Abstract
Background
Oral Submucous Fibrosis (OSMF) is a potentially malignant disorder and burning sensation is the initial complaint among these patients. Scientific literature has established that mast cells play a major role in various inflammatory disorders. However, OSMF being an inflammatory disorder, the role of mast cells is yet to be established. Hence the aim of this study was to evaluate mast cells and burning sensation in various stages of Oral Submucous Fibrosis.
Methods
The study population comprised of forty subjects, thirty were clinically confirmed cases of OSMF and ten healthy individuals served as control. A complete history and clinical examination followed by an incisional biopsy was performed. Samples obtained were subjected to routine histopathological examination and mast cells evaluation.
Results
Mean number of total mast cells in Stage I, II and III OSMF were 8.5 ± 0.7, 11.31 ± 8.8 and 24.7 ± 21.2 respectively. There was a significant difference in total mast cell count between cases and controls. Degranulated mast cells was a significant predictor (p = 0.028), indicating role in clinical staging of OSMF. Degranulated mast cells had a significant role and was a positive predictor (B = 0.763, OR 2.145[95%CI 1.055–4.630]) at moderate levels of burning sensation. A significant difference (p = 0.029) in burning sensation across histopathological grades was also observed in the study.
Conclusion
Degranulated mast cells were found to have a significant influence in mild to moderate levels of burning sensation among OSMF patients. Role of degranulated mast cells were also found to be significant in various clinical stages of OSMF.
Keywords: Mast cells, Oral submucous fibrosis, Regression analysis, Toluidine blue
1. Introduction
Oral Submucous Fibrosis is a chronic inflammatory disorder predominant in Southeast Asian region.1 The prevalence of OSMF in India has increased from 0.1% to 30%.2 The clinical definition of OSMF by Chandramani More and Naman Rao (2019) as ‘a debilitating, progressive, irreversible collagen metabolic disorder induced by chronic chewing of areca nut and its commercial preparations; affecting the oral mucosa and occasionally the pharynx and oesophagus; leading to mucosal stiffness and functional morbidity; and has a potential risk of malignant transformation.3 Burning sensation is an early complaint in patients with OSMF.4 Literature has established the role of mast cells in various inflammatory disorders such as pulpitis, periapical inflammation, gingivitis and oral lichen planus.5, 6, 7 However role of mast cell in burning sensation of OSMF is still being evaluated across various population subsets of the region.
Several theories have been postulated regarding the etiopathogenesis of OSMF. Areca nut and its various commercial preparations has been the widely accepted theory.8 The alkaloid components of areca nut such as arecoline, arecaidine, guvacoline, guvacine stimulate fibroblasts in the connective tissue to produce collagen. The flavanoids component inhibits collagenase and thereby decreases collagen degradation leading to fibrosis.9
The pathological definition of OSMF was given by Jens J. Pindborg and Satyavati M. Sirsat (1966) as ‘an insidious chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with a juxta-epithelial inflammatory reaction followed by a fibro-elastic change of the lamina propria, with epithelial atrophy leading to stiffness’.10
The objectives of this study were (1) Mast cell estimation among cases in different stages of OSMF. (2) Comparison of mast cells between case and control. (3) To find predictors among mast cells in different stages of OSMF. (4) To find predictors among intact, degranulated mast cells for burning sensation. (5) To find significance of burning sensation on clinical staging and histopathological grading.
2. Material and methods
The present study was conducted in the dental outpatient Department of Oral Medicine and Radiology. Prior to enrolling the subjects, ethical clearance was obtained from the university. The inclusion criteria comprised of subjects above 20 years of age, irrespective of gender and those with a habit of areca nut chewing. Subjects who reportedly underwent treatment for OSMF previously, as well as subjects with systemic conditions were excluded from the study. A total of forty subjects comprised the study population; of these thirty subjects were clinically confirmed cases of OSMF and ten subjects presenting with a clinically normal mucosa along with no history of adverse habits comprised the control group.
