Significance
As the proportion of land area covered by arid land vegetation continues to expand and water limitations for plants increase, understanding if and how desert shrubs are responding to environmental change has become more urgent. Among two populations of Mojave Desert shrubs, we found that intrinsic water-use efficiency has increased substantially over the last three decades in response to increasing aridity and CO2 concentration. While increases in intrinsic water-use efficiency have been widely assumed to mitigate negative effects of decreasing water availability, precise effects on plant productivity, reproduction, and survival remain unknown.
Keywords: Mojave Desert, iWUE, stomatal conductance, carbon isotopes, climate change
Abstract
While tree rings have enabled interannual examination of the influence of climate on trees, this is not possible for most shrubs. Here, we leverage a multidecadal record of annual foliar carbon isotope ratio collections coupled with 39 y of survey data from two populations of the drought-deciduous desert shrub Encelia farinosa to provide insight into water-use dynamics and climate. This carbon isotope record provides a unique opportunity to examine the response of desert shrubs to increasing temperature and water stress in a region where climate is changing rapidly. Population mean carbon isotope ratios fluctuated predictably in response to interannual variations in temperature, vapor pressure deficit, and precipitation, and responses were similar among individuals. We leveraged the well-established relationships between leaf carbon isotope ratios and the ratio of intracellular to ambient CO2 concentrations to calculate intrinsic water-use efficiency (iWUE) of the plants and to quantify plant responses to long-term environmental change. The population mean iWUE value increased by 53 to 58% over the study period, much more than the 20 to 30% increase that has been measured in forests [J. Peñuelas, J. G. Canadell, R. Ogaya, Glob. Ecol. Biogeogr. 20, 597–608 (2011)]. Changes were associated with both increased CO2 concentration and increased water stress. Individuals whose lifetimes spanned the entire study period exhibited increases in iWUE that were very similar to the population mean, suggesting that there was significant plasticity within individuals rather than selection at the population scale.
Desert and semidesert ecosystems cover a large proportion of global land area (1, 2) and are expected to grow as global temperatures increase and precipitation patterns change (3, 4). Because deserts have sparse vegetation and low primary productivity (1, 5, 6), desert ecosystems have traditionally been studied less intensively in the context of climate change than that of more densely vegetated ecosystems, particularly forests and grasslands (7). However, the response of desert vegetation to climate change has important implications for land management decisions (8, 9), species abundance and biodiversity (10, 11), and future organic and inorganic carbon storage (12, 13). Carbon isotope ratios in tree rings serve as an invaluable record of gas exchange patterns (14) in forested ecosystems and have been extensively used to understand changes in tree water-use dynamics in response to environmental perturbations. In contrast, perennial vegetation in desert ecosystems is characterized by shrubs with a multistem (suffrutescent) growth form instead of a single persistent main stem, limiting study of climate responses. In forests, long-term tree ring studies have found that intrinsic water-use efficiency (iWUE), defined as photosynthetic rate (A) divided by stomatal conductance to CO2 (gs) (15–17), increased (18, 19) in response to increasing aridity (20) and rising CO2 concentrations (21, 22) over the last century. However, it is unclear if similar responses have occurred in desert ecosystems. Increases in intrinsic water-use efficiency associated with rising CO2 are generally expected to alleviate drought stress as plants could maintain similar rates of carbon assimilation with reduced demand for water.
iWUE serves as an indicator of the trade-off between photosynthetic carbon assimilation and water loss, mediated by both gs and A (23). Since both the photosynthetic uptake of CO2 and the loss of water vapor are regulated via stomata, iWUE is related to the intercellular (ci) and atmospheric (ca) concentrations of CO2. Carbon isotope ratios record information about the ratio of ci to ca (24, 25) and can therefore be used to calculate iWUE (Methods). Increased water limitation, associated with either decreased soil moisture or increased leaf-atmosphere vapor pressure saturation deficit of air (VPD) (26), typically triggers partial stomatal closure and reductions in gs (20, 23). Assuming leaf photosynthetic capacity does not instantly change, reduced gs leads to decreased ci/ca ratios and therefore increased iWUE and increased carbon isotope ratio values. Studies have found that carbon isotope ratios in desert shrubs increase as water availability decreases across spatial gradients (27, 28), indicating a relationship between plant stress response and local water availability.
In addition to responding to water stress, iWUE can also respond to changes in atmospheric CO2 concentration via adjustments in both A and gs. Studies of forests have generally found that increases in CO2 concentration can lead to increases in iWUE (29–31) and enhanced net primary productivity. Early theoretical predictions suggested that highly water-limited ecosystems like deserts would respond most strongly to CO2-driven increases in iWUE, and this CO2 fertilization effect has been cited as one cause of satellite-observed vegetation “greening” in warm, arid regions (32). However, a lack of in situ and long-term studies of desert ecosystems has limited evaluation of those predictions (7, 33). Several free-air CO2 enrichment studies of shrubs in the Mojave Desert have documented variable increases in A (34–37), increases in iWUE (38), and/or decreases in gs in response to elevated CO2. However, these studies were conducted over four or fewer growing seasons, most of the effects have been weaker than expected, and, in many cases, the responses were only observed in particularly wet seasons (33). The relatively short duration and variable findings of these studies make it difficult to forecast long-term responses of desert shrubs to CO2 and aridity increases.
