Of the 1800 known yeast species, it is estimated that 5% are oleaginous (Sitepu et al. 2014a; Vu et al. 2016; Kamineni and Shaw 2020). Yarrowia, Candida, Rhodotorula, Rhodosporidium, Cryptococcus, Trichosporon and Lipomyces are typical genera (Ageitos et al. 2011). Recently, new species and strains belonging to the genera Scheffersomyces, Kurtzmaniella, Myxozyma, Cuniculitrema, Filobasidium, Tremella, Prototheca, Hannaella have been recognized as being oleaginous (Sitepu et al. 2013).
The search for oleaginous yeasts which can be used as an oil source for the production of biodiesel has been directed to environments rich in lignocellulosic materials such as soils, wastewaters, dairy products, flowers and other habitats (Nakase et al. 1976; Olasupo et al. 2003; Oyamada et al. 2008; Pan et al. 2009). Over the last few years, production of microbial oil from lignocellulosic biomass has been studied to develop a lignocellulosic single cell oil (SCO) biorefinery. For this purpose, the use of microorganisms able to assimilate glucose and xylose, sugars derived from cellulose and hemicellulose, respectively, is desirable (Jin et al. 2015). Current practice is to use the glucose released from the enzymatic hydrolysis of cellulose in the production of ethanol; however, its production from xylose released from the hydrolysis of hemicellulose has not been considered feasible (Zhang et al. 2013; Komesu et al. 2020). In this context, conversion of xylose into oil by yeasts can contribute to the use of the whole lignocellulosic biomass in biorefineries. Xylose-assimilating yeasts such as Rhodosporidium toruloides, Rhodotorula minuta, Rhodotorula glutinis, Cutaneotrichosporon oleaginosus—formely known as Trichosporon cutaneum and Cryptococcus curvatus (Bracharz et al. 2017; Liu et al. 2015), Lipomyces starkeyi, Candida sojae, Candida pseudointermedia and Meyerozyma caribbica have been commonly isolated from xylose-rich soil and lignocellulosic biomass (Ayadi et al. 2018; Masoud et al. 2004; Nakase et al. 1976; Olasupo et al. 2003; Oyamada et al. 2008; Pan et al. 2009; Polburee et al. 2015). For instance, most studies on lipid production from xylose have been conducted on R. toruloides, R. glutinis, R. minuta, L. starkeyi and C. oleaginosus (Awad et al. 2019; Lin et al. 2014; Pan et al. 2009; Zhang et al. 2016).
Thus, the aims of this work were to: (1) isolate and screen oleaginous yeasts from two National Parks in Brazil (Caparaó and Serra dos Órgãos) with the potential to produce lipid from xylose; (2) optimize the lipid production by the best yeast lipid producer from culture media containing xylose as the sole carbon source. To the best of our knowledge, the biotechnological potential of yeast found in these parks has not yet been addressed, opening perspectives of isolating strains with metabolic features considered desirable in bioprocesses. Herein, the best isolate in terms of lipid production was physiologically characterized and its lipid production from xylose optimized.
Materials and methods
Yeast strains and maintenance
The yeasts used in this work were isolated from soil samples from two National Parks in Brazil: Caparaó, located on the border of the States of Minas Gerais and Espírito Santo; and Serra dos Órgãos, located in the State of Rio de Janeiro. For long-term conservation, the yeast isolates were stored in yeast peptone (YP) culture medium [1% (w/v) yeast extract, 1% (w/v) peptone] with 50% (v/v) glycerol at – 80 °C. These isolates belong to the culture collection of Microbial Physiology Laboratory of the Department of Microbiology at the Federal University of Viçosa (UFV).
Culture media, growth conditions and inoculum preparation
SS2 culture medium described by Tanimura et al. (2014), which consists of (g/L): (NH4)2SO4 0.7, MgSO4 0.5, NaCl 0.1, CaCl2 0.1, yeast extract 0.1, was used in the steps of inoculum preparation, screening of isolates, evaluation of biomass concentration, lipid accumulation, consumption of sugars and ammonia, optimization of lipid production and analysis of fatty acid profile. SS2 medium was supplemented with different carbon sources: glucose, xylose and a mixture of both, being referred to as SS2G, SS2X, SS2GX, respectively (Table 1).
Table 1.
Composition of SS2 culture media for the batch cultivation of the P. laurentti UFV-1 yeast. SS2G, SS2X and SS2GX correspond to the SS2 culture media containing glucose; xylose and glucose–xylose mixture as carbon sources, respectively. Concentrations of each component are represented in g/L. In these SS2 culture media the C/N ratio was equal to 48
| Component | SS2 culture media | ||
|---|---|---|---|
| SS2G | SS2X | SS2GX | |
| Glucose | 30 | – | 21.42 |
| Xylose | – | 30.62 | 9.19 |
| (NH4)2SO4 | 0.7 | 0.7 | 0.7 |
| NaCl | 0.1 | 0.1 | 0.1 |
| CaCl2 | 0.1 | 0.1 | 0.1 |
| MgSO4 | 0.5 | 0.5 | 0.5 |
| Yeast Extract | 1 | 1 | 1 |
For inoculum preparation, the culture stock was transferred to 125-mL Erlenmeyer flasks containing 30 mL of SS2 supplemented with 30 g/L glucose—SS2G medium. The yeast cultures were incubated at 30 °C at 200 rpm for 18 h in a G25 rotary shaker (New Brunswick, Edison, Nova Jersey, USA). Subsequently, the yeast culture was centrifuged at 12,000 × g at 4 °C for 10 min (Sorval RC5C, Marshall Scientific, Hampton, New Hampshire, USA) and then, the pellet was washed twice with 0.1% (w/v) peptone water. Next, the wet pellet was resuspended in 0.1% (w/v) peptone water and then it was used to adjust the initial inoculum concentration.
Pre-culture for yeast isolation was performed in glycerol-enriched culture medium as described by Pan et al. (2009) containing (in g/L): glycerol 100, (NH4)2SO4 1, KH2PO4 1, MgSO4·7H2O 0.5, and yeast extract 0.2. Glycerol was used as carbon source in the pre-culture step, because we intend, in a future study, to evaluate the capability of the yeast isolates to accumulate lipids in culture media containing glycerol as the sole carbon source. Pre-cultivations were performed in a 250-mL Erlenmeyer flask containing 50 mL of this culture medium, at 30 °C, with a stirring rate of 200 rpm for 24 h.
The isolation step was performed on agar culture medium containing (in g/L): xylose 20, (NH4)2SO4 5, KH2PO4 1, MgSO4·7H2O 0.5, yeast extract 0.5, streptomycin (Sigma-Aldrich, San Luis, Missouri, USA) 0.05 and agar 20.
In the screening step, yeasts were cultivated in 1,000-mL Erlenmeyer flasks filled with 100 mL of the SS2G culture medium at 30 °C, with a stirring rate of 200 rpm for 120 h. The 1:9 ratio between media:head space of the flask was used to ensure better aeration in Erlenmeyer flasks.
To evaluate biomass concentration, lipid production, consumption of sugars and ammonia, P. laurentii UFV-1 was cultured in SS2G, SS2X and SS2GX culture media (Table 1). Batch cultures were performed in 1,000-mL Erlenmeyer flasks containing 100 mL of these culture media, at 30 °C, with a stirring rate of 200 rpm for 96 h. According to Papanikolaou and Aggelis (2011), an initial C/N ratio greater than 20 is sufficient for lipid accumulation by oleaginous microorganisms. Thus, the composition of the aforementioned culture media was defined to ensure a moderated C/N ratio of 48 (Table 1), to observe if, even under this condition, the yeast could display the oleaginous phenotype. The C/N ratio was calculated based on the mass fraction of C in glucose and xylose, and the mass fraction of N in (NH4)2SO4 and yeast extract [total nitrogen equivalent to 10% (w/w) − product code: RM027, HiMedia Laboratories, Vadhani Industrial Estate, Lal Bahadur Shastri Marg, Mumbai, India].
Papiliotrema laurentii UFV-1 was cultivated in SS2G, SS2X and SS2GX culture media (Table 1) to evaluate its fatty acid profile. Batch cultures were performed in 1000-mL Erlenmeyer flasks containing 100 mL of these culture media at 30 °C for 48 h and 200 rpm.
