ABSTRACT
Background
Gestational weight gain (GWG) has important health implications for both the mother and offspring. Maternal diet during pregnancy may play an important role in achieving adequate GWG, although its precise role is unclear.
Objectives
Associations between maternal dietary components (fruits and vegetables, added sugar, percentage energy from fat, dairy) and GWG were examined in 327 pregnant women from the Archive for Research on Child Health cohort.
Methods
Self-reported usual dietary intake was assessed with validated dietary screening tools at the first prenatal visit. GWG was obtained from the birth certificate and was categorized as inadequate, adequate, or excessive according to the Institute of Medicine recommendations. Associations between dietary components and GWG were assessed using multivariable regression models, stratified by maternal prepregnancy BMI category.
Results
Only 31.5% of women had adequate GWG, with 24.8% gaining insufficient weight and 43.7% gaining excessively. Women who consumed more fruits and vegetables were suggestively less likely to have excessive GWG (OR: 0.86; 95% CI: 0.75, 1.00) in the minimally adjusted model, but the association became nonsignificant after adjusting for covariates (OR: 0.89; 95% CI: 0.77, 1.03). In stratified models, higher fruit and vegetable intake was linked to lower likelihood of excessive GWG among women with obesity (OR: 0.77; 95% CI: 0.60, 0.97), whereas higher added sugar intake was linked to a slight reduction in likelihood of excessive GWG (OR: 0.91; 95% CI: 0.84, 0.99) among women with a prepregnancy BMI in the normal range. Other dietary components were not significantly associated with GWG.
Conclusions
These results suggest that consuming fruits and vegetables during pregnancy may reduce risk of excessive GWG among women with obesity. With the rising prevalence of obesity among women of reproductive age, interventions to increase fruit and vegetable intake during pregnancy may have broad public health impact by improving maternal and child health outcomes.
Keywords: diet, fruit, sugar, pregnancy, weight gain, obesity
Findings from this prospective study suggest that fruit and vegetable consumption during pregnancy may reduce risk of excessive gestational weight gain in women with prepregnancy obesity.
Introduction
Inadequate or excessive gestational weight gain (GWG) in pregnancy has been shown to have deleterious consequences for the short- and long-term health of mother and child. For example, insufficient GWG increases the risk of preterm birth and low birth weight, whereas excessive GWG is associated with increased risk of pregnancy-induced hypertension, pre-eclampsia, emergency cesarean delivery, hyperglycemia, and macrosomia (1–4). Moreover, excessive GWG is linked to an elevated risk of postpartum weight retention and of developing overweight and obesity in both the mother (2, 5–8) and her children (3, 9–12). Although some evidence suggests that the maternal and infant health risks associated with GWG are independent of maternal prepregnancy BMI (7), the impact of GWG on maternal and infant health outcomes may depend on the mother's prepregnancy BMI (13). For instance, less GWG results in more favorable outcomes for women with higher BMI, whereas inadequate GWG for women with a normal or low BMI may be detrimental (13). Thus, the Institute of Medicine (IOM) and National Research Council (NRC) developed specific GWG recommendations to optimize health outcomes based on women's prepregnancy BMI (5). Even after the release of these IOM/NRC guidelines in 2009, the prevalence of excessive GWG continued to rise, as nearly half of all pregnant women in the United States gained above the recommended weight in 2012–2013 (14). The increasing prevalence of excessive GWG and well-documented health consequences of both inadequate and excessive GWG underscore the urgent need to identify modifiable factors to promote appropriate weight gain in pregnancy.
Maternal diet during pregnancy may play an important role in achieving adequate GWG. Indeed, a meta-analysis of 13 randomized trials suggested a significant reduction in GWG of −1.92 kg and a trend toward reduction in excessive GWG with various dietary interventions, including calorie restriction and nutritional counseling (15). However, most of the reviewed intervention studies included additional lifestyle changes, such as increased physical activity. Moreover, prior studies have been heterogeneous in study design and have assessed disparate dietary components and outcomes. Finally, previous studies have largely ignored potential heterogeneity in associations based on maternal prepregnancy BMI. Given these limitations, a review article of randomized trials and observational epidemiologic studies evaluating the role of maternal diet on excessive GWG concluded that the role of diet in GWG was largely unclear (16).
Understanding to what extent adverse health outcomes associated with GWG can be modified by dietary intake during pregnancy is of major public health importance. Moreover, with the rising prevalence of obesity among women of reproductive age (17), identifying ways to improve pregnancy outcomes is becoming increasingly important. Therefore, the aim of this study was to examine associations between specific dietary components during pregnancy and GWG stratified by maternal prepregnancy BMI in the prospective Archive for Research in Child Health (ARCH) cohort study.
Methods
Study participants
ARCH was established to be a low-cost, low-participant-burden cohort study, relying on archived information (medical records, birth certificates, newborn blood spots), brief interviews, and clinically obtained specimens (extra tubes of blood and urine collected in addition to those used for routine clinical purposes). Pregnant women were enrolled and interviewed for the ARCH study at their first prenatal care visit (mean gestational age at enrollment was 13.4 wk). Recruitment occurred from 2008 to 2015 in 3 clinics in Lansing, Michigan, enrolling 801 pregnant women. In 2016, ARCH recruitment was expanded to additional prenatal care clinics in other areas of Michigan (Detroit, Grand Rapids, Flint, and Traverse City), yielding a total ARCH enrollment of 1042 women from all locations combined. Few exclusion criteria (<18 y of age or non–English speaking) resulted in a sample of women reflecting the local population, unselected for any specific health conditions or sociodemographic criteria. Follow-up is ongoing and includes phone surveys at 1 mo and 1 y postpartum and annually thereafter. ARCH participants consented to use of the collected data for future research; investigators in this study accessed only de-identified data for the protection and privacy of subjects. ARCH mother–infant dyads are now being followed with more intensive methods under the auspices of the NIH Environmental influences on Child Health Outcomes (ECHO) program (https://www.nih.gov/echo). The ECHO program is supporting multiple pregnancy cohorts, including ARCH, with the goal of investigating environmental exposures on positive health and 4 pediatric issues that have a large public health impact: perinatal outcomes, neurodevelopment, obesity, and upper and lower airway conditions.
For this analysis, we included a subset of pregnant women (n = 472) enrolled in the ARCH study after 2013 when diet assessment was added to the baseline questionnaire. We excluded 82 women with missing diet assessment and 63 women missing information on GWG, leaving a final analytic sample of 327 women (Figure 1). This study was approved by the Institutional Review Board (IRB) at Michigan State University (IRB# LEGACYC07–1201), and all participants provided written informed consent agreeing to the use of data for future research.
FIGURE 1.