Informed consent was obtained from each subject after explaining the procedure and outcomes in detail. A complete case history including medical history was recorded with an emphasis on their adverse habits (areca nut and its combination). Burning sensation was recorded using a self-assessment descriptive rating as mild, moderate and severe.11 This was followed by a clinical examination to record the features such as blanching of oral mucosa, restricted mouth opening (in mm) and palpable vertical fibrotic bands (Fig. 1). The subjects were categorized clinically into three stages based on mouth opening as follows:12
Fig. 1.
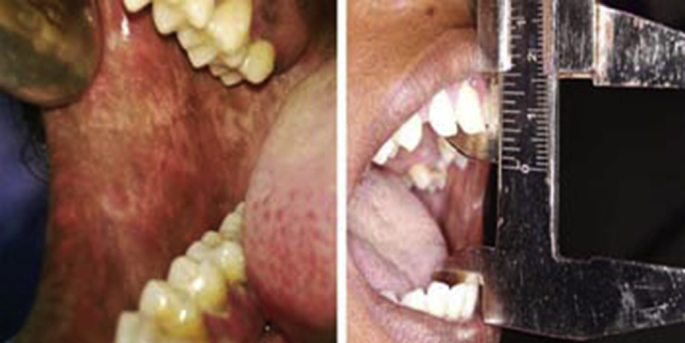
Clinical presentation of OSMF.
Stage I- Mouth opening ≥45 mm.
Stage II- Mouth opening 20–44 mm.
Stage III- Mouth opening ≤20 mm.
Routine complete blood count was performed for all the subjects. On confirming with the normal blood report, biopsy was performed. An incisional biopsy approximately 1 × 1 cm was done under local anaesthesia for clinically diagnosed cases of OSMF. Ten samples of normal oral mucosa were obtained from the surgical site of an asymptomatic impacted tooth. The tissue sample collected was divided into two sections; one section was stained with Hematoxylin and Eosin, the other section of tissue sample was stained using 1% toluidine blue for evaluation of mast cells.13
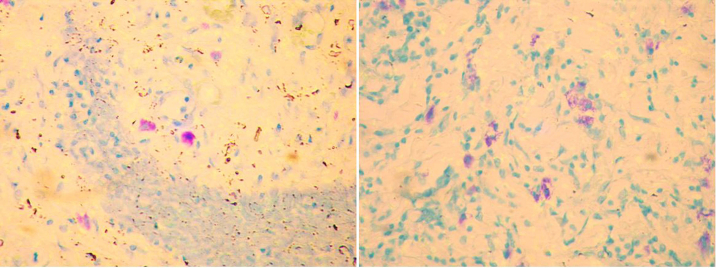
Evaluation: On histological confirmation of OSMF, it was classified into mild, moderate and severe.14 Mast cells were identified in 100× magnification and counted using an occulometer grid field under a magnification of 400× by a step ladder fashion. This was expressed as number of cells per square millimetre (Fig. 2). Mast cells are spindle to oval shaped cells and their granules are purplish red in colour. The nuclei of mast cells appear blue in colour.
Fig. 2.
Photomicrograph showing intact and degranulated mast cells at X400 power.
Statistical analysis: The data obtained was transferred to an MS Excel sheet, and statistical analysis was carried out using statistical package for social sciences (version 16.0 for Windows, SPSS Inc., Chicago, IL, USA). In the study, the independent variable was measured on continuous scale (Mast cells) and the dependent variable (clinical staging and burning sensation) were on ordinal scale. Hence an apt statistical application would be ordinal regression. Where the variables did not satisfy the ordinal regression fit, multinomial regression was adopted. To assess histopathological and clinical significance with burning Kruskal Wallis H test (since both are ordinal scale) was used along with other descriptive statistics and Independent sample t-test separately.
3. Results
The mean age was 35.0 ± 14.1 and 37.0 ± 9.9 among cases and controls respectively. There was a male predominance observed among the cases with a male-to-female ratio of 4:1. Mean of total mast cells in Stage I, II and III were 8.5 ± 0.7, 11.31 ± 8.8 and 24.7 ± 21.2 respectively. There was significant difference (p < 0.05) in intact, degranulated and total number of mast cells between case and control analysed using unpaired t-test (Table 1).