The carbon isotope ratio of a plant is partially genetically determined and thus heritable (16, 23, 39–41). As water-use strategies can play a role in successful establishment, survival, and reproduction, increased frequency of particular carbon isotope ratios can result from selection on water-use traits under different climatic conditions (42). For instance, plants with high δ13C values are more likely to survive a drought, but plants with low δ13C values are able to grow more quickly following competitive release (43–45). Because of this, we may expect population-scale shifts in leaf carbon isotope ratios during periods of high water availability or following drought. In addition to population-scale shifts, individuals do not have fixed iWUE values but rather can exhibit substantial plasticity in iWUE as conditions change (46). While the presence of plasticity suggests that individual plants may be able to acclimate to increasing water stress to a certain degree, plasticity could be the result of either active or passive processes (47), and iWUE acclimation may or may not be advantageous.
So far, very few studies have considered the interactive effects of rising CO2 and increased aridity in deserts, and none have been conducted over multidecadal timescales. Projected precipitation trends in the United States are spatially variable (48), but vapor pressure deficits and temperature are increasing in the desert southwest more quickly than in many other areas (49, 50). Recently, Williams et al. found that 2000 to 2018 was the second driest 19-y period in the desert southwest in the last 1,200 y, in large part due to anthropogenic warming (51). The Mojave Desert is therefore a particularly useful study location for understanding the impacts of rapid and advanced climate change on vegetation in arid ecosystems.
We have conducted continuous annual monitoring of Encelia farinosa, a common drought deciduous shrub, at two undisturbed, geographically distinct sites in the Mojave Desert over the last 39 y (52). These observational data coupled with an extensive dataset of foliar carbon isotope ratios from the populations provide insight into the water-use dynamics of a widespread woody shrub in a highly resource-limited environment. Just as tree ring records have shed light on iWUE trends in forests, these multidecadal leaf isotope chronologies allow us to evaluate the response of desert shrubs to climate change. Specifically, we address the following hypotheses: 1) carbon isotope ratios vary predictably within a population in response to short-term climate fluctuations, and 2) plants will acclimate to long-term trends of increasing water stress through plastic increases in iWUE.
Results
The Climate Is Changing.
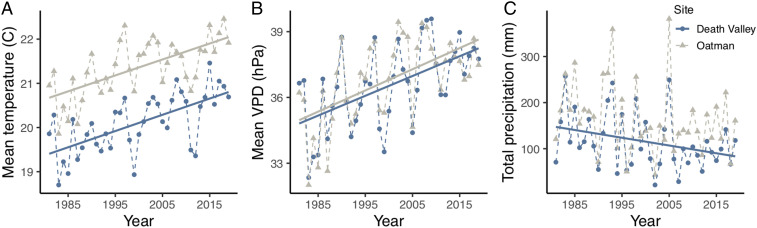
Three distinct aspects of climate have changed over the past 39 y at the Mojave Desert study sites. There have been significant and substantial 1) increases in mean annual temperature, 2) increases in mean daily maximum VPD, and 3) decreases in total annual precipitation (Fig. 1) (for full linear regression results, see SI Appendix, Table S1). At both sites, temperature has increased at a rate of ∼0.04 °C per year, and VPD has increased at a rate of ∼0.1 hPa per year. While total annual precipitation trends have been more variable from year to year, on average, precipitation has decreased by about ∼1.7 mm per year at both sites over the study period. In addition to these climatic changes, CO2 concentration increased by ∼58.5 parts per million (ppm) in the region over the 1991 to 2019 period, at a rate of ∼2.1 ppm per year. Overall, the study site located near Oatman, AZ was warmer and received slightly more precipitation than the study site located in Death Valley, CA. VPD values were very similar between the two sites. Interannual fluctuations in temperature, VPD, and precipitation at the study sites were tied to the El Niño Southern Oscillation (ENSO). El Niño events were associated with cooler, wetter conditions and La Niña events with warmer, drier conditions. Independent of ENSO-related interannual variation in temperature, VPD, and precipitation, long-term climate trends were very similar between the two sites.
Fig. 1.
Annual mean temperature (A) and the annual mean of daily maximum VPD (B) have significantly increased over the 1981-to-2019 study period at the Oatman site (gray triangles) and Death Valley sites (blue circles; P < 0.001). Total annual precipitation (C) has significantly decreased over the same period at the Death Valley (P = 0.049) site, but the decrease was not statistically significant at the Oatman site (P = 0.128). Climate variables were aggregated from April to March in each year to align with the relevant survey period. See SI Appendix, Table S1 for full regression results.