Cultivations for the optimization of lipid production by P. laurentii UFV-1 were carried out in SS2 culture media containing xylose as the sole carbon source with different C/N ratios. For a detailed description of culture media, see the optimization of the lipid production section.
Soil sampling and yeast isolation
Samples were collected from four locations at different altitudes from Caparaó Park [1273 m (20° 25.2′ 91ʺ S 41° 51.2′ 08ʺ W); 1971 m (20° 24.5′ 95ʺ S 0.41° 50.2′ 05ʺ W); 2372 m (20° 25.2′ 26ʺ S 0.41° 48.6′ 21ʺ W) and 2,857 m (20° 26′ 0.60" S 0.41° 47.7′ 80" W)] and Serra dos Órgãos Park [404 m (22° 29′ 43.6ʺ S 43° 00′ 03.7ʺ W); 1016 m (22° 26′ 52.4′ S 42° 59′ 08.8ʺ W); 1658 m (22° 27′ 01.2′ S 43° 00′ 52.7" W) and 2124 m (22° 27′ 33.1′ S 43° 01′ 40.0ʺ W)]. Table S1 depicts the pictures of the harvest sites and the description of the vegetation of these sites. For each compost sample, three simple samples were collected from the rhizosphere (0–20 cm deep) to form a composite sample. The pre-culture preparation was carried out as described by Pan et al. (2009): 1 g of each soil sample was added to a 250-mL Erlenmeyer flask containing 50 mL of glycerol-enriched culture medium. One mL pre-cultured yeast was used to perform tenfold serial dilutions. Aliquots of 0.1 mL from each serial dilution ranging from 10−1 to 10−5 were spread onto agar culture medium and incubated at 30 °C for 2–4 days. Isolated colonies were streaked and restreaked on fresh agar culture medium to obtain pure cultures.
Screening of oleaginous yeasts
The isolated yeasts were screened for their lipid-producing ability by a relative quantification analysis using the Nile Red Fluorometric method (Sitepu et al. 2012). As described in the “Culture media, growth conditions and inoculum preparation” section, yeast isolates were grown in SS2G culture medium and then, the inoculum concentration was adjusted in a fresh SS2G medium to optical density at 600 nm (OD600) equal to 1.0, using a DU 640 UV–visible spectrophotometer (Beckman Coulter Life Sciences, Indianapolis, USA). For each isolate, 250 μL was transferred to a 96-well black microplate in triplicate. Subsequently, 25 μL of dimethyl sulfoxide (DMSO):SS2 (1:1, v/v) was added to each well. Initial fluorescence was measured in a SpectraMax M5 spectrofluorometer (Molecular Devices, San Jose, California, USA), and the excitation and emission wavelengths were set at 488 and 585 nm, respectively. Next, 25 μL of 0.05-mg/mL Nile Red solution (5-μg/mL Nile red) was mixed into the culture for the second fluorescence measurement after 10 min of reaction (Sitepu et al. 2012).
Identification of oleaginous yeasts
Taxonomic identification of the selected yeasts in the step screening was determined by sequencing the D1/D2 domains of the gene encoding subunit 26S of ribosomal DNA. Total DNA was extracted according to the rapid isolation of the yeast DNA protocol (Sambrook and Russel 2001). The universal primers NL-1 (5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL-4 (5′-GGTCCGTGTTTCAAGACGG-3′) were used for D1/D2 amplification (Lachance et al. 2003). Sequencing reactions were generated using the Big Dye Kit version 3.1 (Applied Biosystems, Foster City, California, USA) in combination with the ABI 3730 automated sequencing system (Applied Biosystems). The nucleotide sequences obtained were analyzed and compared with the sequences deposited in the National Center for Biotechnology Information (NCBI), using the Basic Local Alignment Search Tool (BLASTn). The yeast strain selected was subsequently referred to as Papiliotrema laurentii UFV-1.
Profiles of biomass concentration, lipid accumulation, consumption of sugars and ammonia of Papiliotrema laurentii UFV-1
Papiliotrema laurentii UFV-1, the best yeast lipid producer, was cultivated in SS2G, SS2X and SS2GX as described previously. Cell growth was monitored by measuring OD600. The lipid accumulation profile was estimated by fluorescence (RFU/OD unit), whilst the lipid contents were determined by gravimetric analysis. Consumption of glucose and xylose were analyzed in the culture supernatants by determining the concentration of these sugars by high-performance liquid chromatography (HPLC). Consumption of ammonia was also analyzed in the culture supernatants. The method proposed by Chaney and Marbach (1962) was used to determine the ammonical nitrogen concentration during cultivation. This experiment was performed in triplicate.
Optimization of lipid production
Response surface methodology (RSM) with a face-centered central composite design (CCF) was applied to optimize the effect of the four independent factors (agitation, initial biomass concentration, C/N ratio and pH), listed in Table 2, with reference to the lipid content (dependent variable). These independent factors were selected based on previous studies that showed their relevance to lipid accumulation in oleaginous yeasts (Jiru et al. 2017; Li et al. 2008; Papanikolaou and Aggelis 2011). Temperature was not used as an independent factor as previous experiments showed that in temperatures above 30 °C the formation of biomass is drastically reduced, making directing displacement towards the optimal regions difficult. This study adopted CCF because it allows the consideration of results from a factorial design and includes extreme levels of all the evaluated factors. The experimental design consisted of 24 units corresponding to combinations of the encoded levels + 1 and − 1, plus central point with three repetitions, making 27 assays in total (Table 2 and Table S2). Batch cultures were carried out using SS2 medium containing xylose 40 g/L as the sole carbon source, and different concentrations of (NH4)2SO4 to obtain the C/N ratios determined by the levels of the independent variables (Table 2).
Table 2.
Levels of the independent variables used for CCF design
| Independent variable | Code level | ||
|---|---|---|---|
| − 1 | 0 | + 1 | |
| Agitation Rate (rpm) | 150 | 225 | 300 |
| Initial Cell Biomass (OD600) | 0.2 | 0.5 | 0.8 |
| C/N ratio | 40:1 | 55:1 | 70:1 |
| pH | 5.0 | 6.0 | 7.0 |
The effects of agitation (150, 225 and 300 rpm), initial biomass concentration (OD600 0.2, 0.5 and 0.8), C/N ratio (40:1, 55:1 and 70:1) and pH (5.0, 6.0 and 7.0) on lipid production were identified by culturing in 500-mL Erlenmeyer flasks, using cotton plugs as flask caps, containing 100 mL of medium. The cultures were incubated in a G25 rotary shaker (New Brunswick) at 30 °C. After 72 h of growth, the cells were harvested by centrifugation and lyophilized for 24 h (LioTop L101, Liobras, São Carlos, São Paulo, Brazil). The lipid extractions were carried out following the absolute lipid quantification approach.
The data of lipid content [% (w/w)] obtained from RSM were subjected to analysis of variance (ANOVA). The experimental results of RSM were fitted to a response surface regression procedure, using the following second-order polynomial described in the ensuing Eq. 1:
| 1 |
where y represents the response variable, Xi and Xj, the independent variables as described in Table 2 and β0, βi, and βj, the estimate coefficients. The polynomial accuracy model was evaluated as measured by the coefficient of determination (R2). Data were analyzed using the Minitab®17 software package (Minitab Inc., State College, Pennsylvania, USA).
Specific growth rate and determination of dry weight
Cell growth was monitored by measuring the OD600. The specific growth rate (μ) was determined by linear regression between ln values of OD600 and time (h) using the R 3.2.5 software program (https://www.r-project.org). To determine the dry weight of biomass, a calibration curve of the dry cell weight (DCW) versus optical density was constructed. The yeast was transferred to 10 mL of SS2G culture medium and incubated at 30 °C at 200 rpm for 18 h. Subsequently, the yeast culture was centrifuged at 12,000 × g at 4 °C for 10 min and the wet pellet was resuspended in 6 mL of distilled water. Four 1-mL aliquots were harvested and dried at 105 °C for 24 h. In parallel, 1-mL aliquot of the cell suspensions was diluted (1 × 10–2, 3 × 10–2, 4 × 10–2, 5 × 10–2, and 1 × 10–1) and the OD600 measured. The DCW was calculated from the equation obtained from the linear regression of the plot OD600 versus DCW (mg/mL). At an OD600 of 1, the concentration was 0.04 g/L of weight (DCW).