Table 1.
Mast cell estimation in various stages of OSMF and comparison between case and control using unpaired t-test.
| Stage I (Mean ± S.D) | Stage II (Mean ± S.D) | Stage III (Mean ± S.D) | |||||
|---|---|---|---|---|---|---|---|
| Intact mast Cells | 7.5 ± 0.7 | 7.9 ± 8.8 | 15.7 ± 14.5 | ||||
| Degranulated mast cells | 1 ± 0.0 | 3.4 ± 3.1 | 9.0 ± 7.9 | ||||
| Total No. of Mast cells | 8.5 ± 0.7 | 11.3 ± 8.8 | 24.7 ± 21.2 | ||||
| Parameter Estimate | |||||||
| Group |
N |
Mean |
Std. Deviation |
Std. Error |
t-value |
p-Value |
|
| Intact mast Cells | Case | 30 | 9.7 | 10.4 | 1.9 | 2.74 | 0.01* |
| Control | 10 | 3.7 | 3.1 | 1.0 | |||
| Degranulated mast cells | Case | 30 | 4.6 | 5.0 | 0.9 | 4.57 | 0.01* |
| Control | 10 | 0.4 | 0.2 | 0.0 | |||
| Total No. of Mast cells | Case | 30 | 14.3 | 13.5 | 2.4 | 3.80 | 0.01* |
| Control | 10 | 4.2 | 3.2 | 1.0 | |||
Inference: Statistical result using unpaired t-test revealed a significant difference in intact, degranulated and total number of mast cells between case and control.
*p-value < 0.05.
The study variables satisfied the assumptions for ordinal regression analysis (Table 2); to analyse role of intact mast cells and degranulated cells in clinical staging and burning sensation associated with OSMF. Model fitting (p = 0.013) indicates overall fit of the model. Among mast cells, parameter estimates of ordinal regression highlighted the role of degranulated mast cell as a positive coefficient and significant positive predictor (clinical staging B = 0.277, p = 0.028, burning sensation B = 0.205, p = 0.034). Test of parallel lines assumption was satisfied for clinical staging p = 0.267 where as this assumption was not satisfied for burning sensation p = 0.002 under ordinal regression. Hence multinomial logistic regression was adopted to further establish the influence of mast cells on burning sensation.
Table 2.
Ordinal regression to analyse role mast cells in clinical staging and multinomial regression to analyse role of mast cells in burning sensation.
| Parameter Estimates (Ordinal regression) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Std. Error | df | Sig. (p-Value) | 95% Confidence Interval |
||||||
| Lower Bound | Upper Bound | |||||||||
| Threshold | Stage I | −1.445 | 0.909 | 1 | 0.112 | −3.226 | 0.337 | |||
| Stage II | 3.109 | 1.054 | 1 | 0.003 | 1.043 | 5.176 | ||||
| Location | Intact mast cells | 0.044 | 0.055 | 1 | 0.418 | −0.063 | 0.151 | |||
| Degranulated mast Cells | 0.277 | 0.126 | 1 | 0.028* | 0.031 | 0.524 | ||||
| Parameter Estimate (Multinomial regression) | ||||||||||
| Burning sensation* | B | Std. Error | Df | Sig. (p-Value) | Exp (B) | 95% Confidence Interval | ||||
| Lower Bound |
Upper Bound |
|||||||||
| Moderate | Intact mast cells | −0.021 | 0.082 | 1 | 0.793 | 0.979 | 0.834 | 1.149 | ||
| Degranulated mast cells | 0.763 | 0.362 | 1 | 0.035* | 2.145 | 1.055 | 4.360 | |||
| Severe | Intact mast cells | −0.061 | 0.093 | 1 | 0.516 | 0.941 | 0.784 | 1.130 | ||
| Degranulated mast cells | 0.634 | 0.357 | 1 | 0.076 | 1.885 | 0.936 | 3.794 | |||
Model fitting information- Chi Square test (8.740), p = 0.013*.