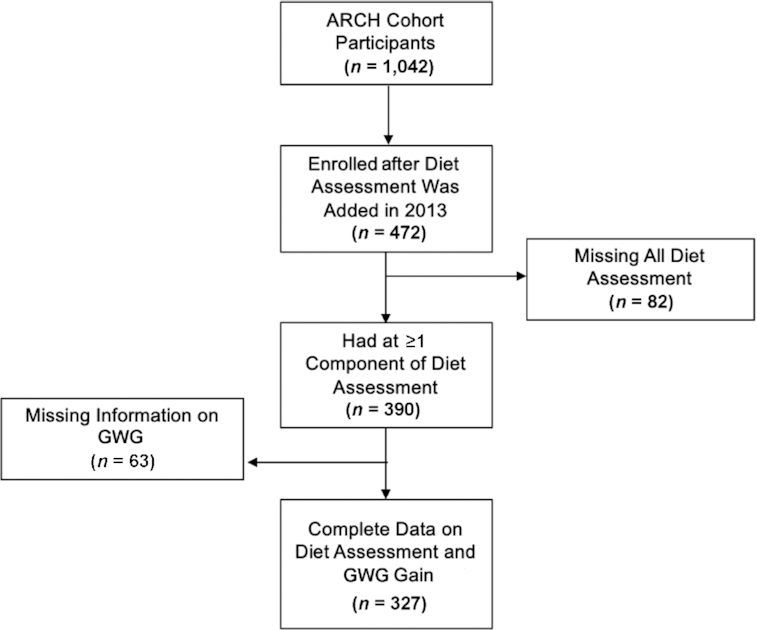
Inclusion of study participants flowchart. GWG, gestational weight gain.
Dietary assessment
Dietary intake was assessed at the first prenatal clinic visit when women were enrolled in the study, using interviewer-administered survey questions. Dietary components from the consensus measures of the Phenotypes and eXposures (PhenX: RTI International)) toolkit, version 22.0, were used because they are validated, well-established, broadly applicable, and low-burden (18–21). The dietary questions were part of the “Five Factor Screener” developed by the National Cancer Institute to assess an individual's approximate intake of several food items over the past month, and have been validated against the 24-h dietary recall (21). These survey questions were used to estimate usual intakes of fruits and vegetables (cups/d), added sugars (teaspoons/d), energy from fat (%), and dairy food (servings/d), according to the analytic steps detailed in the PhenX Toolkit, version 30.0 (8 April, 2020). The fruits and vegetables composite score comprised 100% fruit juice, fruit, salad, white potatoes, cooked dried beans, other vegetables (e.g., tomatoes, string beans, cabbage), tomato sauce, and salsa. In secondary analysis, we considered a modified fruit and vegetable consumption variable by removing French fry consumption from the measure. Dairy foods included milk and cheese. Added sugar sources included soda, fruit-flavored drinks with sugar, pastries (donuts, sweet rolls, Danish), and cookies, cake, pie, or brownies. Frequencies of an individual's consumption of the following foods predictive of percentage energy were assessed: cereals, skim milk, eggs, sausage or bacon, margarine or butter, fruit juice, fruit, hot dogs, cheese, French fries, mayonnaise, salad dressing, and rice. Consumption of these foods over the past month were scored across 8 response categories, ranging from “Never” to “2 or more times per day.” Scoring of dietary variables was defined according to established PhenX protocols, and these scores were utilized as continuous variables in the analysis. Because some of the dietary data necessary to calculate component measures were missing, sample sizes varied slightly across the dietary components assessed.
GWG assessment
GWG was defined by calculating the difference between prepregnancy weight and the last weight before delivery measured in pounds (lbs). Maternal weight measurements were obtained from the child's birth certificate and derived from prepregnancy weight and delivery weight measured by medical staff, although self-reported prepregnancy weight from the enrollment questionnaire was used for 2 women missing this information in the birth certificate. We categorized GWG as inadequate, adequate, or excessive according to the IOM recommendations (22). The guidelines recommend weight gain of 28–40 lbs for underweight women [BMI (in kg/m2) < 18.5], 25–35 lbs (11.3-15.9 kg) for normal-weight women (BMI 18.5–24.9), 15–25 lbs (6.8-11.3 kg) for overweight women (BMI 25.0–29.9), and 11–20 lbs (5.0-9.1 kg) for women with obesity (BMI ≥ 30).
Covariates
Information on covariates was collected from either the enrollment questionnaire or the birth certificate. Data on maternal age (continuous), race (non-Hispanic white; non-Hispanic Black; Hispanic; other), marital status (married and living with infant's father; married and not living with infant's father; unmarried and living with infant's father; unmarried and not living with infant's father), educational attainment (less than high school; high school or equivalent; some college; college or more), household income (<$25,000; $25,000–$49,999; $50,000–$74,999; ≥$75,000), and participation in vigorous physical activity over the last month (yes; no; unknown) were self-reported at study enrollment. Pregnancy-related nausea and vomiting was assessed at study enrollment using the Pregnancy-Unique Quantification of Emesis and Nausea Index (PUQE) and Modified-PUQE survey (23), with scores < 6 coded as mild, 7 to <12 as moderate, and ≥12 as severe. Depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (≥10 for possible depression compared with <10 for no depression) (24). Weeks of gestation (continuous), fetal sex (male/female), maternal smoking history (yes/no), receipt of WIC food during pregnancy (yes/no), mother's prepregnancy weight (continuous), mother's weight at delivery (continuous), and mother's height (continuous) were ascertained from the birth certificate data. Prepregnancy BMI was calculated from height and weight measures obtained from the birth certificate. Whereas height can be measured during pregnancy, prepregnancy weight is often self-reported because by the time women present for prenatal care, it is too late to get a measured prepregnancy weight. Thus, even though birth certificate records use maternal weights derived from prenatal records, those prenatal records often include self-reported prepregnancy weights, which have been shown to be valid measures of measured weight and height in a prior study (25). Maternal BMI was categorized as follows: underweight (<18.5), normal (18.5 to <25), overweight (25 to <30), class I obesity (30 to <35), class II obesity (35 to <40), and class III obesity (≥40). However, because of insufficient sample size and because there are no specific IOM GWG recommendations based on obesity classes, obesity classes I, II, and III were combined into a single category (i.e., BMI ≥ 30) in statistical models.
Statistical analysis
We evaluated mean intake of dietary components across demographic and pregnancy characteristics and assessed differences using ANOVA tests. Multivariable logistic regression models were used to estimate the relation between each dietary intake factor and odds of excessive or inadequate GWG compared with adequate GWG based on the IOM/NRC guidelines. We first evaluated minimally adjusted models accounting for gestational age at delivery. Potential confounders were included in the multivariable model based on prior knowledge of factors associated with dietary intake and GWG (1, 26). The multivariable model included the following factors: weeks of gestation at delivery, physical activity, prepregnancy BMI, maternal age, educational level, fetal sex, and marital status. Given that maternal prepregnancy BMI is an important determinant of GWG (5, 27) and that associations between GWG and adverse health outcomes differ according to prepregnancy BMI (17), we evaluated models stratified by BMI category and statistically compared models with and without relevant interaction terms between these factors and dietary components using the likelihood ratio test. In secondary analysis, we used multivariable linear regression models to examine the relation between each dietary factor and a continuous measure of GWG in lbs, using the same set of covariates as in the logistic models. To minimize potential confounding and assess potential heterogeneity related to smoking status, we also examined associations stratified by maternal smoking history. In sensitivity analyses, we examined associations for fruit and vegetable consumption and GWG excluding French fries from the composite fruit and vegetable score. Pearson's correlation coefficients were used to examine the linear relation between dietary indicators. All analyses were conducted using SAS software version 9.4 (SAS Institute, Inc.), with α < 0.05 used to determine significance.