Analytical methods
Lipid absolute quantification
Polar and non-polar lipids were quantified following the method described by Bligh and Dyer (1959). Fifty mg of lyophilized biomass were used to extract the lipid fraction, to which 1 mL of a methanol:chloroform solution (2:1, v/v) was added. The suspension was homogenized using Tissuelyser II equipment (Qiagen, Hidden, North Rhine-Westphalia, Germany) with a vibrating frequency of 30 shakes per second for 5 min. Following this procedure, the solution was centrifuged 3 times at 14,000 × g for 5 min to ensure total extraction of lipids from the initial biomass. The supernatants were collected and stored in 15-mL centrifuge glass tubes. Next, 3 mL of 100% chloroform were added and the mixture homogenized. Two mL of a 1% (w/v) saline solution was added and the system was homogenized to obtain a two-phase liquid system. The phases were separated by centrifugation at 1464 × g for 20 min. The lower phase was transferred to previously weighed microtubes. The samples were then evaporated in a Concentrator plus (Eppendorf AG, Hamburg, Germany) at 60 °C for 24 h, and the lipid content was determined gravimetrically.
Glucose and xylose quantifications
Glucose and xylose concentrations were quantified by HPLC, in a TA-20 Shimadzu chromatograph (Shimadzu Co. Nakagyo-ku, Kyoto, Japan). The culture supernatant was passed through a 0.13-mm syringe filter (0.45 μm, Merck Milipore Co., Darmstadt, Germany) prior to injection into the HPLC. Twenty microliters of sample were injected and passed through an Aminex ion exchange column (HPX-87H 300 × 7.8 mm, 9 µm, Bio-Rad, Munich, Germany) with 5 mM H2SO4 as eluent at 0.7 mL/min, at 40 °C, and detected by a refractive index detector (RID-20A, Shimadzu). Quantification of all sugars was performed by calibration and verification with external standards d-glucose and d-xylose purchase from Sigma-Aldrich.
Ammoniacal nitrogen consumption
Nitrogen consumption during cultivation was determined by the method proposed by Chaney and Marbach (1962). Five μL of culture supernatant (previously diluted in deionized water, when it was necessary) was mixed with 100 μL of solution A [phenol 5% (w/v) and sodium nitroprusside 0.025% (w/v)] and 100 μL of solution B [NaOH 2.5% (w/v) and NaOCl 1% (w/v)]. This solution was incubated at 39 °C for 20 min and ammoniacal nitrogen was quantified by absorbance measured at 630 nm on the Multiskan GO plate reader (ThermoScientific, Wilmington, Delaware, USA). The standard curve was prepared using solutions with ammonium chloride (Sigma-Aldrich) concentrations ranging from 0 to 12 mM. This experiment was performed with three technical replicates.
Determination of fermentation parameters
The following parameters were determined: biomass concentration [DCW (g/L)] and lipid content [% (w/w), g/100 g of DCW] based on gravimetric method, lipid titer (g/L), and volumetric lipid productivity (g/L h).
Fatty acids methyl ester profile
Samples of P. laurentii UFV-1 cultured in SS2G, SS2X and SS2GX were harvested at 48 h of cultivation (maximum lipid accumulation estimated by fluorescence). The pellet obtained by centrifugation at 12,000 × g at 4 °C for 10 min was lyophilized. The lipids in the yeast cell samples (4–5 mg of dry weight) were saponified, methylated, and the fatty acid methyl esters (FAMES) were extracted following instructions in the Sherlock Microbial Identification System Operating Manual, version 6.0 (Microbial ID, Inc., Newark, Delaware, USA). The resulting methyl ester mixtures were separated using an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, California, USA), with a crosslinked 5% phenyl methyl silicone fused silica capillary column and a flame ionization detector following the default configuration of the Sherlock MIDI system. FAMES were identified using the MIDI identification system and the RTSBA6 library (Microbial ID, Inc.).
Data and statistical analysis
Graphical representations were constructed using SigmaPlot version 12.0 from Systat Software, Inc. (San Jose California USA, https://www.systatsoftware.com), and a statistical analysis was carried out using the R software program (https://www.r-project.org). The significance level used in the statistical tests was 5%.
Prediction of fatty acid quality for biodiesel production
From the fatty acid profiles determined in this work, the following biodiesel features were estimated: cetane number (NC), iodine value, cloud point (°C) higher heating value (MJ/kg), kinematic viscosity (mm2/s) and density (g/cm3) according to Sergeeva et al. (2017).
Results
Isolation, screening, and taxonomic identification of oleaginous yeasts
A total of 126 yeasts were isolated from soil samples from two Brazilian National Parks: Caparaó (CA) and Serra dos Órgãos (SO). In the screening step, we evaluated their ability to accumulate lipids using a method based on Nile red fluorescence (Table S3). From this group, isolates SO91 and SO19 stood out as being the best lipid producers after 120 h of cultivation in SS2 medium.
The sequencing of the D1/D2 region of the large subunit of the ribosomal gene allowed us to identify these two promising isolates as P. laurentii, previously classified as Cryptococcus laurentii (Liu et al. 2015) (Table 3).
Table 3.
Taxonomic identification of the oleaginous yeasts that stood out as the best lipid producers
| Collection code | Species | Strain | CBS reference number | Access number | Identity |
|---|---|---|---|---|---|
| SO91 | P. laurentii | UFV-1 | CBS 139 | AF075469.1 | 579/581 (99%) |
| SO19 | P. laurentii | UFV-2 | CBS 139 | AF075469.1 | 561/562 (99%) |
Lipid accumulation by these yeasts, which were cultivated in glucose and/or xylose as a carbon source, was determined by the gravimetric method. It is of particular interest that the P. laurentii strains UFV-1 and UFV-2 accumulated 43 and 40% of their dry weight as lipids, respectively, at 48 h of cultivation in culture medium containing glucose as the sole carbon source. In culture medium containing xylose as the sole carbon source, the maximum lipid content achieved was 30 and 21% (w/w), for UFV-1 and UFV-2, respectively. Since the lipid accumulation of the UFV-1 strain was superior to that obtained for UFV-2, further studies were carried out on the UFV-1 strain to evaluate its potential for use as an oil source for fatty acid-derived biofuels.
Physiological characterization and evaluation lipid production by P. laurentii UFV-1
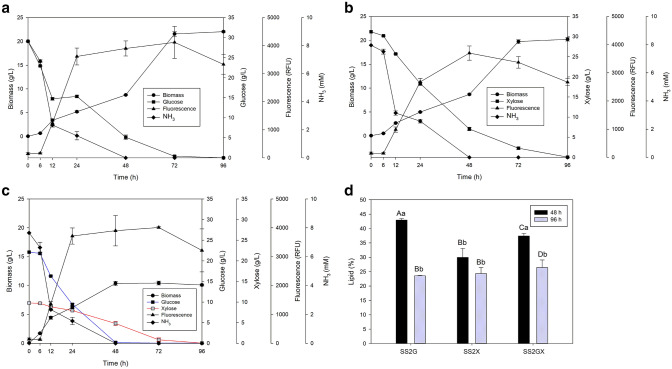
We evaluated the P. laurentii UFV-1 growth and lipid accumulation in SS2 culture medium with a carbon–nitrogen ratio of 48:1 over the 96 h of cultivation. Notably, this yeast triggered the lipid accumulation process during the late exponential growth phase (Fig. 1). It should be noted that the ammonia was completely consumed at 48 h in the three-growth media. Furthermore, we observed that the fluorescence decreased after 72 h of growth, indicating that there was degradation of accumulated neutral lipids (Fig. 1). Consistent with this, the lipid content decreased after 72 h in all growth media. We observed in the SS2GX culture medium that the consumption of both glucose and xylose started at 6 h of growth; however, glucose was consumed faster than xylose (Fig. 1c). After the depletion of glucose, the consumption of xylose increased, indicating that glucose repressed the genes involved in xylose metabolism. Indeed, the growth of P. laurentii UFV-1 was impaired on agar medium containing xylose and 2-deoxy-d-glucose (data not shown), confirming the repression effect exerted by glucose on xylose metabolism.
Fig. 1.