Test of Parallel lines p = 0.267.
Inference: Degranulated mast cells had a positive coefficient (0.277) and is a significant predictor (p = 0.028) in clinical staging of OSMF, indicating increasing odd with staging. Degranulated mast cells were found to be positive coefficient at moderate (0.763) and severe levels (0.634) of burning sensation, significant positive predictor at moderate levels (p = 0.035), as well as indicating lesser influence at severe levels of burning sensation.
*p-value < 0.05; aThe reference category is: mild.
Goodness-of-Fit, p = 0.636 and Likelihood Ratio Tests p = 0.001* (Degranulated mast cells).
Goodness of fit (p = 0.63) indicates overall fit of the model, likelihood ratio indicates significance p = 0.001 (p < 0.05) of degranulated mast cells on the overall model. Parameter estimates indicate statistically significant role (p = 0.035) of degranulated mast cell and is also a positive predictor at moderate levels (B = 0.763, OR 2.145[95%CI 1.055–4.360]) and not so at severe (B = 0.634, OR 1.885[95%CI 0.936–3.794]) levels of burning sensation. Mild burning sensation served as reference category in the model.
Kruskal-Wallis H test was used to analyse clinical staging and histopathological grading with burning sensation (Table 3) which showed no significant difference p = 0.089(p > 0.05) in burning sensation between various clinical stages, whereas significant difference p = 0.029 (p < 0.05) in burning sensation was evident between histopathological grading, post hoc analysis for same was not carried out as a look at mean ranks suggests significant difference lying between the moderate and severe levels of grading.
Table 3.
Kruskal Wallis H Test to analyse significance between clinical staging, histopathological grading and burning sensation.
| Ranks and Test Statistics | |||
|---|---|---|---|
| Clinical staging | N | Mean Rank | |
| Burning sensation | Stage I | 2 | 16.50 |
| Stage II | 21 | 13.45 | |
| Stage III | 7 | 21.36 | |
| Total | 30 | ||
| Burning sensation | |||
| Chi-Square | 4.836 | ||
| Df | 2 | ||
| Asymp. Sig. | 0.089 | ||
| Ranks and Test Statistics | |||
| Histopathological grading |
N |
Mean Rank |
|
| Burning sensation | Mild | 14 | 13.29 |
| Moderate | 10 | 13.80 | |
| Severe | 6 | 23.50 | |
| Total | 30 | ||
| Burning sensation | |||
| Chi-Square | 7.053 | ||
| Df | 2 | ||
| Asymp. Sig. (*p-value < 0.05) | 0.029* | ||
Inference: A significant difference (p = 0.029) in burning sensation across various histopathological grades was observed, mean ranks suggests a significance between moderate and severe levels of grading. Clinical staging and burning sensation were non-significant (p = 0.089).
4. Discussion
Oral Submucous Fibrosis is a chronic disorder characterised by clinical features such as blanching of oral mucosa, vesicles, ulceration, burning sensation aggravated on spicy foods, palpable fibrotic bands and restricted mouth opening. Histological features of OSMF include epithelial atrophy and presence of chronic inflammatory cell infiltrate. Fibroelastic changes in the connective tissue include an overall increase in production of collagen and decreased collagen degradation thus leading to fibrosis.15 There are several hypotheses regarding burning sensation in OSMF patients among which the role of mast cells is not well established. Mast cell is a constituent of connective tissue that are commonly increased in inflammatory conidtions.
The mean age in our study was reported to be around 35 years among the cases. OSMF is commonly known to occur or reflect in the middle age. The age group from our study was consistent with the study of Rahul et al., who reported the maximum number of OSMF cases in the age group of 30–40 years in their study.16 Similarly, Chandramani et al., reported predominance between 18 and 35 years and Nigam et al., reported an age predominance between 34 and 40 years.3,11 This age group is more vulnerable to stress as well as peer influence. Oral cavity would have been exposed to the variable of interest such as betel nut chewing in various forms for a certain number of years prior to onset of clinical symptoms.