Results
Participant characteristics
Of the 327 women in this study, 60.9% were overweight or obese entering pregnancy. The mean age at enrollment was 27 y, and the racial/ethnic, educational, and income distributions were reflective of the local populations. For example, 66.7% of women were non-Hispanic white, 20.1% non-Hispanic Black, 10.1% Hispanic, and 3.1% of another racial/ethnic group. Whereas 59.3% of women in this study had at least some college education, 48.4% reported annual household incomes <$25,000, likely reflecting a mix of the respective working-class and graduate student populations in the capital city of Lansing, MI, and the nearby college town of East Lansing, MI. Only 31.5% of women had adequate GWG over pregnancy, with 24.8% of women gaining insufficient weight and 43.7% gaining excessively. Women consumed daily means of 3.14 cups of fruits and vegetables, 17.1 teaspoons of added sugar, 33.0% energy from fat, and 1.79 servings of dairy (Table 1). Mean fruit and vegetable consumption was highest among women with class III obesity and lowest among underweight women (3.74 compared with 2.03 daily cups). Non-Hispanic Black women and women of other race/ethnicity reported higher fruit and vegetable consumption (3.62 and 4.21 daily cups, respectively) than non-Hispanic white and Hispanic women (2.97 and 2.81 daily cups, respectively). Fruit and vegetable consumption was also higher among women with adequate GWG (3.49 daily cups) than among those with inadequate GWG (2.97 daily cups) or excessive GWG (2.98 daily cups) and among those who delivered a male (3.37 daily cups) as opposed to a female (2.85 daily cups) infant. Added sugar intake tended to decrease with increasing maternal age, educational attainment, and household income and was higher among unmarried women, those with an unplanned pregnancy, women with a history of smoking, and women receiving WIC benefits. Underweight (41.1%) and non-Hispanic Black (35.1%) women had higher percentage energy from fat than women in other BMI and racial categories. Dairy consumption was slightly higher among married than among unmarried women, with the highest intake of 2.05 daily servings among married women who were not living with the infant's father. Dietary components assessed in this study were weakly correlated with each other [Pearson correlation coefficients ranged from −0.21 between fruit and vegetable consumption and percentage energy from fat (P = 0.001) to 0.20 between added sugar and percentage energy from fat (P = 0.001)].
TABLE 1.
Characteristics of pregnant women in the Archive for Research on Child Health study according to dietary indicators1
| Daily cup equivalents of fruits and vegetables | Teaspoons of added sugar | Percentage energy from fat | Daily servings of dairy | |||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | Mean ± SD | |
| Total sample | 309 | 3.14 ± 1.7 | 314 | 17.1 ± 9.6 | 265 | 33.0 ± 5.0 | 327 | 1.79 ± 1.1 |
| Maternal age category, y | ||||||||
| <20 | 22 | 2.77 ± 1.6 | 23 | 15.9 ± 7.7 | 21 | 33.1 ± 4.8 | 25 | 1.84 ± 1.0 |
| 20–29 | 183 | 3.12 ± 2.0 | 187 | 19.3 ± 10.8 | 162 | 33.6 ± 5.6 | 196 | 1.78 ± 1.1 |
| 30–34 | 14 | 3.64 ± 1.3 | 16 | 14.2 ± 6.8 | 13 | 32.0 ± 4.2 | 16 | 1.77 ± 0.9 |
| ≥35 | 17 | 3.21 ± 1.1 | 17 | 12.2 ± 3.3 | 14 | 32.2 ± 3.8 | 17 | 1.83 ± 1.1 |
| P = 0.59 | P = 0.01 | P = 0.62 | P = 0.99 | |||||
| Prepregnancy BMI category2 | ||||||||
| Underweight | 6 | 2.03 ± 1.6 | 6 | 16.6 ± 4.4 | 5 | 41.1 ± 12.3 | 6 | 1.61 ± 1.0 |
| Normal | 116 | 3.28 ± 1.5 | 118 | 16.4 ± 9.7 | 95 | 32.5 ± 4.8 | 122 | 1.90 ± 1.2 |
| Overweight | 81 | 2.83 ± 1.6 | 84 | 18.0 ± 10.3 | 74 | 33.1 ± 4.3 | 88 | 1.78 ± 1.1 |
| Obese I | 48 | 3.53 ± 2.2 | 49 | 17.1 ± 7.2 | 44 | 32.4 ± 4.1 | 51 | 1.76 ± 0.9 |
| Obese II | 30 | 2.45 ± 1.0 | 31 | 20.0 ± 12.6 | 22 | 34.8 ± 6.9 | 31 | 1.60 ± 1.3 |
| Obese III | 28 | 3.74 ± 2.2 | 26 | 14.6 ± 6.1 | 25 | 32.4 ± 4.4 | 29 | 1.72 ± 1.0 |
| P = 0.01 | P = 0.31 | P = 0.003 | P = 0.78 | |||||
| Race/ethnicity | ||||||||
| White, non-Hispanic | 203 | 2.97 ± 1.3 | 207 | 15.7 ± 8.4 | 169 | 32.3 ± 4.2 | 212 | 1.90 ± 1.1 |
| Black, non-Hispanic | 62 | 3.62 ± 2.4 | 59 | 19.7 ± 10.8 | 54 | 35.1 ± 6.9 | 64 | 1.51 ± 1.0 |
| Hispanic | 27 | 2.81 ± 1.8 | 29 | 19.8 ± 9.9 | 27 | 32.8 ± 3.7 | 32 | 1.76 ± 1.2 |
| Other | 9 | 4.21 ± 3.5 | 10 | 23.8 ± 15.9 | 7 | 32.6 ± 4.4 | 10 | 1.49 ± 1.8 |
| P = 0.02 | P = 0.003 | P = 0.01 | P = 0.13 | |||||
| Educational level | ||||||||
| Less than HS | 40 | 3.02 ± 2.1 | 41 | 18.5 ± 9.3 | 38 | 34.0 ± 4.4 | 46 | 1.73 ± 0.9 |
| HS or equivalent | 81 | 3.22 ± 2.2 | 82 | 20.2 ± 10.7 | 71 | 32.9 ± 6.5 | 85 | 1.67 ± 1.1 |