Plants within Populations Exhibited Similar Interannual Fluctuations in Foliar Carbon Isotope Ratios.
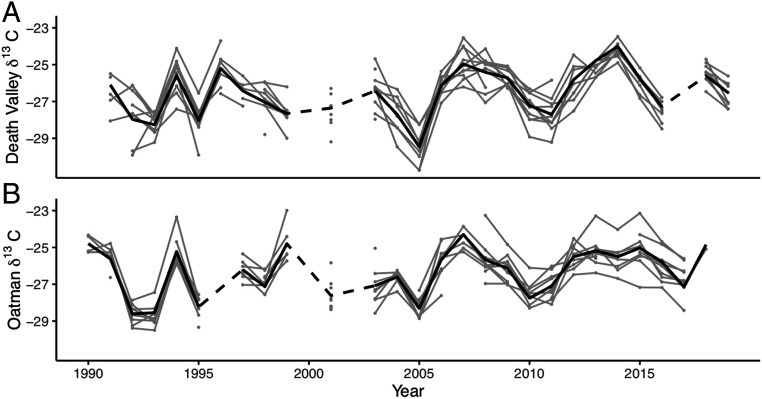
Twenty-seven years of samples were analyzed from each population for a total of 1,863 unique leaf samples from the Death Valley population and 1,346 samples from the Oatman population. To examine within-population variability, we included annual measurements of 10 individuals with the most continuous leaf sample records available from 1991 to 2019 for each population (Fig. 2). These time series demonstrated that fluctuations in foliar δ13C values were similar in timing, direction, and magnitude among individuals within each population. Between-year variance in population mean δ13C values (variance = 1.6 per mil for both Death Valley and Oatman) exceeded the average between-plant variance in δ13C values within each year (variance = 0.7 per mil in Death Valley and. 0.6 per mil in Oatman), and the δ13C values of the continuously measured plants did not deviate substantially from the population mean δ13C values. There was no difference between the annual population mean δ13C values of plants at the Death Valley and Oatman sites (P = 0.37, t = −0.90), despite the climatic differences between the two sites, and there were no significant temporal trends in the mean δ13C value of either population (P = 0.18 for Oatman and P = 0.34 for Death Valley).
Fig. 2.
Leaf δ13C values (per mil) of 10 long-lived E. farinosa plants from the (A) Death Valley and (B) Oatman populations vary over time and in a similar manner among plants. The black line represents the population mean, and the gray lines and points represent individual plants. Dashed lines connect the population mean across years without isotope data.
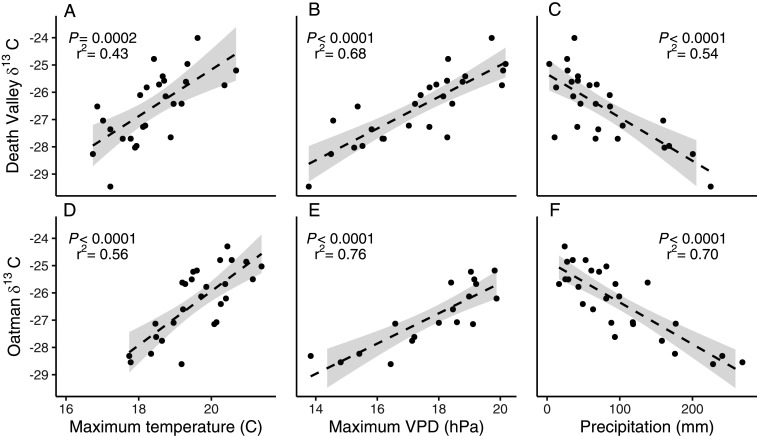
The similarity of interannual fluctuations in δ13C values among individuals implied that common factors, such as temperature, VPD, or precipitation, were driving a large portion of the interannual variations in δ13C values. There were significant positive relationships between population mean δ13C values and mean growing season maximum daily temperature (Fig. 3 A and D) and maximum daily VPD (Fig. 3 B and E), and a significant negative relationship between δ13C values and total growing season precipitation (Fig. 3 C and F). Multiple regressions of the population mean carbon isotope ratio by precipitation, temperature, and VPD explained 70% of the variation in the mean δ13C value of the Death Valley population and 77% in the Oatman population (SI Appendix, Table S2) (P < 0.0001 for both) although VPD was the only significant predictor for either population.
Fig. 3.
Leaf δ13C values (per mil) of E. farinosa in the Death Valley population are positively related to (A) maximum daily temperature and (B) maximum daily VPD, and negatively related to C total precipitation over the November-to-March growing season. δ13C values in the Oatman population follow the same patterns; they are positively related to (D) temperature and (E) VPD, and negatively related to (F) precipitation. Light gray bands represent the 95% CI of the regressions.