Profiles of sugar consumption (g/L); ammoniacal nitrogen consumption (mM); biomass concentration (g/L), and lipid accumulation, estimated through fluorescence (RFU/OD unit), of P. laurentii UFV-1 cultured in SS2 culture medium containing as carbon sources: a glucose (SS2G), b xylose (SS2X), and c a mixture of glucose and xylose (70:30, % G:X) (SS2GX); at 30 °C and 200 rpm for 96 h. The C/N ratio of these media was equal to 48. d Lipid contents of P. laurentii UFV-1 measured at 48 and 96 h of growth in SS2G, SS2X and SS2GX. Equal capital letters for the same culture medium and lowercase letters for the same time of cultivation do not differ significantly from each other by Tukey's multiple comparison test (p value > 0.05). Error bars in the a–d represent the standard deviation
Under unoptimized conditions, the maximum lipid content [43% (w/w)] was obtained in the culture medium containing glucose as the sole carbon source. In the presence of xylose and a mixture of glucose and xylose, the lipid contents were 30 and 37.4% (w/w), respectively. According to the fluorescence results, we observed, using the gravimetric method, the lipid content of P. laurentii UFV-1 decreased at 96 h for all carbon sources (Fig. 1d).
Optimization of lipid production by P. laurentii UFV-1 from xylose
To improve the lipid content (dependent variable) in P. laurentii UFV-1, we evaluated the effects of the agitation rate, initial cell biomass, C/N ratio, and pH (independent variables). We used an RSM considering a CCF fitted to a second-order polynomial regression. The values of the regression coefficients were calculated and the model for lipid content was fitted (p value = 0.05), in which the cofactors agitation rate and biomass displayed quadratic coefficients (Eq. 2):
| 2 |
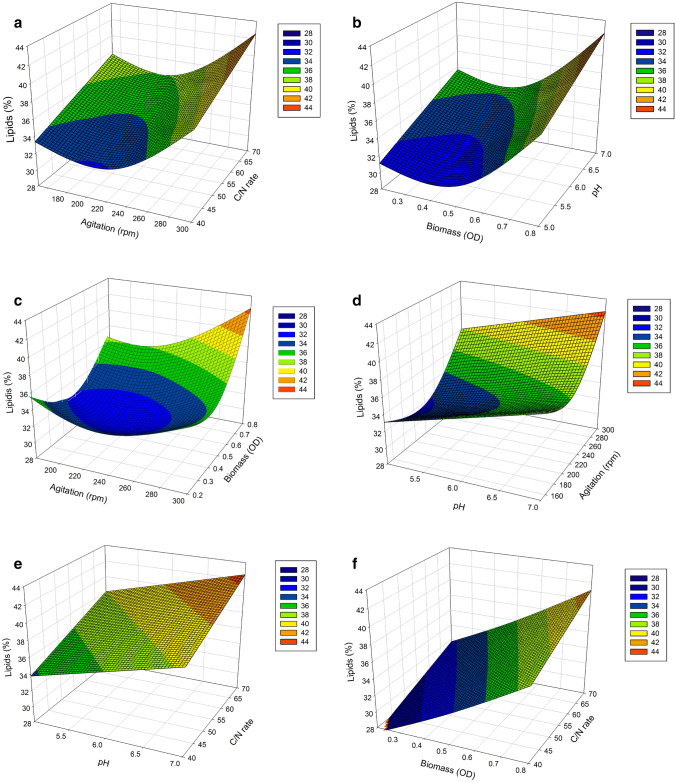
According to the ANOVA F test (Table S4), the model obtained was significant, i. e, the coefficients of the linear cofactors (C/N ratio, pH, agitation, and initial biomass) were significant (p value < 0.05). With reference to the quadratic effects of the agitation and biomass cofactors, we observed that the interaction terms were also significant (p value < 0.05). Despite the lack of fit (p value = 0.026), the model presented high quality, expressed by the coefficient of determination (R2), which was 0.75 (Eq. 2). To evaluate the effects and interactions of the independent and dependent variables, we fitted a predicted response surface from the model (Fig. 2).
Fig. 2.
Responses surfaces of lipid contents [% (w/w)] as a function of: a agitation and C/N ratio, b biomass and pH, c agitation and biomass, d pH and agitation, e pH and C/N ratio, and f biomass and C/N ratio. For an analysis of the effect of factors two to two on lipid content response the other factors were fixed at their higher coded levels (C/N ratio = + 1, biomass = + 1, agitation = + 1, and pH = + 1). Gradient color legends correspond to the variation in lipid contents [% (w/w)]. Blue and red surfaces represent the lowest and highest responses, respectively
We observed that the interaction between agitation and initial biomass presented a positive effect, i.e., lipid accumulation rises with increases in these variables (Fig. 2c). The best levels of the initial biomass and agitation that favor lipid accumulation were OD600 = 0.8 and 300 rpm, respectively. As regards this agitation and biomass, we observed an increase in lipid accumulation of high values for both factors (Fig. 2c).
Taking the C/N ratio and pH effects into account, the increase in the level of these factors suggests an increase in the response (Fig. 2e). C/N ratio and pH coefficients showed the most important effects in relation to the accumulation of lipids by the P. laurentii UFV-1 yeast. The predicted model indicated that values of pH higher than 7.0 maximize the response variable; however, it was not possible to work with higher values under our experimental conditions because of precipitate formation in the culture medium. This precipitation was the result of the formation of insoluble calcium and magnesium salts in the presence of HPO42− ions from the buffer used (citrate–phosphate). These salts present low solubility constant in aqueous medium. In addition, C/N ratio values above 70 favor an increase in the lipid content. As such, an additional experiment in displacement of the C/N was performed under optimized conditions (30 °C, 300 rpm, OD600 = 0.8 and pH 7.0). We observed that maximum lipid content [63.5% (w/w)] was obtained at a C/N ratio of 100:1, in which the biomass, lipid titer and volumetric lipid productivity were 9.31 g/L, 5.90 g/L and 0.082 g/L h, respectively (Table 4).
Table 4.
Average of lipid contents [% (w/w)], biomass concentration (g/L), lipid titer (g/L) and volumetric lipid productivity (g/L h) achieved by Papiliotrema laurentii UFV-1 cultivated in different C/N ratios under agitation of 300 rpm, at 30 °C, pH equal to 7.0 for 72 h
| C/N ratio | Accumulated lipids [% (w/w)] | Biomass concentration (g/L) | Lipid titer (g/L) | Volumetric lipid productivity (g/L.h) |
|---|---|---|---|---|
| 70:1 | 42.0 ± 0.49 | 9.42 ± 0.41 | 4.00 ± 0.24 | 0.056 ± 0.0034 |
| 85:1 | 60.7 ± 2.17 | 8.94 ± 0.23 | 5.42 ± 0.14 | 0.075 ± 0.0019 |
| 100:1 | 63.5 ± 1.05 | 9.31 ± 0.33 | 5.90 ± 0.28 | 0.082 ± 0.0039 |
| 115:1 | 50.2 ± 1.87 | 9.44 ± 0.16 | 4.74 ± 0.35 | 0.066 ± 0.0049 |
| 130:1 | 39.2 ± 1.99 | 9.25 ± 0.48 | 3.63 ± 0.36 | 0.050 ± 0.0050 |
| 145:1 | 40.2 ± 1.30 | 9.30 ± 0.67 | 3.74 ± 0.27 | 0.052 ± 0.0038 |
The effect of the C/N ratio on lipid production under the conditions previously optimized (30 °C, 300 rpm, OD600 = 0.8 and pH 7.0 for 72 h) was evaluated and the new data were represented by a second-order polynomial regression (Eq. 3), whose R2 was 0.92. This model allowed us to estimate the best conditions for maximum lipid accumulation (Table S6).
| 3 |
Thus, the best C/N ratio that confers the greatest theoretical accumulation of lipids is 97.64:1, which predicted an accumulation of 62% (w/w), when the pH value, initial biomass and agitation were 7.0, OD600 = 0.8 and 300 rpm, respectively.
Fatty acid profile of P. laurentti UFV-1 and prediction of biodiesel properties
We evaluated the P. laurentii UFV-1 fatty acid profile to investigate whether it was appropriate to biodiesel production. The most abundant fatty acids found in P. laurentii UFV-1 were oleic, palmitic and stearic. Importantly, high proportions of oleic acid (approximately 59%), and palmitic acid (around 28%) were found in the fatty acid profiles of P. laurentii UFV-1 regardless of the growth media used (Table 5).
Table 5.