| Some college | 79 | 2.90 ± 1.5 | 83 | 17.9 ± 10.3 | 70 | 33.1 ± 4.8 | 86 | 1.73 ± 1.2 |
| College or more | 104 | 3.37 ± 1.4 | 103 | 13.5 ± 6.5 | 82 | 32.4 ± 3.9 | 105 | 2.00 ± 1.1 |
| P = 0.33 | P < 0.0001 | P = 0.40 | P = 0.16 | |||||
| Marital status | ||||||||
| Married, cohabitating | 121 | 3.21 ± 1.3 | 120 | 13.9 ± 6.7 | 92 | 32.0 ± 4.1 | 122 | 1.98 ± 1.1 |
| Married, not cohabitating | 16 | 3.67 ± 2.3 | 16 | 15.6 ± 6.4 | 13 | 31.1 ± 3.4 | 17 | 2.05 ± 1.3 |
| Unmarried, cohabitating | 96 | 2.86 ± 1.6 | 100 | 18.8 ± 10.8 | 88 | 34.1 ± 5.3 | 105 | 1.79 ± 1.1 |
| Unmarried, not cohabitating | 76 | 3.26 ± 2.4 | 78 | 20.2 ± 10.8 | 72 | 33.3 ± 5.8 | 83 | 1.47 ± 1.0 |
| P = 0.21 | P < 0.0001 | P = 0.02 | P = 0.01 | |||||
| Household income, $ | ||||||||
| <25,000 | 145 | 3.22 ± 2.1 | 145 | 19.3 ± 9.8 | 127 | 33.5 ± 5.9 | 154 | 1.73 ± 1.1 |
| 25,000– 49,000 | 68 | 2.98 ± 1.5 | 72 | 18.3 ± 11.0 | 59 | 32.9 ± 4.1 | 74 | 1.80 ± 1.1 |
| 50,000– 74,999 | 29 | 3.11 ± 1.1 | 29 | 12.1 ± 3.4 | 24 | 31.5 ± 3.3 | 29 | 1.81 ± 1.1 |
| ≥75,000 | 59 | 3.18 ± 1.2 | 61 | 12.1 ± 4.6 | 47 | 32.3 ± 3.9 | 61 | 1.95 ± 1.1 |
| P = 0.83 | P < 0.0001 | P = 0.21 | P = 0.65 | |||||
| Gestational weight gain | ||||||||
| Inadequate | 77 | 2.97 ± 1.3 | 75 | 16.2 ± 8.4 | 66 | 32.9 ± 5.6 | 81 | 1.85 ± 1.2 |
| Adequate | 99 | 3.49 ± 2.1 | 101 | 17.7 ± 10.2 | 83 | 33.2 ± 4.2 | 103 | 1.83 ± 1.1 |
| Excessive | 133 | 2.98 ± 1.6 | 138 | 17.2 ± 9.7 | 116 | 32.9 ± 5.3 | 143 | 1.74 ± 1.1 |
| P = 0.05 | P = 0.58 | P = 0.86 | P = 0.71 | |||||
| Depression | ||||||||
| Yes | 59 | 3.37 ± 2.3 | 58 | 19.0 ± 8.4 | 48 | 34.1 ± 5.4 | 61 | 1.65 ± 1.0 |
| No | 247 | 3.07 ± 1.6 | 253 | 16.6 ± 9.6 | 214 | 32.7 ± 4.9 | 263 | 1.83 ± 1.1 |
| P = 0.23 | P = 0.08 | P = 0.08 | P = 0.24 | |||||
| PUQE score last 12 h | ||||||||
| Mild | 236 | 3.07 ± 1.6 | 239 | 16.8 ± 9.7 | 204 | 33.1 ± 4.9 | 248 | 1.81 ± 1.1 |
| Moderate/severe | 71 | 3.34 ± 2.1 | 73 | 18.1 ± 9.4 | 59 | 32.8 ± 5.5 | 77 | 1.76 ± 1.1 |
| P = 0.50 | P = 0.61 | P = 0.78 | P = 0.77 | |||||
| Fetal sex | ||||||||
| Male | 171 | 3.37 ± 1.9 | 170 | 16.8 ± 9.7 | 146 | 32.8 ± 4.8 | 177 | 1.89 ± 1.1 |
| Female | 138 | 2.85 ± 1.4 | 144 | 17.5 ± 9.5 | 119 | 33.2 ± 5.3 | 150 | 1.68 ± 1.1 |
| P = 0.01 | P = 0.50 | P = 0.58 | P = 0.09 | |||||
| Planned pregnancy | ||||||||
| Yes | 135 | 3.16 ± 1.4 | 139 | 15.0 ± 8.5 | 116 | 32.3 ± 4.1 | 143 | 1.84 ± 1.1 |
| No | 171 | 3.08 ± 1.9 | 172 | 18.7 ± 9.8 | 147 | 33.6 ± 5.7 | 181 | 1.76 ± 1.1 |
| P = 0.68 | P = 0.0004 | P = 0.05 | P = 0.55 | |||||
| Maternal history of smoking | ||||||||
| Yes | 93 | 3.25 ± 2.3 | 92 | 21.1 ± 11.1 | 76 | 34.0 ± 6.1 | 96 | 1.76 ± 1.1 |
| No | 216 | 3.09 ± 1.5 | 222 | 15.5 ± 8.3 | 189 | 32.6 ± 4.5 | 231 | 1.81 ± 1.1 |
| P = 0.47 | P < 0.0001 | P = 0.03 | P = 0.70 | |||||
| Vigorous physical activity | ||||||||
| Yes | 61 | 3.20 ± 1.3 | 65 | 15.6 ± 9.9 | 44 | 32.1 ± 5.0 | 65 | 1.86 ± 1.0 |
| No | 234 | 3.09 ± 1.8 | 235 | 17.7 ± 9.6 | 206 | 33.1 ± 5.1 | 246 | 1.75 ± 1.1 |
| P = 0.64 | P = 0.13 | P = 0.26 | P = 0.44 | |||||
| Mother receiving WIC | ||||||||
| Yes | 155 | 3.27 ± 2.1 | 159 | 19.6 ± 10.6 | 145 | 33.4 ± 5.7 | 170 | 1.78 ± 1.0 |
| No | 144 | 3.05 ± 1.2 | 145 | 14.5 ± 7.3 | 114 | 32.3 ± 3.8 | 147 | 1.81 ± 1.2 |
| P = 0.27 | P < 0.0001 | P = 0.07 | P = 0.76 | |||||
Values are ns or means ± SDs unless otherwise indicated. P values from ANOVA test. HS, high school; PUQE, Pregnancy-Unique Quantification of Emesis and Nausea Index; WIC, Women, Infants, and Children nutritional program.
BMI (in kg/m2) categories are as follows: underweight (<18.5); normal (18.5 ≤ BMI < 25); overweight (25 ≤ BMI < 30); obese I (30 ≤ BMI < 35); obese II (35 ≤ BMI < 40); obese III (≥40)
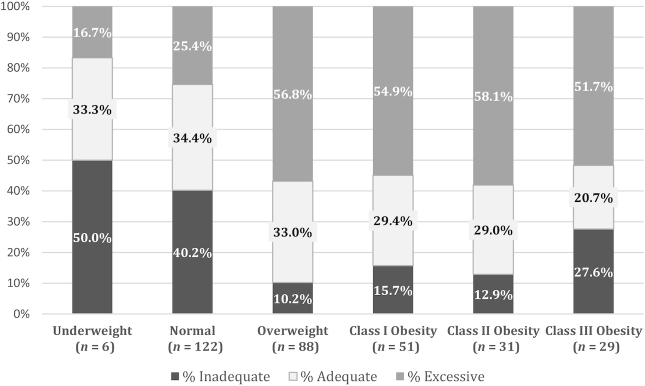
As Figure 2 shows, the majority of overweight and obese women gained in excess of the IOM/NRC guidelines, with the highest percentage of excessive GWG (58.1%) among women in the Class II obesity category (chi-square P < 0.0001). Overall, the prevalence of excessive GWG was higher among overweight or obese women than among underweight women or women in the normal BMI range. Likewise, the percentage of pregnant women with adequate GWG tended to decrease with increasing maternal prepregnancy BMI, with 34.4% of women in the normal BMI range gaining adequate GWG compared with just 20.7% of women in the Class III obese category prepregnancy. Inadequate GWG was most prominent among women with prepregnancy BMI in the underweight (50.0%) and normal (40.2%) ranges. However, women in the severe obesity III class were more likely to gain inadequately (according to the IOM recommendation) than women in the overweight or obesity Class I or II categories.