In addition to leaf-level adjustments in stomatal opening in response to water availability, plant leaf cover saturated when moisture availability was high and fell as VPD increased (SI Appendix, Fig. S1A). Because δ13C values and leaf cover both varied with VPD, population mean δ13C values were negatively related to leaf cover (SI Appendix, Fig. S1B), suggesting that leaf-level responses to water availability (via stomatal opening or closing) were coordinated with plant-level responses (via changes in leaf cover).
Ecophysiological Adjustments Have Occurred Over Time: iWUE Has Increased, and ci/ca Has Decreased.
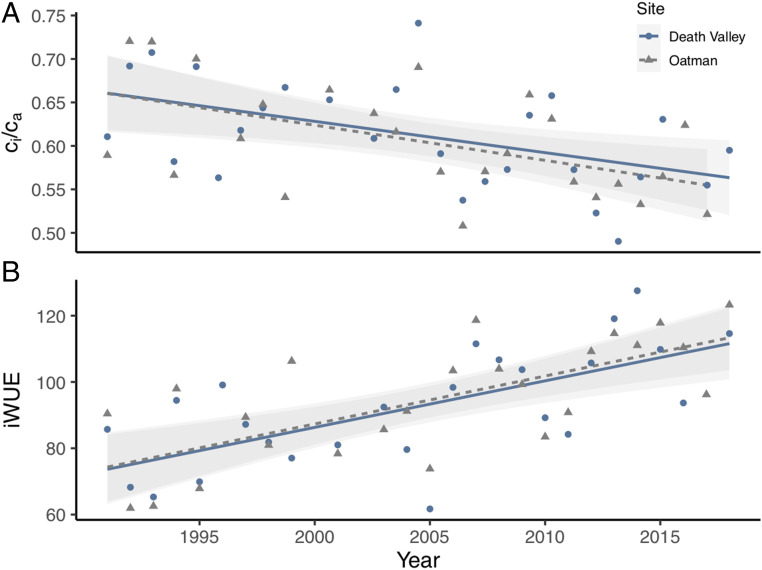
Over the past three decades, significant and similar decreases in population mean ci/ca ratios have occurred in both the Death Valley and Oatman populations (Fig. 4). This trend suggests that limitations due to stomatal control of intracellular CO2 supply may have increased relative to photosynthetic capacity to assimilate carbon (16, 17). Coincident with decreased ci/ca ratios, there have been significant increases in population mean iWUE values among plants at both the Death Valley and Oatman sites (Fig. 4). All plants appeared to become increasingly conservative in their water use relative to photosynthetic gain. A portion of the residual variation in these temporal trends is associated with fluctuations in water availability, parallel to the relationships between climate and δ13C (Fig. 3). For instance, the unusually high values of ci/ca and low values of iWUE observed in 2005 were coincident with exceptionally low VPD (13.8 hPa in Death Valley and Oatman). Similarly, the low values of ci/ca and high values of iWUE observed in 2007 were coincident with exceptionally high VPD (20.2 hPa in Death Valley and 21.6 hPa in Oatman).
Fig. 4.
Ecophysiological changes in leaves of E. farinosa were detected between 1991 and 2018. (A) ci/ca ratios have decreased significantly over time in both the Death Valley (blue circles; r2 = 0.21, slope = −0.003, P = 0.011) and the Oatman (gray triangles; r2 = 0.25, slope = −0.004, P = 0.007) populations. (B) iWUE values have increased significantly in both populations (r2 = 0.42, slope = 1.3, P = 0.0002 for Death Valley; and r2 = 0.46, slope = 1.4, P = 0.0001 for Oatman). Light gray bands represent the 95% CI of the regressions.
In theory, the temporal trends in ci/ca ratios and iWUE values could be attributable to a population-level shift in ecophysiological characteristics through selection for plants with particular intrinsic water-use efficiencies. Alternatively, the temporal trends in ci/ca ratios and iWUE values could be attributed to individual-level acclimation to increased water stress or CO2 concentrations. To differentiate between the two explanations, we compared temporal trends in ci/ca ratios and iWUE values for the entire population versus a subset of plants that survived the duration of the study period because temporal trends among the latter group could only reflect physiological acclimation of individuals, and not population-level selection. Long-term trends in ci/ca and iWUE values were nearly identical between the two groups. Over the study period, both the entire population and the subset of long-lived plants underwent a 17% decrease in ci/ca at Oatman and a 15% decrease at Death Valley. iWUE values increased by 58% for both the entire population and the subset of long-lived plants at Oatman, by 52% for long-lived plants at Death Valley, and by 53% for the whole Death Valley population. While it is possible that individuals that died had different iWUE acclimation responses, the consistency among trends for long-lived plants and the entire population suggests that plant-level acclimation responses, rather than population-scale shifts due to recruitment, were the primary drivers of the temporal trends in ci/ca ratios and iWUE values.