In the present study there was a male predominance among OSMF subjects. This was concurrent with studies conducted by Ranganathan et al., and Goel S et al., who observed a male-to-female ratio of 9.9: 1.0 and 5:1 respectively.17,18 This is probably due to females being more conscious in health and aesthetics and also due to ease of accessibility for males to areca nut and its products more frequently than females especially in these regions.
Our study showed an increase in total number of mast cells with increase in severity of clinical staging of OSMF. This was consistent with a study by Bhatt and Dolakia, who reported that mean of mast cells in Grade I and II was 4.5 and 4.9 respectively. The authors reported that itching sensation is related to histamine released following degranulation of mast cells and suggested the concept of histamine mast cell chain.19 Sabarinath et al., also suggested an increase in mast cell density with increase in severity of OSMF.20 Pujari et al., suggested an increase in mast cell density with an increase in severity of OSMF and thus mast cells play a role in initiation and progression of OSMF.21
The intact and degranulated mast cells were significantly higher in case than control suggesting the role of mast cells in progression of OSMF. This was concurrent with the existing literature.20,21
Intact mast cells were found to be a negative predictor for clinical staging of OSMF. Degranulated mast cell was a significant positive predictor with positive coefficient seen increasing with severity of OSMF. Sabarinath et al., suggested that degranulated mast cells were significantly increased in the very early and early stages of OSMF.20
The present study showed a significant increase in degranulated mast cells in mild and moderate scale of burning sensation and subsequent decrease in degranulated mast cells was seen in severe levels of burning sensation. Thus, suggesting that degranulated mast cells may be responsible for mild to moderate burning sensation.
Chronic irritation of oral mucosa due to areca nut may lead to infiltration of inflammatory cells like mast cells and their differentiation in the oral mucosa. Following degranulation of mast cells various inflammatory mediators such as proteases such as tryptase, chymase, cathepsin G and cytokines like Tumour Necrosis Factor, Interleukin; histamine, serotonin and hydrolases are released. It is possible that inflammatory cells releasing cytokines may stimulate the increase in the number of mast cells in OSMF.22
There are several theories regarding burning sensation in OSMF. Presence of inflammatory cell infiltrate in the connective tissue can predispose to burning sensation. Consumption of a normal diet becomes difficult leading to poor nutrition. This can lead to deficiency of various elements such as iron, vitamin B complex and initiate anaemia and cause stomatitis. Anaemia can further be perpetuated by an inadequate intake of food due to fibrosis and trismus that occurs in the later stages.19,23
Literature also suggests that epithelial atrophy increases with increase in severity of OSMF.24,25 This reduces the distance of intra-epithelial nerve endings from the surface making it more sensitive to burning sensation. Salivary mucous gel barrier loss may affect the ‘protective diffusion membrane’ function causing burning sensation of the oral cavity.26
One interesting finding of this study for which there is no available literature to compare with is burning sensation was observed to be higher in clinical stage III than stage I however this finding did not show statistical significance. Burning sensation was significant across the histopathological grading in the present study.
The limitations of this study were subjects representing population from one local community with a short follow up period and sampling bias which can influence the possible outcome of the study. These limitations can be overcome by having a multi-centre trial using a larger sample size, for more efficient internal and external validity. Randomized control trials with antihistamines, would further add to the understanding of possible role of mast cell degranulation, in causing burning sensation among OSMF patients.
5. Conclusion
In summation, degranulated mast cells were a significant predictor and the odds of increase in these cells was seen with increase in clinical stages of OSMF as per our study. Degranulated mast cells were also a positive predictor for burning sensation in the mild to moderate category of burning sensation. Further multi-centered studies can be done with a larger sample size to conclude our findings.