FIGURE 2.
Gestational weight gain adequacy in the Archive for Research on Child Health cohort according to the 2009 Institute of Medicine/National Research Council guidelines.
Associations of maternal dietary components with GWG
We did not observe any significant associations between dietary components and inadequate GWG in minimally adjusted or multivariable-adjusted logistic models (Table 2). Likewise, added sugar, percentage energy from fat, and dairy were not associated with excessive GWG in models. Women who consumed more fruits and vegetables were suggestively less likely to have excessive GWG (OR: 0.86; 95% CI: 0.75, 1.00) in the minimally adjusted model, but the association became nonsignificant after adjusting for covariates (OR: 0.89; 95% CI: 0.77, 1.03). In sensitivity analyses, associations of fruit and vegetable consumption with GWG were similar using the modified fruit and vegetable variable which excluded French fry consumption (e.g., for multivariable-adjusted association with excessive GWG: OR: 0.89; 95% CI: 0.76, 1.03; data not shown). Although formal tests for interaction were not statistically significant (interaction P ≥ 0.20), stratified analyses suggested that associations of diet with GWG may differ by maternal prepregnancy BMI. For example, higher added sugar intake was linked to a slight reduction in likelihood of excessive GWG (OR: 0.91; 95% CI: 0.84, 0.99) among women with a prepregnancy BMI in the normal range (Table 2). Among women with obesity, higher fruit and vegetable consumption was associated with a 23% reduction in the odds of excessive GWG (OR: 0.77; 95% CI: 0.60, 0.97). Dietary components were not significantly associated with GWG in the linear models. Similarly, we did not observe any significant associations between dietary components and GWG in models stratified by maternal smoking history (interaction P ≥ 0.12 for smoking) (Supplemental Table 1).
TABLE 2.
Multivariable regression models for the associations between dietary indicators and GWG in the Archive for Research on Child Health cohort1
| Inadequate GWG | Excessive GWG | Minimally adjusted effect estimate for total weight gain in kg (95% CI) | Multivariable-adjusted effect estimate for total weight gain in kg (95% CI) | |||
|---|---|---|---|---|---|---|
| Minimally adjusted model, OR (95% CI) | Multivariable-adjusted model, OR (95% CI) | Minimally adjusted model, OR (95% CI) | Multivariable-adjusted model, OR (95% CI) | |||
| Overall | ||||||
| Fruits and vegetables | 0.85 (0.70, 1.03) | 0.85 (0.70, 1.04) | 0.86 (0.75, 1.00) | 0.89 (0.77, 1.03) | −0.68 (−1.60, 0.25) | −0.50 (−1.43, 0.44) |
| Added sugar | 0.98 (0.95, 1.02) | 0.98 (0.94, 1.02) | 1.00 (0.97, 1.02) | 0.98 (0.96, 1.01) | 0.06 (−0.10, 0.23) | −0.01 (−0.18, 0.17) |
| % energy from fat | 0.98 (0.92, 1.05) | 0.98 (0.91, 1.05) | 0.98 (0.93, 1.04) | 0.97 (0.91, 1.03) | 0.19 (−0.17, 0.55) | 0.03 (−0.33, 0.39) |
| Dairy | 1.03 (0.79, 1.35) | 0.99 (0.75, 1.31) | 0.93 (0.73, 1.18) | 1.00 (0.77, 1.29) | 0.32 (−1.10, 1.70) | 0.35 (−1.09, 1.80) |
| Normal BMI | ||||||
| Fruits and vegetables | 0.80 (0.59, 1.08) | 0.81 (0.59, 1.10) | 0.68 (0.46, 0.98) | 0.73 (0.49, 1.07) | −0.92 (−2.30, 0.45) | −0.66 (−2.04, 0.72) |
| Added sugar | 0.98 (0.94, 1.02) | 0.96 (0.91, 1.02) | 0.97 (0.93, 1.02) | 0.91 (0.84, 0.99) | 0.05 (−0.16, 0.27) | −0.12 (−0.37, 0.14) |
| % energy from fat | 1.00 (0.89, 1.12) | 1.00 (0.89, 1.12) | 0.98 (0.87, 1.11) | 0.89 (0.76, 1.03) | 0.07 (−0.43, 0.58) | −0.22 (−0.75, 0.30) |
| Dairy | 1.01 (0.72, 1.43) | 1.03 (0.72, 1.48) | 0.65 (0.39, 1.08) | 0.67 (0.39, 1.15) | −0.72 (−2.4, 1.0) | −0.52 (−2.22, 1.18) |
| Overweight | ||||||
| Fruits and vegetables | 0.86 (0.37, 2.04) | 0.93 (0.38, 2.27) | 1.29 (0.92, 1.80) | 1.35 (0.93, 1.96) | 0.43 (−1.9, 2.8) | 0.76 (−1.7, 3.2) |
| Added sugar | 0.97 (0.87, 1.07) | 0.96 (0.86, 1.07) | 1.01 (0.97, 1.06) | 1.00 (0.96, 1.05) | 0.13 (−0.24, 0.49) | 0.10 (−0.29, 0.48) |
| % energy from fat | 0.73 (0.52, 1.02) | 0.64 (0.41, 1.00) | 0.99 (0.88, 1.12) | 0.99 (0.87, 1.13) | 0.85 (−0.07, 1.80) | 0.82 (−0.14, 1.78) |
| Dairy | 0.94 (0.47, 1.89) | 0.90 (0.42, 1.93) | 0.98 (0.64, 1.48) | 1.03 (0.66, 1.59) | −0.07 (−3.4, 3.2) | 0.50 (−3.1, 4.1) |
| Obese | ||||||
| Fruits and vegetables | 0.75 (0.53, 1.08) | 0.71 (0.48, 1.03) | 0.75 (0.59, 0.95) | 0.77 (0.60, 0.97) | −0.94 (−2.40, 0.52) | −0.95 (−2.44, 0.55) |
| Added sugar | 1.00 (0.93, 1.08) | 1.03 (0.94, 1.13) | 0.99 (0.95, 1.04) | 1.00 (0.95, 1.06) | 0.04 (−0.29, 0.36) | 0.03 (−0.31, 0.37) |
| % energy from fat | 0.98 (0.84, 1.15) | 1.03 (0.84, 1.27) | 0.99 (0.91, 1.09) | 1.01 (0.92, 1.12) | 0.07 (−0.60, 0.74) | −0.01 (−0.71, 0.68) |
| Dairy | 0.95 (0.47, 1.95) | 1.17 (0.50, 2.72) | 1.15 (0.76, 1.75) | 1.31 (0.82, 2.09) | 1.91 (−0.86, 4.70) | 1.51 (−1.41, 4.43) |
1Multivariable model for logistic regression and linear regression models both adjusted for weeks of gestation at delivery, physical activity, prepregnancy BMI, maternal age, educational level, fetal sex, and marital status. P values for interaction by maternal prepregnancy BMI for inadequate GWG: by fruits/vegetables, Pint = 0.88; added sugar, Pint = 0.20; percentage energy from fat, Pint = 0.79; and dairy, Pint = 0.85. P values for interaction by maternal prepregnancy BMI for excessive GWG: by fruits/vegetables, Pint = 0.32; added sugar, Pint = 0.25; percentage energy from fat, Pint = 0.22; and dairy, Pint = 0.22. GWG, gestational weight gain.