Changes in VPD and CO2 Concentration Are Driving Trends in ci/ca Ratios and iWUE.
Both VPD and atmospheric CO2 concentration have increased over the study period, and both are likely to drive changes in ci/ca ratios and iWUE values. We evaluated the influence of VPD and CO2 concentration on ci/ca and iWUE by comparing the marginal effect of each independent variable on ci/ca and iWUE while holding the other constant (Fig. 5). There was some covariation between CO2 concentration and VPD across the study period in Oatman (r2 = 0.25, P = 0.006) and Death Valley (r2 = 0.05, P = 0.14), but covariation was minimal enough to enable analysis of the respective effects of VPD and CO2 on ci/ca and iWUE using partial correlation (variable inflation factors <1.5).
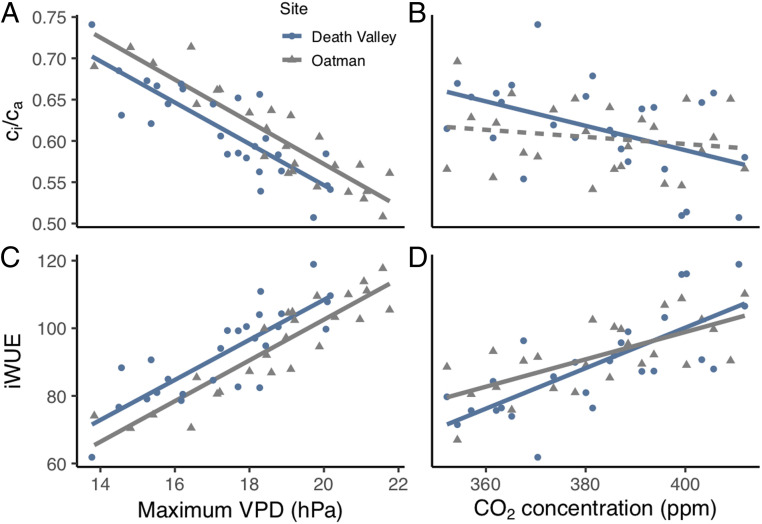
Fig. 5.
Effects plots showing the relative impact of VPD (A and C) and CO2 concentration (B and D) on the population mean ci/ca ratio (A and B) and iWUE (C and D) of E. farinosa leaves for the Death Valley population (blue circles) and the Oatman population (gray triangles) when the other variable is held constant at the mean value. Significant relationships (P < 0.05) are denoted by solid lines, and insignificant relationships are denoted with dashed lines. See Results for regression statistics.
After controlling for CO2 concentration, there was a strong, negative relationship between VPD and ci/ca ratios (Fig. 5A) for the Death Valley (r2 = 0.67, P < 0.0001) and Oatman populations (r2 = 0.77, P < 0.0001). After controlling for VPD, there was no significant relationship between CO2 concentrations and ci/ca ratios in the Oatman population (Fig. 5B) (partial correlation, P = 0.47). In contrast to the Oatman data, there was a significant relationship between CO2 concentrations and ci/ca ratios in the Death Valley population after controlling for VPD (Fig. 5B) (partial correlation, r2 = 0.21 and P = 0.022), indicating that a constant ci/ca ratio was not maintained as ca increased. These results indicate that CO2 concentration and VPD synergistically drove the decrease in ci/ca ratios in the Death Valley population, but that VPD exclusively drove the decrease in ci/ca ratios in Oatman.
CO2 concentration played a larger role in influencing iWUE values than ci/ca ratios. Both VPD and CO2 concentration contributed substantially to increases in iWUE values (Fig. 5 C and D) although VPD drove a larger proportion of the increase than did CO2 (partial correlations, r2 = 0.66 for VPD and 0.52 for CO2, P < 0.0001 in Death Valley; and r2 = 0.77, P < 0.0001 for VPD and r2 = 0.39, P = 0.0012 for CO2 in Oatman). A multiple regression including VPD and CO2 concentration explained 80% of the variability in iWUE in Death Valley and 87% in Oatman (SI Appendix, Table S3). Therefore, while other variables associated with the environment (such as soil moisture availability) or characteristics of the population (such as plant age) may also influence iWUE, we found that two environmental parameters accounted for a large majority of iWUE variability in these populations.
Discussion
There is general consensus that aridity will continue to increase in the North American Southwest due to both decreasing precipitation and increasing evaporative demand (49, 51, 53–55), but current understanding of how desert shrubs will respond to climate change lags behind understanding of forest responses. Using a multidecadal record of foliar δ13C values, we identified similarities in carbon isotope ratio fluctuations among individuals, strong relationships between carbon isotope ratios and growing season climate, and substantial increases in iWUE associated with increasing water stress and CO2 concentration.