Fatty acid profile of P. laurentii UFV-1 grown in the SS2 culture media containing glucose (SS2G), xylose (SS2X), and mixture of glucose–xylose (SS2GX) as carbon sources
| Fatty acid | Fatty acid profile [% (w/w)] | ||
|---|---|---|---|
| SS2G | SS2X | SS2GX | |
| Myristic acid (14:0) | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| Palmitic acid (16:0) | 29.4 ± 0.7 | 28.8 ± 0.02 | 28.4 ± 0.3 |
| Stearic acid (18:0) | 9.8 ± 0.9 | 11 ± 0.8 | 9.5 ± 1.4 |
| Arachidic acid (20:0) | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.1 |
| Palmitoleyl alcohol/cis-10-palmitoleic acid (16:1) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Oleic acid (18:1) | 59.3 ± 0.7 | 58.6 ± 0.8 | 60.5 ± 1.5 |
The profile was determined at the time of maximum lipid accumulation (48 h) from cultivation at 30 °C and 200 rpm
With reference to the predictive equations reported previously by Sergeeva et al. (2017), we predicted the physical properties of biodiesel from P. laurentii UFV-1 (Table 6). Iodine value (IV), cetane number (CN), higher heating value (HHV), viscosity, and density were similar between FAME obtained from cultivation in SS2G, SS2X and SS2GX, and showed the ability to adequately meet biodiesel quality standards from Europe (standard EN 14214), USA (standard ASTM D 6751), and Brazil (RANP 45).
Table 6.
Predicted properties of biodiesel from the fatty acid profile of Papiliotrema laurentii UFV-1 and quality standards of biodiesel production in Europe, USA and Brazil
| Biodiesel properties | Treatments | Standards | ||||
|---|---|---|---|---|---|---|
| SS2G | SS2X | SS2GX | Europe (EN 14214:2012) | USA (ASTM D 6751-07b) | Brazil (RANP 45–2014) | |
| Iodine value (IV) | 50.989 | 50.390 | 52.017 | ≤ 120 | ≤ 120 | – |
| Cetane Number (CN) | 67.568 | 67.590 | 67.388 | ≥ 51 | ≥ 47 | ≥ 42 |
| Higher heating value (MJ/kg) | 39.844 | 39.769 | 39.846 | ≥ 35a | – | – |
| Kinematic viscosity (mm2/s) | 4.626 | 4.622 | 4.624 | 3.5–5.0 | 1.9 – 6.0 | 3.0–6.0 |
| Density (g/cm3) | 0.870 | 0.868 | 0.870 | 0.86–0.90 | 0.86 – 0.91 | 0.85–0.90 |
aEN 14213:2003
Discussion
Several studies have explored the microbial community found in soil to isolate oleaginous yeasts (Sláviková and Vadkertiová 2003; Pan et al. 2009; Kitcha and Cheirsilp 2011; Chandran and Das 2012; Takashima et al. 2012; Schulze et al. 2014). However, there are no reports on the isolation of oleaginous yeasts from soil samples from Caparaó and Serra dos Órgãos, the two Brazilian National Parks featured in our study. These parks are located in the Atlantic Rainforest in Brazil, one of the largest rainforests in the Americas, extending along the Brazilian coast. This biome, considered a hotspot region (Myers et al. 2000; Morais et al. 2013), favors a high degree of biodiversity, endemism and a wide diversity of species that still require scientific description. Even though we isolated 126 yeasts from these parks, their diversity was not assessed because our aim was to characterize only the best yeast lipid producer selected at the screening stage. Research should be undertaken in the future to evaluate whether other isolates are taxonomically related, providing insights into yeast diversity in these parks.
It has been reported that oleaginous yeasts usually initiate the lipid accumulation process in the late exponential phase and continue into the stationary phase (Beopoulos et al. 2011). Consistent with this, our results show that lipid accumulation in P. laurentii UFV-1 began during the late exponential growth phase, at 12 h (Fig. 1). The maximum lipid content achieved by P. laurentii UFV-1 was at 48 h of growth, which is considered a short period. Overall, the highest lipid contents displayed by other oleaginous yeasts such as Yarrowia lipolytica ATCC 20460 (Tai and Stephanopoulos 2013), L. starkeyi AS 2.1560 (Wang et al. 2016), T. cutaneum 2.1374 (Liu et al. 2013), Trichosporon fermentans CICC 1368 (Zhu et al. 2008), Rhodotorula toruloides CBS 14 (Tiukova et al. 2019) and Rhodotorula kratochvilovae SY89 (Jiru et al. 2017) were reached in periods beyond 48 h of growth.
At 96 h of growth, the lipid content of P. laurentii UFV-1 decreased, which is likely related to the depletion of both carbon and nitrogen. Under this condition of starvation, lipid degradation may have occurred through the β-oxidation pathway to generate energy for cell maintenance and homeostasis (Kohlwein 2010). Recently, it has been demonstrated in the P. laurentii RY1 strain cultivated under conditions of nitrogen limitation that genes involved with the β-oxidation pathway are up-regulated (Sarkar et al. 2018). These authors claimed that this is a response aimed at generating the energy amount required and carbon for yeast growth.
The majority of studies involving oleaginous yeasts are carried out on Y. lipolytica. Despite its remarkable ability to grow in hydrocarbons, it is not able to use xylose as the sole carbon source (Rodriguez et al. 2016). Xylose is one of the most abundant pentose sugars found in nature (Pan et al. 2009; Li et al. 2019). In the context of biofuels, the production of ethanol from xylose is still economically unfeasible (Zhang et al. 2013; Komesu et al. 2020). As such, the production of microbial oil from xylose is an option where this pentose can be used in biorefinery. As mentioned previously, lipid production by P. laurentii UFV-1 from glucose was superior to xylose. However, it should be noted that the oleaginous phenotype was also observed in culture medium containing xylose as the sole carbon source, even under unoptimized conditions. This difference may be related to the requirement for NADPH in the xylose metabolism, as the first reaction, which is catalyzed by xylose reductase, requiring it to be a reducing agent (Tiukova et al. 2019). Such an assumption is based on the fact that NADPH is the reducing power source for fatty acid synthesis. It is noteworthy that three copies of the gene encoding this enzyme were predicted in the P. laurentti UFV-1 genome (Fig. S1). Thus, the growth in xylose requires higher availability of NADPH than in glucose, likely impairing the synthesis of fatty acids.
The optimization of lipid production by the P. laurentti UFV-1 strain from xylose increased the lipid content approximately twofold, i.e., 63.5% (w/w) of the cell dry biomass, highlighting its potential for use in oil production from this sugar, which is a promising route in biorefinery. This lipid content was higher than those obtained from xylose by L. starkeyi AS 2.1560 (57%) (Gong et al. 2012), Rodhosporidium toruloides CBS14 (45%) (Wiebe et al. 2012), Rhodotorula graminis (17%) (Galafassi et al. 2012), Cryptococcus albidus var. albidus CBS 4517 (33%) (Hansson and Dostfilek 1986), and R. toruloides DMKU-RE16 (17.7%) (Poontawee et al. 2017). Moreover, biomass accumulation by P. laurentii UFV-1 using 40-g/L xylose as the sole carbon source reached 9.31 g/L, close to values obtained from optimized cultivations using high glucose concentrations, such as R. toruloides DMKU3-TK16 (13.33 g/L) with 70-g/L glucose (Kraisintu et al. 2010); Rhodosporidium paludigenum DMKU-RE61 (9.4 g/L) with 70-g/L glucose (Poontawee et al. 2017), and Rhodosporidium fluviale DMKU-RK253 (10.3 g/L) with 70-g/L glucose (Poontawee et al. 2017).