Discussion
In this prospective cohort study, higher fruit and vegetable consumption was associated with a reduced likelihood of excessive GWG only among women with obesity. Among women with a prepregnancy BMI in the normal range, higher added sugar intake was associated with slightly lower odds of excessive GWG. Percentage energy from fat and dairy consumption were not associated with GWG in this study. These findings suggest that consuming fruits and vegetables during pregnancy may reduce the risk of excessive GWG in high-risk populations of women with prepregnancy obesity, although future epidemiologic studies are needed to confirm these findings. Given that 41% of women of reproductive age in the United States were overweight or obese in 2016 (17), increasing fruit and vegetable consumption during pregnancy may be an impactful strategy for reducing GWG and GWG-related health complications for mother and infant.
Women in this study had dietary intakes that were generally comparable with those of other US women. For example, an analysis of NHANES data from 2007–2010 reported that women aged 19–50 y consumed ∼1.4 servings of dairy per day, compared with 1.7 daily servings of dairy in our study (28). In a recent analysis from NHANES 2001–2014 data, pregnant women consumed 21.2 teaspoons of added sugars daily and 33.0% energy from fat (29), which are also comparable with the mean 17.1 teaspoons of added sugar daily and 33.0% energy from fat observed in our study population. Finally, women in our study consumed a mean of 3.14 cup equivalents of fruits and vegetables daily, which is slightly higher than the 2.5 daily cup mean consumption among US adults in NHANES data from 2013–2016 (30). Adherence to the IOM GWG recommendations in our study was in line with national trends, but more women in our study had inadequate gain and fewer women had excessive weight than other US women. Specifically, in our study, 24.8% of women had inadequate gain, 31.5% had adequate gain, and 43.7% excessive GWG, whereas of other women in the United States, 20.4% had inadequate, 32.1% had adequate, and 47.5% had excessive GWG (14). We observed a similar pattern when comparing the women in our study with those in Michigan in 2012–2013, 19.2% of whom had inadequate gain, 30.6% had adequate gain, and 50.3% had excessive gain (14). In our study, women with overweight and obesity were more likely to gain in excess of the IOM/NRC guidelines, which is similar to what has been reported in other studies (13, 14). Moreover, our findings that nearly 30% of women with class III obesity achieved inadequate GWG were similar to the 32% reported among US women with class III obesity overall (13, 14).
Associations of fruit and vegetable intake with maternal GWG
The major finding from our study is that higher consumption of fruits and vegetables is associated with a lower likelihood of excessive GWG, but only in women with obesity. Thus far, there have been mixed findings on associations of fruit/vegetable intakes and GWG from observational studies (31–34). For example, similarly to our study, a large prospective study of 595 pregnant women in the United States observed inverse associations of fruit and vegetable consumption with GWG (34). Likewise, vegetable, but not fruit intake, was inversely associated with GWG in a cross-sectional analysis of 490 pregnant NHANES participants (32). However, fruit and vegetable consumption was not linked to GWG in other prospective studies (31, 33). Although our study did not evaluate diet quality specifically, high fruit and vegetable consumption is consistently associated with higher diet quality (35); and diet quality has been linked with reduced GWG in several (32, 36–38), but not all (39–41) prior studies. The underlying cause of these discrepancies between fruit/vegetable consumption or diet quality and GWG is unclear. However, our findings suggest that diet quality or the consumption of fruits and vegetables may be especially important in vulnerable women. Therefore, future studies should consider maternal prepregnancy BMI, and potentially other health and lifestyle factors, when evaluating associations between fruit/vegetable intake and GWG.
Associations of dairy, added sugar, and fat consumption with GWG
In our study, consumption of dairy foods was not associated with GWG, which is discordant with findings from previous studies. For example, total dairy intake was associated with increased GWG and higher odds of excessive GWG in Project Viva participants (31), although excess calorie intake was shown to mediate part of the associations. Similarly, dairy consumption was also linked to excessive gain in several other observational studies (31, 42, 43). It is possible that the dissimilarities between these studies and ours are due to sociodemographic differences or differences in the instruments used to assess dairy intake. For example, women in our study were primarily non-Hispanic white, but with a low family income, which is a unique population compared with previous studies. In addition, the dairy category in our dietary assessment tool only considered milk and cheese, which differs from previous studies that included other dairy products such as yogurt, ice cream, cottage cheese, and cream cheese. Therefore, additional studies are needed to understand whether associations between dairy consumption and GWG are unique to certain populations and to specific types of dairy foods.
In previous studies, sweets, processed food (31, 32, 42), sweetened beverages (44, 45), snacks, fish, and bread (45) have been shown to be associated with excessive GWG. In this study, however, we observed a suggested inverse association between added sugar intake and GWG in normal-weight women, and no association in overweight or obese women. Whereas this finding conflicts with data from nonpregnant populations linking sugar-sweetened beverages with increased obesity risk (34), it is consistent with findings from the Project Viva pregnancy cohort (31), which also reported a slight trend toward an inverse relation between the consumption of sugar-sweetened beverages and GWG (31). These findings may partially reflect observed inverse associations of carbohydrate intake with GWG (46), although future studies are needed to understand whether and how associations may vary by maternal prepregnancy BMI.
In our study, we also did not observe associations between percentage energy from fat and GWG. Whereas higher overall energy intake is associated with increased GWG (47), findings for associations between fat intake and GWG are mixed. In 1 study, percentage energy from monounsaturated fat was inversely related with risk of excess GWG, whereas percentage energy from protein, saturated fat, polyunsaturated fat, and trans-fat was positively associated with excess gain—but CIs for these associations were wide (31). However, a randomized controlled trial of 46 pregnant overweight or obese US women compared low-fat (25% energy from fat) with low-carbohydrate (35% energy from fat) diets and observed no significant differences in GWG (48).
The association between GWG and adverse health outcomes differs according to prepregnancy BMI, with more optimal outcomes observed with lower weight gains for women with higher BMI (13). Our data extend previous findings on the role of diet in GWG by examining differences in associations by maternal prepregnancy BMI category. Moreover, in this study we characterized dietary intake according to categories of maternal prepregnancy BMI (including within classes of obesity) and evaluated GWG adequacy across all obesity categories. This is an important strength because the 2009 GWG guidelines did not distinguish GWG recommendations within obesity categories because of the lack of data in the published literature (at the time) for women with BMIs ≥ 35 (49). Similarly, few studies have specifically evaluated associations of diet with GWG in women with class II and III obesity, making it difficult to identify which interventions are most effective in this subgroup. Thus, our analysis attempts to provide preliminary data that could contribute to informing future recommendations for GWG for the growing number of morbidly obese adults in the United States.