Contribution details
Laliytha Bijai Kumar: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Manuscript preparation, Manuscript editing, Manscript review, Guarantor. Philips Mathew: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Manuscript preparation, Manuscript editing, Manscript review, Guarantor. Nirmal Madhavan: Concepts, Design, Definition of intellectual content, Literature search, Clinical studies, Manuscript preparation, Manuscript editing, Manscript review, Guarantor. Sabin Siddique: Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manscript review, Guarantor. Nandita Kshetrimayum: Data analysis, Statistical analysis, Manuscript preparation, Manuscript editing, Manscript review, Guarantor. Kiran Iyer: Concepts, Design, Definition of intellectual content, Data analysis, Statistical analysis, Manscript review, Guarantor.
Source of funding
Self-funded
Declaration of competing interest
None declared.
Acknowledgment
The authors extend their sincere thanks to all participants in this study.
Contributor Information
Laliytha Bijai Kumar, Email: laliythaoralmed@gmail.com.
Philips Mathew, Email: drphilipsmathew@yahoo.com.
Nirmal Madhavan, Email: rmnirmal@gmail.com.
Sabin Siddique, Email: drsabinsiddique@gmail.com.
Nandita Kshetrimayum, Email: drnanditak@gmail.com.
Kiran Iyer, Email: drkiraniyer8@gmail.com.
References
- 1.More C.B., Das S., Patel H., Adalja C., Kamatchi V., Venkatesh R. Proposed clinical classification for oral submucous fibrosis. Oral Oncol. 2012;48:200–202. doi: 10.1016/j.oraloncology.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Rao N.R., Villa A., More C.B. Oral submucous fibrosis: a contemporary narrative review with a proposed inter-professional approach for an early diagnosis and clinical management. J of Otolaryngol - Head & Neck Surg. 2020;49(3) doi: 10.1186/s40463-020-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.More C.B., Rao N.R. Proposed clinical definition for oral submucous fibrosis. J Oral Biol Craniofac Res. 2019;9(4):311–314. doi: 10.1016/j.jobcr.2019.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.More C.B., Gavli N., Chen Y., Rao N.R. A novel clinical protocol for therapeutic intervention in oral submucous fibrosis: an evidence based approach. J Oral Maxillofac Pathol. 2018;22(3):382–391. doi: 10.4103/jomfp.JOMFP_223_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Telagi N., Mujib B.R.A., Kulkarni P.G., Naik R. The master switch: comparative study of mast cell in oral epithelial dysplasia. Oral Submucous Fibrosis and Oral Squamous Cells Carcinoma and Assoc. Inflam. Angiogen. 2015;19(1):25–29. doi: 10.4103/0973-029X.157196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinakar G., Ganesh A., Kumar M.S.P., Sabesan M., Narasimhan M., Deivanayagam K. Immunohistochemical quantification of mast cells in inflamed and non inflamed pulp tissue. J Oral Maxillofac Pathol. 2018;22(1):73–77. doi: 10.4103/jomfp.JOMFP_206_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheethal H.S., Hema K.N., Smitha T., Chauhan K. Role of mast cells in inflammatory and reactive pathologies of pulp, periapical area and periodontium. J Oral Maxillofac Pathol. 2018;22(1):92–97. doi: 10.4103/jomfp.JOMFP_278_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta P.C. Areca nut use in India. Indian J Med Sci. 2007;61:317–319. [PubMed] [Google Scholar]
- 9.Aziz S.R. Coming to America: betel nut and oral sub mucous fibrosis. J Am Dent Assoc. 2010;141(4):423–428. doi: 10.14219/jada.archive.2010.0194. [DOI] [PubMed] [Google Scholar]