According to our study, several works have been able to optimize the lipid production by oleaginous yeasts through RSM (Awad et al. 2019; Dasgupta et al. 2017; El-Shall et al. 2018; Kim et al. 2019; Selvakumar and Sivashanmugam 2018). Awad et al. (2019) evaluated the effect of 12 nitrogen and 10 carbon sources on lipid production by C. oleaginosus employing RSM. The highest lipid content [49.74 ± 5.16% (w/w)] was achieved using lactose and yeast extract as carbon and nitrogen sources, respectively, at C/N ratio of 120:1. Kim et al. (2019) also evaluated the effect of the medium composition on biomass and lipid production by Ettlia sp. YC001 using Plackett–Burman design (PBD) and RSM. The best results, 43.88 ± 0.92-g/L biomass and lipid content of 13.8%, respectively, were obtained using fructose and yeast extract as of carbon and nitrogen source sources, respectively, after 9 days of cultivation. The authors pointed out that this high biomass value was related to water evaporation due to the prolonged cultivation time. Dasgupta et al. (2017) adopted RSM (Box–Behnken design) to optimize the lipid production by Rhodotorula mucilaginosa IIPL32. In this work the effects of aeration rate, time and sugar concentration were evaluated. These authors used pentose-rich sugarcane bagasse hydrolysate as carbon source and recorded 8.6 g of lipid per 100 g of sugar consumed under optimized conditions. El-Shall et al. (2018) employed two statistical designs (PBD and RSM) to maximize the lipid production by Drechslera sp. First, they evaluate 'one variable at a time' strategy to select the most significant factors that influenced the lipid accumulation. Afterwards, the statistical designs defined the optimal parameter values: incubation time 144 h, FeSO4 0.015 g/L, pH 8.5 and yeast extract 2 g/L. Under these conditions, the lipid content was 40.75% (w/w). Selvakumar and Sivashanmugam (2018) evaluated the effects of pH, temperature, agitation and carbon source concentration on lipid production by Naganishia liquefaciens NITTS2. They adopted a CCD in a RSM methodology and optimized the lipid content, which was 55.7% (w/w).
An important goal in bioprocesses is to increase volumetric productivity. Importantly, P. laurentii UFV-1, under optimized conditions, achieved a volumetric lipid productivity of 0.082 g/L.h, which was higher than those obtained by oleaginous yeasts such as Rhodotorula kratochvilovae SY89—0.060 g/L.h, cultivated in culture medium containing glucose 50 g/L with a C/N ratio of 120:1 (Jiru et al. 2017); R. toruloides DMKU3-TK16—0.055 g/L.h, cultivated in culture medium containing glucose 70 g/L with a C/N ratio of 140:1 (Kraisintu et al. 2010); R. toruloides CBS14—0.04 g/L h, cultivated in culture medium containing xylose 30 g/L with a C/N ratio of 100:1 (Wiebe et al. 2012); and L. starkeyi AS 2.1560—0.076 g/L h, cultivated in culture medium containing xylose 70 g/L with a C/N ratio of 90:1 (Gong et al. 2012).
It should be noted that the highest lipid content was reached when the C/N ratio was high (100:1), since the limitation of nitrogen and high amounts of sugar are pivotal to ensuring a metabolic overflow to lipid syntheses (Beopoulos et al. 2011). Similarly, high C/N ratios, above 100, also improved the lipid contents reached by the other following oleaginous yeasts: Trichosporom fermentans (Zhu et al. 2008), R. toruloides DMAKU3-TK16 (Kraisintu et al. 2010) and Rhodotorula kratochvilovae (Jiru et al. 2017). The highest agitation ratio evaluated in this work (300 rpm) favored lipid accumulation. In fact, the increase in the agitation rate enhances the dissolved oxygen level in the culture medium, which in turn improves lipid production (Enshaeieh et al. 2013). Increases in pH values improved lipid production by P. laurentii UFV-1 and the highest lipid content level was achieved at pH 7. Contrary to P. laurentii, the lowest lipid content achieved by R. kratochvilovae was at pH 5.5 (Jiru et al. 2017). The size of the inoculum has an important effect on lipid production by oleaginous yeast (Kitcha and Cheirsilp 2011). In our study, the highest initial inoculum evaluated (OD600 = 0.8) allowed for reaching the highest lipid content, and avoiding a long lag phase.
The fatty acid profile used for biodiesel production plays an important role in determining its quality, mainly in terms of the length and degree of unsaturation of fatty acids. Overall, the presence of C16 and C18 fatty acids is positive where a low degree of unsaturation is desirable. Suitable compositions of fatty acids for biodiesel production consist mainly of palmitic (16:0), stearic (18:0), oleic (18:1), linoleic (18:2) and linolenic (18:3) (Knothe 2009). Interestingly, the fatty acid composition of P. laurentti UFV-1 is similar to the following vegetable oils used in the production of biodiesel: soybean (Glycine max), palm (Elaeis guineenses), Madhuca indica, Calophyllum inophyllum and Azadirachta indica, (Ramos et al. 2009; Pinzi et al. 2009; Sajjadi et al. 2016). It is important to point out that a high amount of olei acid, (59%) was found in the fatty acid profile of this yeast. This is a desirable feature since methyl oleate is the main constituent of biodiesel (Sitepu et al. 2014b). Moreover, oleic acid and palmitoleic are important for ensuring oxidation stability (Pinzi et al. 2009). Another important characteristic for the oxidation stability of biodiesel is the presence of reduced amounts of polyunsaturated acids (Deeba et al. 2016). Additionally, low levels of polyunsaturated acids in biodiesel are desirable because the cetane number, which is an indicator of combustion speed of fuel, increases as the number of unsaturated bonds decreases (Knothe 2005). Notably, the content of polyunsaturated fatty acids was low in P. laurentti UFV-1 (Table 5). The prediction of biodiesel properties from the fatty acid composition of P. laurentti UFV-1 showed that viscosity, density, and HHV oil were similar to other oleaginous yeasts, such as P. laurentii AM113 (Wang et al. 2018), L. starkeyi NBRC 10381 and R. toruloides NBRC 0559 (Tanimura et al. 2014) and to oils from soy, palm and canola (Hoekman et al. 2012). With regard to iodine value, P. laurentti UFV-1 displayed lower values compared to microbial and plant oils (Hoekman et al. 2012; Tanimura et al. 2014; Wang et al. 2018). The cetane number was similar to P. laurentii AM113 (Wang et al. 2018), and higher than L. starkeyi NBRC 10381 and R. toruloides NBRC 0559 (Tanimura et al. 2014) and plant oils (Hoekman et al. 2012). Therefore, the predicted parameters are in agreement with the quality standards of biodiesel production in Brazil, Europe and USA. Taken together, our results indicate that the oil produced by P. laurentti UFV-1 can be used in the biodiesel industry.
To conclude, in the present study, for the first time, oleaginous yeasts were isolated from soil samples from two National Parks in Brazil (Caparaó and Serra dos Órgãos). Under unoptimized conditions, the lipid production by P. laurentii UFV-1, the best lipid-producing, was higher in glucose than in xylose. The optimization strategy adopted in this work allowed us to improve the lipid production from xylose, achieving a lipid content of 63.5% (w/w). This improved lipid content was obtained under the following conditions: carbon/nitrogen ratio of 100:1, pH value = 7.0, initial OD600 = 0.8 and agitation = 300 rpm. Moreover, the prediction of the properties of biodiesel based on the fatty acid profile of P. laurentii UFV-1 underscores that P. laurentii’s oil is suitable for producing high-quality biodiesel. Our study highlights the potential for utilization of this yeast strain in the conversion of xylose into oil in a lignocellulosic biorefinery.
Accession number AF075469.1—Cryptococcus laurentii 26S ribosomal RNA gene.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the two Brazilian National Parks, Caparaó and Serra dos Órgãos, for allowing us to collect soil samples (Registration number 46270-2, Instituto Chico Mendes/Ministry of Environment/Brazil) and to Professor Carlos Rosa for supporting us with the taxonomic identification of the isolates.
Author contributions
NMV and RCVS participated in the study design, conducted analysis and manuscript preparation; VKCG participated of the optimization of lipid production; RZV participated in the analysis of experimental data and manuscript preparation; ELMA participated in the execution of physiological characterization; FAS participated in the statistical analysis and manuscript preparation; JIRJ designed optimization of lipid production experiments; and WBS coordinated study design, analysis and manuscript preparation.
Funding
This study was financed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)—Process 444480/2014-5, Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) and Coordenacão de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest in the publication.
Informed consent
Informed consent was obtained from all individual participants included in the study
Footnotes
Nívea Moreira Vieira and Raquel Cristina Vieira dos Santos have contributed equally to this paper.