Time series of the δ13C values of individuals measured repeatedly throughout the study period supported the hypothesis that interannual variations in leaf carbon isotopes are similar among individuals within a population and that these year-to-year fluctuations can be largely explained by climate variability. The observed patterns were very similar in two geographically distinct populations. Specifically, δ13C values increased as water stress increased, driven by either evaporative demand (VPD and temperature) or water supply (precipitation). While VPD was a stronger predictor of δ13C values than either temperature or precipitation, precipitation is an imperfect proxy for soil moisture, precluding the conclusion that δ13C values in E. farinosa are more strongly influenced by evaporative demand than soil moisture. However, we found that VPD successfully captured the impacts of both precipitation and temperature on shrub intrinsic water-use efficiencies over the growing season in this interior desert ecosystem and would expect similar relationships in other arid regions in which VPD is closely related to soil moisture, especially over annual or longer timescales.
The decrease in ci/ca ratios over time was strongly associated with increasing water stress in both populations, but the response of ci/ca ratios to increasing CO2 concentration varied between populations. Most previous studies of the response of carbon isotope ratios to CO2 enrichment find that plants regulate stomata so as to maintain an approximately constant ci/ca ratio after controlling for fluctuations in water availability (24, 56, 57), leading to increased iWUE but relatively constant carbon isotope ratios. Data from the Oatman population agreed with those findings: CO2 concentration was not significantly related to ci/ca ratios after controlling for VPD, indicating that a constant ci/ca ratio would be maintained in the absence of increasing atmospheric water demand. In contrast, other studies have found inconsistent responses (58) or decreases in ci/ca (59) in response to rising CO2 concentration, which we observed in the Death Valley population. There was a significant but weak negative relationship between CO2 concentration and ci/ca ratios in the Death Valley population after controlling for VPD (r2 = 0.21 and P = 0.022), indicating that there is potential for CO2 concentration to drive changes in ci/ca ratios under the CO2 increases that occurred over the study period (about 60 ppm). If future ci/ca ratios were to decrease in response to increasing ca, as occasionally observed in other studies (59, 60), larger global increases in iWUE would be expected (61) than if ci/ca ratios remained constant. While the possibility of CO2-driven changes in ci/ca ratios merits additional study, VPD accounted for the majority of the temporal trend in ci/ca ratios in both populations over this time period.
As a result of changes in ci and ca, iWUE has increased by 53 and 58% over the study period in the Death Valley and Oatman populations, respectively. The iWUE increase is related to both VPD and CO2 concentration although the increase in VPD explains a larger proportion of the iWUE trend than the increase in CO2 concentration. Many tree ring studies have identified an increase in iWUE associated with rising CO2 concentration over the last century (18, 21, 22, 31, 59, 62, 63), but most estimates of the change in iWUE have been substantially lower than those measured in this study. A metaanalysis of studies using tree ring δ13C values to examine iWUE increases across a variety of biomes from the 1960s to the early 2000s found that estimates of iWUE increases ranged from 5.6 to 36.2%, with an average increase of 20.5% (18). A subsequent review, also considering studies of tree ring δ13C values across biomes, reported an average iWUE increase of about 26% between 1960 and 2010 (21). iWUE changes among desert shrubs have not previously been quantified over long timescales or under natural conditions, but a CO2 enrichment study found that three Mojave shrubs increased iWUE by an average of 48% in elevated (∼550 ppm) versus ambient (∼360 ppm) conditions (38). The magnitude of that increase is comparable to the iWUE increases measured in this study under a much smaller increase in CO2. Our data indicate that the increase in iWUE among desert shrubs may be considerably larger than the average response of forests, compounded by simultaneous increases in both CO2 and water stress. Although the two E. farinosa populations had slightly different responses to increasing CO2 concentration, most of the trends in, and drivers of, δ13C values, ci/ca ratios, and iWUE were nearly identical despite the fact that they are located over 200 km apart from one another. This consistency suggests that the observed patterns are likely widespread rather than an anomalous result restricted to a single microsite.
Selection for particular δ13C values in response to variations in water availability has been identified in E. farinosa previously (42, 46), and we expected genetic changes to play a role in driving long-term population-scale trends in iWUE. However, temporal changes in the iWUE of individual plants were not different from those of the population, suggesting that selection did not significantly contribute to the population-level changes in iWUE values over the study period. Earlier analysis found that 90% of E. farinosa die by age 24 (52), and the vast majority of the plants present in the 2019 populations were recruited after 1991. However, substantial recruitment events (>50 individuals) have only occurred in 3 y since 1991 in Death Valley (1992, 2005, and 2016) and in 5 y in Oatman (1992, 1998, 2003, 2005, and 2008), providing relatively little opportunity for selection. The infrequency of establishment events likely means that the 39-y study period was too short to capture evolutionary changes in iWUE values.