Strengths and limitations
Strengths of this study include the prospective assessment of diet and the use of a validated dietary screener tool. In addition, we were able to assess potential heterogeneity in associations of diet and GWG by mother's prepregnancy BMI and were able to account for multiple independent predictors of GWG in our model. Nevertheless, residual confounding by unmeasured health behaviors is possible, particularly given the clustering of unhealthy habits (50), although the multivariable model adjusted for measured confounders did not alter results materially compared with the minimally adjusted model. This study relied on self-reported dietary assessment, and differential self-report, particularly among women with obesity, is possible (51). In addition, the reliance on a minimal set of dietary food groups precluded our ability to assess overall dietary quality or patterns, or to adjust for total energy intake. However, dietary recommendations based on food groups may be more easily amenable than empirically derived dietary patterns. Although we were not statistically powered to stratify models or evaluate statistical interaction in associations across obesity classes in this study, we plan on following up on these preliminary analyses in our larger cohort study being conducted as part of the ongoing US ECHO program. Finally, owing to the large number of associations examined as part of this study, we cannot rule out the possibility of observing several observations by chance.
Conclusions
In conclusion, higher fruit and vegetable consumption during pregnancy among women with obesity may be linked to a lower risk of excessive GWG and, thereby, improved maternal and child health outcomes. Once GWG recommendations are available for class-specific obesity categories, larger studies may be needed to evaluate these associations using the new recommendations.
Supplementary Material
ACKNOWLEDGEMENTS
With much appreciation to the ARCH and CHARM (Child Health Advances from Research with Mother) study team at Michigan State University (MSU) and all the mothers and children who participated from the following clinics in Michigan; MSU College of Human Medicine Department of Obstetrics and Gynecology Faculty practice, Sparrow/MSU Obstetrics and Gynecology Residency Clinic, Ingham County Health Department Community Health Center - Women's Health, Wayne State University Physician Group, Hurley Residency Clinic, Cherry Street Health Center, Grand Traverse Women's Clinic. The authors’ responsibilities were as follows—KAH: analyzed the data and wrote the paper; and all authors: designed the research and read and approved the final manuscript.
Notes
Supported in part by the Michigan State University (MSU) Office of the Vice President for Research, MSU College of Human Medicine, MSU Center for Research in Autism, Intellectual and Neurodevelopmental Disabilities, USDA National Institute of Food and Agriculture and Michigan AgBioResearch, and NIH Environmental influences on Child Health Outcomes (ECHO) program grants UG3 OD023285 and UH3 OD023285. ECHO is a nationwide research program supported by the NIH, Office of the Director, to enhance child health.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/cdn/.
Abbreviations used: ARCH, Archive for Research on Child Health; ECHO, Environmental influences on Child Health Outcomes; GWG, gestational weight gain; IOM, Institute of Medicine; IRB, Institutional Review Board; NRC, National Research Council; PUQE, Pregnancy-Unique Quantification of Emesis and Nausea Index; WIC, Women, Infants, & Children nutritional program.
Contributor Information
Kelly A Hirko, Email: khirko@epi.msu.edu, Department of Epidemiology and Biostatistics, College of Human Medicine, Michigan State University, East Lansing, MI, USA.
Sarah S Comstock, Department of Food Science and Human Nutrition, Michigan State University, East Lansing, MI, USA.
Rita S Strakovsky, Department of Food Science and Human Nutrition, Michigan State University, East Lansing, MI, USA.
Jean M Kerver, Department of Epidemiology and Biostatistics, College of Human Medicine, Michigan State University, East Lansing, MI, USA.
References
- 1. Herring SJ, Oken E. Weight gain during pregnancy: importance for maternal and child health. Ann Nestlé. 2010;68:17–28. [Google Scholar]
- 2. Harrison CL. Gestational weight gain and its association with infant birth weight. Obesity. 2017;25:1468–9. [DOI] [PubMed] [Google Scholar]
- 3. Sridhar SB, Darbinian J, Ehrlich SF, Markman MA, Gunderson EP, Ferrara A, Hedderson MM. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014;211:259.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kominiarek MA, Peaceman AM. Gestational weight gain. Am J Obstet Gynecol. 2017;217:642–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines Weight gain during pregnancy: reexamining the guidelines. Rasmussen KM, Yaktine AL, editors. Washington (DC): National Academies Press (US); 2009. [PubMed] [Google Scholar]
- 6. Fraser A, Tilling K, Macdonald-Wallis C, Hughes R, Sattar N, Nelson SM, Lawlor DA. Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the Avon Longitudinal Study of Parents and Children (ALSPAC). Am J Clin Nutr. 2011;93:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siega-Riz AM, Viswanathan M, Moos M-K, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009;201:339.e1–14. [DOI] [PubMed] [Google Scholar]
- 8. Mannan M, Doi SAR, Mamun AA. Association between weight gain during pregnancy and postpartum weight retention and obesity: a bias-adjusted meta-analysis. Nutr Rev. 2013;71:343–52. [DOI] [PubMed] [Google Scholar]
- 9. Ensenauer R, Chmitorz A, Riedel C, Fenske N, Hauner H, Nennstiel-Ratzel U, von Kries R. Effects of suboptimal or excessive gestational weight gain on childhood overweight and abdominal adiposity: results from a retrospective cohort study. Int J Obes. 2013;37:505–12. [DOI] [PubMed] [Google Scholar]
- 10. Dello Russo M, Ahrens W, De Vriendt T, Marild S, Molnar D, Moreno LA, Reeske A, Veidebaum T, Kourides YA, Barba G et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: the IDEFICS project. Int J Obes (Lond). 2013;37:914–19. [DOI] [PubMed] [Google Scholar]
- 11. Hull HR, Thornton JC, Ji Y, Paley C, Rosenn B, Mathews P, Navder K, Yu A, Dorsey K, Gallagher D. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Voerman E, Santos S, Patro Golab B, Amiano P, Ballester F, Barros H, Bergström A, Charles M-A, Chatzi L, Chevrier C et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: an individual participant data meta-analysis. PLoS Med. 2019;16:e1002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siega-Riz AM, Bodnar LM, Stotland NE, Stang J. The current understanding of gestational weight gain among women with obesity and the need for future research. A National Academy of Medicine Discussion Paper. Washington (DC): National Academy of Medicine; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deputy NP, Sharma AJ, Kim SY. Gestational weight gain – United States, 2012 and 2013. MMWR Morb Mortal Wkly Rep. 2015;64:1215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanentsapf I, Heitmann BL, Adegboye ARA. Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy Childbirth. 2011;11:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tobias DK, Bao W. Diet during pregnancy and gestational weight gain. Curr Nutr Rep. 2014;3:289–97. [Google Scholar]
- 17. Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and extreme obesity among adults aged 20 and over: United States, 1960–1962 through 2013–2014. National Center for Health Statistics, Health E-Stats. Hyattsville, MD: National Center for Health Statistics; 2016. [Google Scholar]