References
- Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG. Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol. 2011;90:1219–1227. doi: 10.1007/s00253-011-3200-z. [DOI] [PubMed] [Google Scholar]
- Awad D, Bohnen F, Mehlmer N, Brueck T. Multi-factorial-guided media optimization for enhanced biomass and lipid formation by the oleaginous yeast Cutaneotrichosporon oleaginosus. Front Bioeng Biotechnol. 2019;7:54. doi: 10.3389/fbioe.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayadi I, Belghith H, Gargouri A, Guerfali M. Screening of new oleaginous yeasts for single cell oil production, hydrolytic potential exploitation and agro-industrial by-products valorization. Process Saf Environ Prot. 2018;119:104–114. doi: 10.1016/j.psep.2018.07.012. [DOI] [Google Scholar]
- Beopoulos A, Nicaud JM, Gaillardin C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol. 2011;90:1193–1206. doi: 10.1007/s00253-011-3212-8. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- Bracharz F, Beukhout T, Mehlmer N, Brück T. Opportunities and challenges in the development of Cutaneotrichosporon oleaginosus ATCC 20509 as a new cell factory for custom tailored microbial oils. Microb Cell Fact. 2017;16:178. doi: 10.1186/s12934-017-0791-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspeta L, Nielsen J. Economic and environmental impacts of microbial biodiesel. Nat Biotechnol. 2013;31:789. doi: 10.1038/nbt.2683. [DOI] [PubMed] [Google Scholar]
- Chandran P, Das N. Role of plasmid in diesel oil degradation by yeast species isolated from petroleum hydrocarbon-contaminated soil. Environ Technol. 2012;33:645–652. doi: 10.1080/09593330.2011.587024. [DOI] [PubMed] [Google Scholar]
- Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–132. doi: 10.1093/clinchem/8.2.130. [DOI] [PubMed] [Google Scholar]
- Christophe G, Kumar V, Nouaille R, et al. Recent developments in microbial oils production: A possible alternative to vegetable oils for biodiesel without competition with human food? Braz Arch Biol Technol. 2012;55:29–46. doi: 10.1590/S1516-89132012000100004. [DOI] [Google Scholar]
- Dasgupta D, Sharma T, Bhatt A, Bandhu S, Ghosh D. Cultivation of oleaginous yeast Rhodotorula mucilaginosa IIPL32 in split column airlift reactor and its influence on fuel properties. Biocatal Agric Biotechnol. 2017;10:308–316. doi: 10.1016/j.bcab.2017.04.002. [DOI] [Google Scholar]
- Deeba F, Pruthi V, Negi YS. Converting paper mill sludge into neutral lipids by oleaginous yeast Cryptococcus vishniaccii for biodiesel production. Bioresour Technol. 2016;213:96–102. doi: 10.1016/j.biortech.2016.02.105. [DOI] [PubMed] [Google Scholar]
- El-Shall H, Abu-Elreesh G, El-Sabbagh S, Abd-El-Haleem D. Isolation and optimization of lipid production from Drechslera sp. and feasibility of using orange peel as a substrate for growth. J Adv Biotechnol. 2018;7:1019–1043. doi: 10.24297/jbt.v7i1.7589. [DOI] [Google Scholar]
- Enshaeieh M, Abdoli A, Nahvi I. Medium optimization for biotechnological production of single cell oil using Yarrowia lipolytica M7 and Candida sp. J Cell Mol Res. 2013;5:17–23. doi: 10.22067/jcmr.v5i1.16611. [DOI] [Google Scholar]
- Galafassi S, Cucchetti D, Pizza F, et al. Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour Technol. 2012;111:398–403. doi: 10.1016/j.biortech.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Gong Z, Wang Q, Shen H, et al. Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresour Technol. 2012;117:20–24. doi: 10.1016/j.biortech.2012.04.063. [DOI] [PubMed] [Google Scholar]
- Hansson L, Dostfilek M. Influence of cultivation conditions on lipid production by Cryptococcus albidus. Appl Microbiol Biotechnol. 1986;24:12–18. [Google Scholar]
- Hoekman SK, Broch A, Robbins C, et al. Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev. 2012;16:143–169. doi: 10.1016/j.rser.2011.07.143. [DOI] [Google Scholar]
- Jin M, Slininger PJ, Dien BS, et al. Microbial lipid-based lignocellulosic biorefinery: feasibility and challenges. Trends Biotechnol. 2015;33:43–54. doi: 10.1016/j.tibtech.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Jiru TM, Groenewald M, Pohl C, et al. Optimization of cultivation conditions for biotechnological production of lipid by Rhodotorula kratochvilovae (syn, Rhodosporidium kratochvilovae) SY89 for biodiesel preparation. 3 Biotech. 2017;7:1–11. doi: 10.1007/s13205-017-0769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamineni A, Shaw J. Engineering triacylglycerol production from sugars in oleaginous yeasts. Curr Opin Biotechnol. 2020;62:239–247. doi: 10.1016/j.copbio.2019.12.022. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee B, Kim HS, Nam K, Moon M, Oh H-M, Chang YK. Increased biomass and lipid production of Ettlia sp. YC001 by optimized C and N sources in heterotrophic culture. Sci Rep. 2019;9:6830. doi: 10.1038/s41598-019-43366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitcha S, Cheirsilp B. Screening of oleaginous yeasts and optimization for lipid production using crude glycerol as a carbon source. Energy Procedia. 2011;9:274–282. doi: 10.1016/j.egypro.2011.09.029. [DOI] [Google Scholar]
- Knothe G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol. 2005;86:1059–1070. doi: 10.1016/j.fuproc.2004.11.002. [DOI] [Google Scholar]
- Knothe G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ Sci. 2009;2:759–766. doi: 10.1039/b903941d. [DOI] [Google Scholar]
- Kohlwein SD. Triacylglycerol homeostasis: Insights from yeast. J Biol Chem. 2010;285:15663–15667. doi: 10.1074/jbc.R110.118356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komesu A, Oliveira J, Neto JM, et al (2020) Xylose fermentation to bioethanol production using genetic engineering microorganisms. In: Kuila A, Sharma V (eds) Genetic and metabolic engineering for improved biofuel production from lignocellulosic biomass. Elsevier, Amsterdam, pp 143–154. 10.1016/B978-0-12-817953-6.00010-5
- Kraisintu P, Yongmanitchai W, Limtong S. Selection and optimization for lipid production of a newly isolated oleaginous yeast, Rhodosporidium toruloides DMKU3-TK16. Kasetsart J Nat Sci. 2010;44:436–445. [Google Scholar]
- Lachance MA, Daniel HM, Meyer W, et al. The D1/D2 domain of the large-subunit rDNA of the yeast species Clavispora lusitaniae is unusually polymorphic. FEMS Yeast Res. 2003;4:253–258. doi: 10.1016/S1567-1356(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Leung DYC, Wu X, Leung MKH. A review on biodiesel production using catalyzed transesterification. Appl Energy. 2010;87:1083–1095. doi: 10.1016/j.apenergy.2009.10.006. [DOI] [Google Scholar]
- Li Q, Du W, Liu D. Perspectives of microbial oils for biodiesel production. Appl Microb Biotechnol. 2008;80:749–756. doi: 10.1007/s00253-008-1625-9. [DOI] [PubMed] [Google Scholar]
- Li X, Chen Y, Nielsen J. Harnessing xylose pathways for biofuels production. Curr Opin Biotechnol. 2019;57:56–65. doi: 10.1016/j.copbio.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhao ZK, Bai F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol. 2007;41:312–317. doi: 10.1016/j.enzmictec.2007.02.008. [DOI] [Google Scholar]
- Lin J, Li S, Sun M, et al. Microbial lipid production by oleaginous yeast in d-xylose solution using a two-stage culture mode. RSC Adv. 2014;4:34944–34949. doi: 10.1039/c4ra01453g. [DOI] [Google Scholar]
- Liu X-Z, Wang Q-M, Göker M, et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol. 2015;81:85–147. doi: 10.1016/j.simyco.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gao Y, Chen J, et al. Analysis of metabolic fluxes for better understanding of mechanisms related to lipid accumulation in oleaginous yeast Trichosporon cutaneum. Bioresour Technol. 2013;130:144–151. doi: 10.1016/j.biortech.2012.12.072. [DOI] [PubMed] [Google Scholar]
- MacDonald J (2016) Coal and gas to stay cheap, but renewables still win race on costs. In: Bloom. New energy financ. https://about.bnef.com/blog/coal-and-gas-to-stay-cheap-but-renewables-still-win-race-on-costs/s