Notably, iWUE acclimation could either reflect an active and advantageous adjustment of gas exchange dynamics to optimize carbon–water trade-offs or simply a passive and nonadaptive stomatal response. While it is intuitive that iWUE increases may improve plant tolerance of increased aridity, increased iWUE in other ecosystems has not been universally associated with increased plant growth, increased reproduction, or reduced mortality (18, 21). Moreover, the transitions in species composition and biotic interactions that are accompanying abiotic changes in many desert regions may mediate the effects of iWUE increases. Further studies will be required to identify any potential benefits of observed increases in iWUE and to determine if phenotypic iWUE increases are sufficient for long-term survival of the population as water stress increases. Despite remaining uncertainty regarding the implications of increased iWUE on plant growth and survival, this study fills a major gap in the understanding of long-term patterns in the water use of desert shrubs and reveals that E. farinosa leaves have undergone substantial acclimation in response to changing climate.
Methods
Site Descriptions, Sample Collection, and Data Collection.
Two near-monospecific stands of E. farinosa were identified and marked with cairns in the early 1980s for continuous, long-term monitoring. The first site is located ∼21 km southwest of Shoshone, CA, covering about 450 m2 of a south-facing slope in Death Valley National Park (referred to as the “Death Valley” site). The second site is about 315 m2 and is located on a slope ∼8 km southwest of Oatman, AZ (referred to as the “Oatman” site). A map of the approximate locations of the study sites is provided as SI Appendix, Fig. S2. There were no visual indications that human impacts affected the populations over the survey period.
The sites were surveyed annually during the last 2 wk of March beginning in 1981 at the Death Valley site and 1982 at the Oatman site. In the first survey year, all mature plants (1 y or older) were tagged and their coordinates on an x,y grid were recorded. Each year, new individuals were tagged and added to the record if they had a woody basal stem, indicating that they were at least 1 y old. Tags were removed, and no further data were recorded following an individual’s death. During each annual survey, data were collected for plant size, flowering status, leaf cover, canopy dieback, and parasite presence (52). Additionally, five to 10 sun leaves from the most recent mature leaf flush were collected annually from each plant for carbon and nitrogen isotope analysis beginning in 1991 at the Death Valley site and in 1989 at the Oatman site. Because E. farinosa plants are drought-deciduous and drop their leaves every summer, leaf carbon isotope ratios reflect only photosynthate produced within the current season. Leaves were not collected from plants that would be damaged by leaf collection, and not all plants had leaves in every year. Annually summarized data that are reported in this manuscript are provided in SI Appendix, Table S4, including data on climate, CO2 concentration and δ13C, population and sample sizes, and population mean δ13C values, ci/ca ratios, and iWUE values.
Isotope Analysis.
Leaf samples were dried upon returning from the field and stored in a cool, dark, dry place until analysis was conducted. Selected samples were ground to <40 mesh and loaded into tin capsules for analysis of carbon isotope ratios. Isotope analyses were conducted using a Carlo-Erba EA-1110 elemental analyzer coupled to a Finnigan Mat Delta+ isotope-ratio mass spectrometer (IRMS) via a continuous flow interface (ThermoFinnigan Conflo III, Bremen, Germany). Laboratory reference materials were calibrated using international standards USGS40 (δ13C = −26.24‰) and USGS41 (δ13C = 37.76‰), and all results are reported in delta notation on the Vienna Pee Dee Belemnite (VPDB) scale. Long-term measurement uncertainty for quality control materials is 0.2‰ for δ13C.
Climate Data.
We acquired monthly data on total precipitation, mean daily maximum VPD, and mean daily minimum, mean, and maximum temperature for each site from the Parameter-Elevation Regressions on Independent Slopes Model (PRISM) Climate Group datasets (4 km resolution, https://prism.oregonstate.edu/, accessed 15 January 2020). Mean temperature in the PRISM dataset is calculated as the average of the minimum and maximum daily temperatures. For the Death Valley population, we used climate data from the grid cell containing Shoshone, CA because the presence of a weather station in Shoshone improved the reliability of the data. For the Oatman population, we used the grid cell containing the site coordinates. Because E. farinosa at these sites typically leaf out in late fall and annual surveys were conducted in late March, we considered the November-to-March period preceding each survey to be the climatic period relevant for leaf δ13C values. Climate data included throughout are averages over this November-to-March growing season, unless otherwise specified.
Calculation of ci/ca and iWUE.
The carbon isotope ratio of a leaf is a function of the δ13C value of atmospheric CO2 (δ13Catm), the ci/ca ratio, and the fractionations associated with CO2 diffusion (a = 4.4‰) and net carboxylase discrimination (b = 27‰) (Eq. 1) (23, 25):
| [1] |
The relationship between iWUE and ci and ca is given in Eq. 2, in which 1.6 is the ratio of the diffusivities of CO2 in air to that of water vapor in air.
| [2] |
There has been a decrease in the δ13C value of atmospheric CO2 over the study period due to fossil fuel combustion, known as the Suess effect (64, 65), which confounds interpretation of long-term trends in plant δ13C. However, the ci/ca ratio and iWUE are unaffected by changes in the δ13C value of the atmosphere and reflect only physiologically relevant trends in plant gas exchange.