- 18. Hamilton CM, Strader LC, Pratt JG, Maiese D, Hendershot T, Kwok RK, Hammond JA, Huggins W, Jackman D, Pan H et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174:253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hendershot T, Pan H, Haines J, Harlan WR, Marazita ML, McCarty CA, Ramos EM, Hamilton CM. Using the PhenX Toolkit to add standard measures to a study. Curr Protoc Hum Genet. 2015;86:1.21.1–1.21.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maiese DR, Hendershot TP, Strader LC, Wagener DK, Hammond JA, Huggins W, Kwok RK, Hancock DB, Whitehead NS, Nettles DS et al. PhenX—establishing a consensus process to select common measures for collaborative research. Research Triangle Park, NC: RTI Press; 2013. [PubMed] [Google Scholar]
- 21. National Cancer Institute (NCI) Five-factor screener: National Health Interview Survey (NHIS) validation results. Bethesda, MD: NCI; 2005. [Google Scholar]
- 22. Institute of Medicine Weight gain during pregnancy: reexamining the guidelines. Washington (DC): National Academies Press; 2009. [PubMed] [Google Scholar]
- 23. Koren G, Boskovic R, Hard M, Maltepe C, Navioz Y, Einarson A. Motherisk—PUQE (pregnancy-unique quantification of emesis and nausea) scoring system for nausea and vomiting of pregnancy. Am J Obstet Gynecol. 2002;186:S228–31. [DOI] [PubMed] [Google Scholar]
- 24. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. [DOI] [PubMed] [Google Scholar]
- 25. Shin D, Chung H, Weatherspoon L, Song WO. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J. 2014;18:1667–74. [DOI] [PubMed] [Google Scholar]
- 26. Martin CL, Siega-Riz AM, Sotres-Alvarez D, Robinson WR, Daniels JL, Perrin EM, Stuebe AM. Maternal dietary patterns during pregnancy are associated with child growth in the first 3 years of life. J Nutr. 2016;146:2281–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Deputy NP, Sharma AJ, Kim SY, Hinkle SN. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet Gynecol. 2015;125:773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quann EE, Fulgoni VL, Auestad N. Consuming the daily recommended amounts of dairy products would reduce the prevalence of inadequate micronutrient intakes in the United States: diet modeling study based on NHANES 2007–2010. Nutr J. 2015;14:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey RL, Pac SG, Fulgoni VL, Reidy KC, Catalano PM. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw Open. 2019;2:e195967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hoy MK, Clemens JC, Martin CL, Moshfegh AJ. Fruit and vegetable consumption of US adults by level of variety, What We Eat in America, NHANES 2013–2016. Curr Dev Nutr. 2020;4, doi: 10.1093/cdn/nzaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stuebe AM, Oken E, Gillman MW. Associations of diet and physical activity during pregnancy with risk for excessive gestational weight gain. Am J Obstet Gynecol. 2009;201:58.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shin D, Bianchi L, Chung H, Weatherspoon L, Song WO. Is gestational weight gain associated with diet quality during pregnancy?. Matern Child Health J. 2014;18:1433–43. [DOI] [PubMed] [Google Scholar]
- 33. Herring SJ, Nelson DB, Davey A, Klotz AA, Dibble LV, Oken E, Foster GD. Determinants of excessive gestational weight gain in urban, low-income women. Womens Health Issues. 2012;22:e439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olson CM, Strawderman MS. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J Am Diet Assoc. 2003;103:48–54. [DOI] [PubMed] [Google Scholar]
- 35. Wallace TC, Bailey RL, Blumberg JB, Burton-Freeman B, Chen C-YO, Crowe-White KM, Drewnowski A, Hooshmand S, Johnson E, Lewis R et al. Fruits, vegetables, and health: a comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit Rev Food Sci Nutr. 2020;60:2174–211. [DOI] [PubMed] [Google Scholar]
- 36. Muktabhant B, Lawrie TA, Lumbiganon P, Laopaiboon M. Diet or exercise, or both, for preventing excessive weight gain in pregnancy. Cochrane Database Syst Rev. 2015(6):CD007145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hillesund ER, Bere E, Haugen M, Øverby NC. Development of a New Nordic Diet score and its association with gestational weight gain and fetal growth – a study performed in the Norwegian Mother and Child Cohort Study (MoBa). Public Health Nutr. 2014;17:1909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ancira-Moreno M, Vadillo-Ortega F, Rivera-Dommarco JÁ, Sánchez BN, Pasteris J, Batis C, Castillo-Castrejón M, O'Neill MS. Gestational weight gain trajectories over pregnancy and their association with maternal diet quality: results from the PRINCESA cohort. Nutrition. 2019;65:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc. 2009;109:1004–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tielemans MJ, Erler NS, Leermakers ETM, van den Broek M, Jaddoe VWV, Steegers EAP, Kiefte-de Jong JC, Franco OH. Apriori and a posteriori dietary patterns during pregnancy and gestational weight gain: the Generation R study. Nutrients. 2015;7:9383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yong HY, Mohd Shariff Z, Mohd Yusof BN, Rejali Z, Tee YYS, Bindels J, van der Beek EM. Pre-pregnancy BMI influences the association of dietary quality and gestational weight gain: the SECOST Study. Int J Environ Res Public Health. 2019;16:3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olafsdottir AS, Skuladottir GV, Thorsdottir I, Hauksson A, Steingrimsdottir L. Maternal diet in early and late pregnancy in relation to weight gain. Int J Obes. 2006;30:492–9. [DOI] [PubMed] [Google Scholar]
- 43. Olsen SF, Halldorsson TI, Willett WC, Knudsen VK, Gillman MW, Mikkelsen TB, Olsen J, NUTRIX Consortium . Milk consumption during pregnancy is associated with increased infant size at birth: prospective cohort study. Am J Clin Nutr. 2007;86:1104–10. [DOI] [PubMed] [Google Scholar]
- 44. Guilloty NI, Soto R, Anzalota L, Rosario Z, Cordero JF, Palacios C. Diet, pre-pregnancy BMI, and gestational weight gain in Puerto Rican women. Matern Child Health J. 2015;19:2453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bärebring L, Brembeck P, Löf M, Brekke HK, Winkvist A, Augustin H. Food intake and gestational weight gain in Swedish women. Springerplus. 2016;5:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lagiou P, Tamimi RM, Mucci LA, Adami H-O, Hsieh C-C, Trichopoulos D. Diet during pregnancy in relation to maternal weight gain and birth size. Eur J Clin Nutr. 2004;58:231–7. [DOI] [PubMed] [Google Scholar]
- 47. Institute of Medicine (US) Committee on Nutritional Status during Pregnancy and Lactation Nutrition during pregnancy: part I weight gain: part II nutritional supplements. Washington (DC): National Academies Press; 1990. [PubMed] [Google Scholar]
- 48. Rhodes ET, Pawlak DB, Takoudes TC, Ebbeling CB, Feldman HA, Lovesky MM, Cooke EA, Leidig MM, Ludwig DS. Effects of a low-glycemic load diet in overweight and obese pregnant women: a pilot randomized controlled trial. Am J Clin Nutr. 2010;92:1306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pronk NP, Anderson LH, Crain AL, Martinson BC, O'Connor PJ, Sherwood NE, Whitebird RR. Meeting recommendations for multiple healthy lifestyle factors. Prevalence, clustering, and predictors among adolescent, adult, and senior health plan members. Am J Prev Med. 2004;27:25–33. [DOI] [PubMed] [Google Scholar]
- 51. Heitmann BL, Lissner L. Dietary underreporting by obese individuals–is it specific or non-specific?. BMJ. 1995;311:986–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.