- 10.Pindborg J.J., Sirsat S.M. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22(6):764–779. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 11.Nigam N.K., Aravinda K., Dhillon M., Gupta S., Reddy S., Raju M.S. Prevalence of oral submucous fibrosis among habitual gutkha and areca nut chewers in Moradabad district. J Oral Biol Craniofac Res. 2014;4(1):8–13. doi: 10.1016/j.jobcr.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar K.K., Sarawathi T.R., Ranganathan K., Devi M.U., Elizabeth J. Oral submucous fibrosis: a clinico-histopathological study in Chennai. Indian J Dent Res. 2007;18:106–111. doi: 10.4103/0970-9290.33785. [DOI] [PubMed] [Google Scholar]
- 13.Churukian C.J., Schenk E.A. Rapid Grocott's methenamine-silver nitrate method for fungi and Pneumocystis carinii. Am J Clin Pathol. 1977;68:427–428. doi: 10.1093/ajcp/68.3.427. [DOI] [PubMed] [Google Scholar]
- 14.Utsunomiya H., Tilakaratne W.M., Oshiro K. Extracellular matrix remodelling in oral submucous fibrosis: its stage-specific modes revealed by immunohistochemistry and in situ hybridization. J Oral Pathol Med. 2005;34(8):498–507. doi: 10.1111/j.1600-0714.2005.00339.x. [DOI] [PubMed] [Google Scholar]
- 15.Rajalalitha P., Valli S. Molecular pathogenesis of oral submucous fibrosis-A collagen metabolic disorder. J Oral Pathol Med. 2005;34:321–328. doi: 10.1111/j.1600-0714.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava R., Jyoti B., Pradhan D., Siddiqui Z. Prevalence of oral submucous fibrosis in patients visiting dental OPD of a dental college in Kanpur: a demographic study. J Fam Med Prim Care. 2019;8(8):2612–2617. doi: 10.4103/jfmpc.jfmpc_465_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranganathan K., Devi M.U., Joshua E., Kumar K.K., Saraswathi T.R. Oral submucous fibrosis: a case-control study in Chennai, south India. J Oral Pathol Med. 2004;33:274–277. doi: 10.1111/j.0904-2512.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- 18.Goel S., Ahmed J., Singh M.P., Nahar P. A clinic-histopathological comparative study in population of Southern Rajasthan. J Carcinog Mutagen. 2010;1:108. [Google Scholar]
- 19.Bhatt A.P., Dholakia H.M. Mast cell density in oral submucous fibrosis. J Indian Dent Assoc. 1977;49:187–191. [Google Scholar]
- 20.Sabarinath B., Sriram G., Saraswathi T.R., Sivapathasundharam B. Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral Submucous fibrosis. Indian J Dent Res. 2011;22:116–121. doi: 10.4103/0970-9290.80009. [DOI] [PubMed] [Google Scholar]
- 21.Pujari R., Vidya N. Mast cell density in oral submucous fibrosis: a possible role in pathogenesis. Int J Health Sci. 2013;7:23–29. doi: 10.12816/0006017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woltman A.M., Kooten C.V. Functional modulation of dendritic cells to suppress adaptive immune responses. J Leukoc Biol. 2003;73:428–441. doi: 10.1189/jlb.0902431. [DOI] [PubMed] [Google Scholar]
- 23.Rajendran R., Vijayakumar T., Vasudevan D.M. An alternative pathogenetic pathway for oral submucous fibrosis. Med Hypotheses. 1989;30(1):35–37. doi: 10.1016/0306-9877(89)90122-9. [DOI] [PubMed] [Google Scholar]
- 24.Rathore A.S., Gupta A., Shetty D.C., Kumar K., Dhanapal R. Redefining epithelial characterization in oral submucous fibrosis using morphometric analysis. J Oral Maxillofac Pathol. 2017;21(1):36–40. doi: 10.4103/0973-029X.203792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mani N.J., Singh B. Studies on oral submucous fibrosis. III. Epithelial changes. Oral Surg Oral Med Oral Pathol. 1976;41:203–214. doi: 10.1016/0030-4220(76)90232-2. [DOI] [PubMed] [Google Scholar]
- 26.Sarode S.C., Sarode G.S. Burning sensation in oral submucous fibrosis and its possible association with mucin secreted by affected minor salivary glands. Oral Oncol. 2013;49:16–17. doi: 10.1016/j.oraloncology.2013.01.004. [DOI] [PubMed] [Google Scholar]