- Masoud W, Cesar LB, Jespersen L, Jakobsen M. Yeast involved in fermentation of Coffea arabica in East Africa determined by genotyping and by direct denaturating gradient gel electrophoresis. Yeast. 2004;21:549–556. doi: 10.1002/yea.1124s. [DOI] [PubMed] [Google Scholar]
- Morais CG, Cadete RM, Uetanabaro APT, et al. D-xylose-fermenting and xylanase-producing yeast species from rotting wood of two Atlantic Rainforest habitats in Brazil. Fungal Genet Biol. 2013;60:19–28. doi: 10.1016/j.fgb.2013.07.003. [DOI] [PubMed] [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, et al. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Nakase T, Komagata K, Fukazawa Y. Candida pseudointermedia sp. nov., isolated from “kamaboko”, a traditional fish-paste product in Japan. J Gen Appl Microbiol. 1976;22:177–182. doi: 10.2323/jgam.22.177. [DOI] [Google Scholar]
- Olasupo NA, Bakre S, Teniola OD, James SA. Identification of yeasts isolated from Nigerian sugar cane peels. J Basic Microbiol. 2003;43:530–533. doi: 10.1002/jobm.200310231. [DOI] [PubMed] [Google Scholar]
- Oyamada H, Ogawa Y, Shibata N, et al. Structural analysis of cell wall mannan of Candida sojae, a new yeast species isolated from defatted soybean flakes. Arch Microbiol. 2008;189:483–490. doi: 10.1007/s00203-007-0339-1. [DOI] [PubMed] [Google Scholar]
- Pan LX, Yang DF, Shao L, et al. Isolation of the oleaginous yeasts from the soil and studies of their lipid-producing capacities. Food Technol Biotechnol. 2009;47:215–220. [Google Scholar]
- Papanikolaou S, Aggelis G. Lipids of oleaginous yeasts. Part II: technology and potential applications. Eur J Lipid Sci Tech. 2011;113:1052–1073. doi: 10.1002/ejlt.201100015. [DOI] [Google Scholar]
- Pinzi S, Garcia IL, Luque de Castro MD, et al. The ideal vegetable oil-based biodiesel composition: a review of social, economical and technical implications. Energy Fuels. 2009;23:2325–2341. doi: 10.1021/ef801098a. [DOI] [Google Scholar]
- Polburee P, Yongmanitchai W, Lertwattanasakul N, et al. Characterization of oleaginous yeasts accumulating high levels of lipid when cultivated in glycerol and their potential for lipid production from biodiesel-derived crude glycerol. Fungal Biol. 2015 doi: 10.1016/j.funbio.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Poontawee R, Yongmanitchai W, Limtong S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017;53:44–60. doi: 10.1016/j.procbio.2016.11.013. [DOI] [Google Scholar]
- Ramos MJ, Fernández CM, Casas A, et al. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour Technol. 2009;100:261–268. doi: 10.1016/j.biortech.2008.06.039. [DOI] [PubMed] [Google Scholar]
- Rodriguez GM, Hussain MS, Gambill L, et al. Engineering xylose utilization in Yarrowia lipolytica by understanding its cryptic xylose pathway. Biotechnol Biofuels. 2016;9:149. doi: 10.1186/s13068-016-0562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi B, Raman AAA, Arandiyan H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: composition, specifications and prediction models. Renew Sust Energ Rev. 2016;63:62–92. doi: 10.1016/j.rser.2016.05.035. [DOI] [Google Scholar]
- Sambrook J, Russel DW. Molecular cloning: a laboratory manual. 3. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Sarkar S, Chakravorty S, Mukherjee A, et al. De novo RNA-Seq based transcriptome analysis of Papiliotrema laurentii strain RY1 under nitrogen starvation. Gene. 2018;645:146–156. doi: 10.1016/j.gene.2017.12.014. [DOI] [PubMed] [Google Scholar]
- Schulze I, Hansen S, Großhans S, et al. Characterization of newly isolated oleaginous yeasts—Cryptococcus podzolicus, Trichosporon porosum and Pichia segobiensis. AMB Express. 2014;4:1–11. doi: 10.1186/s13568-014-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar P, Sivashanmugam P. Study on lipid accumulation in novel oleaginous yeast Naganishia liquefaciens NITTS2 utilizing pre-digested municipal waste activated sludge: a low-cost feedstock for biodiesel production. Appl Biochem Biotechnol. 2018;186:731–749. doi: 10.1007/s12010-018-2777-4. [DOI] [PubMed] [Google Scholar]
- Sergeeva YE, Mostova EB, Gorin KV, et al. Calculation of biodiesel fuel characteristics based on the fatty acid composition of the lipids of some biotechnologically important microorganisms. Appl Biochem Microbiol. 2017;53:807–813. doi: 10.1134/S0003683817080063. [DOI] [Google Scholar]
- Sitepu IR, Garay LA, Sestric R, et al. Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol Adv. 2014;32:1336–1360. doi: 10.1016/j.biotechadv.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Sitepu IR, Ignatia L, Franz AK, et al. An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. J Microbiol Methods. 2012;91:321–328. doi: 10.1016/j.mimet.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitepu IR, Jin M, Fernandez JE, Boundy-Mills KL. Identification of oleaginous yeast strains able to accumulate high intracellular lipids when cultivated in alkaline pretreated corn stover. Appl Microbiol Biotechnol. 2014;98:7645–7657. doi: 10.1007/s00253-014-5944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitepu IR, Sestric R, Ignatia L, et al. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour Technol. 2013;144:360–369. doi: 10.1016/j.biortech.2013.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sláviková E, Vadkertiová R. The diversity of yeasts in the agricultural soil. J Basic Microbiol Int J Biochem Physiol Genet Morphol Ecol Microorg. 2003;43:430–436. doi: 10.1002/jobm.200310277. [DOI] [PubMed] [Google Scholar]
- Tai M, Stephanopoulos G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab Eng. 2013;15:1–9. doi: 10.1016/j.ymben.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Takashima M, Sugita T, Van BH, et al. Taxonomic richness of yeasts in Japan within subtropical and cool temperate areas. PLoS ONE. 2012;7:e50784. doi: 10.1371/journal.pone.0050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Takashima M, Sugita T, et al. Selection of oleaginous yeasts with high lipid productivity for practical biodiesel production. Bioresour Technol. 2014;153:230–235. doi: 10.1016/j.biortech.2013.11.086. [DOI] [PubMed] [Google Scholar]
- Tiukova IA, Brandenburg J, Blomqvist J, et al. Proteome analysis of xylose metabolism in Rhodotorula toruloides during lipid production. Biotechnol Biofuels. 2019;12:137. doi: 10.1186/s13068-019-1478-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu D, Groenewald M, Szöke S, et al. DNA barcoding analysis of more than 9000 yeast isolates contributes to quantitative thresholds for yeast species and genera delimitation. Stud Mycol. 2016;85:91–105. doi: 10.1016/j.simyco.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Liu L, Liang W. Single cell oil production from hydrolysates of inulin by a newly isolated yeast Papiliotrema laurentii AM113 for biodiesel making. Appl Biochem Biotechnol. 2018;184:168–181. doi: 10.1007/s12010-017-2538-9. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu R, Wang R, et al. Overexpression of ACC gene from oleaginous yeast Lipomyces starkeyi enhanced the lipid accumulation in Saccharomyces cerevisiae with increased levels of glycerol 3-phosphate substrates. Biosci Biotechnol Biochem. 2016;80:1214–1222. doi: 10.1080/09168451.2015.1136883. [DOI] [PubMed] [Google Scholar]
- Wiebe MG, Koivuranta K, Penttilä M, Ruohonen L. Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol. 2012;12:26. doi: 10.1186/1472-6750-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Li L, Zhang J, et al. Improving ethanol and xylitol fermentation at elevated temperature through substitution of xylose reductase in Kluyveromyces marxianus. J Ind Microbiol Biotechnol. 2013;40:305–316. doi: 10.1007/s10295-013-1230-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Skerker JM, Rutter CD, et al. Engineering Rhodosporidium toruloides for increased lipid production. Biotechnol Bioeng. 2016;113:1056–1066. doi: 10.1002/bit.25864. [DOI] [PubMed] [Google Scholar]
- Zhu LY, Zong MH, Wu H. Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresour Technol. 2008;99:7881–7885. doi: 10.1016/j.biortech.2008.02.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.