δ13C Value and Concentration of Atmospheric CO2.
Publicly available data on the δ13C (66) and concentration (67) of atmospheric CO2 in Wendover, UT were obtained from the National Oceanic and Atmospheric Administration (NOAA) Earth System Research Laboratory Global Monitoring Division (ESRL). Although other NOAA ESRL sites have longer term datasets, we chose to use data from the Wendover site as it is similar to the study sites in terms of latitude, aridity, vegetation, and proximity to urbanized areas. Data on the δ13C values of CO2 were available for 1993 to 2014, and data on CO2 concentrations were available for 1993 to 2018. Average δ13CO2 values were strongly linearly correlated with year (r2 = 0.965) so we extrapolated values for 1991, 1992, and 2015 to 2019 based on the linear regression between δ13CO2 and year (y = −0.028597x + 49.031911). We also extrapolated CO2 concentration for 1991, 1992, and 2019 based on the linear regression between CO2 and year (r2 = 0.995; y = 2.091x − 3,811).
Statistical Analysis.
All data analyses were conducted in R version 3.6.1 (R Core Team). Unless otherwise specified, statistical tests including δ13C, ci/ca, and iWUE values were applied to annual population means using all collected data (provided in SI Appendix, Table S4) (n = 26 y for Death Valley and 25 y for Oatman). Single linear regressions of mean annual temperature, mean daily maximum VPD, total annual precipitation, CO2 concentration, and population mean δ13C, ci/ca, and iWUE values by year were applied for each population to identify temporal trends. For comparison, we also conducted linear regression of ci/ca, and iWUE values by year for a subset of plants that survived the duration of the study period (n = 322 observations from 10 plants in Death Valley and n = 302 observations from nine plants in Oatman). We compared annual population mean δ13C values between the Death Valley and Oatman populations using a t test. The effects of temperature, VPD, and precipitation on population mean δ13C values were assessed using single and multiple linear regression. Partial correlation and multiple regression were used to identify the independent and cumulative effects of VPD and CO2 concentration on population mean ci/ca, and iWUE values.
Supplementary Material
Acknowledgments
This work was supported by NSF Grant DEB-1950025. We thank the numerous individuals that have assisted with data collection during annual field surveys; David R. Bowling and Steven A. Kannenberg for feedback on early drafts of the manuscript; and the Stable Isotope Ratio Facility for Environmental Research (University of Utah) for conducting carbon isotope analyses.
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008345117/-/DCSupplemental.
Data Availability.
All data that are reported in this manuscript are provided in SI Appendix, Table S4, including data on climate, CO2 concentration and δ13C, population and sample sizes, and population mean δ13C values, ci/ca ratios, and iWUE values.
References
- 1.Lal R., Carbon sequestration in dryland ecosystems. Environ. Manage. 33, 528–544 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Intergovernmental Panel on Climate Change (IPCC) , Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems, P. R. Shukla et al., Eds., in press. https://www.ipcc.ch/site/assets/uploads/2019/11/SRCCL-Full-Report-Compiled-191128.pdf. Accessed 11 July 2020. [Google Scholar]
- 3.Huang J., Yu H., Guan X., Wang G., Guo R., Accelerated dryland expansion under climate change. Nat. Clim. Chang. 6, 166–171 (2016). [Google Scholar]
- 4.Schlesinger W. H., et al. , Biological feedbacks in global desertification. Science 247, 1043–1048 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Ehleringer J., Mooney H. A., “Productivity of desert and mediterranean-climate plants” in Physiological Plant Ecology IV: Ecosystem Processes: Mineral Cycling, Productivity and Man’s Influence, Lange O. L., Nobel P. S., Osmond C. B., Ziegler H., Eds. (Encyclopedia of Plant Physiology, Springer, 1983), pp. 205–231. [Google Scholar]
- 6.Grace J., Understanding and managing the global carbon cycle. J. Ecol. 92, 189–202 (2004). [Google Scholar]
- 7.Lioubimtseva E., Adams J. M., Possible implications of increased carbon dioxide levels and climate change for desert ecosystems. Environ. Manage. 33, S388–S404 (2004). [Google Scholar]
- 8.Curtin C. G., Sayre N. F., Lane B. D., Transformations of the Chihuahuan borderlands: Grazing, fragmentation, and biodiversity conservation in desert grasslands. Environ. Sci. Policy 5, 55–68 (2002). [Google Scholar]
- 9.Svejcar T., et al. , Carbon fluxes on North American rangelands. Rangeland Ecol. Manag. 61, 465–474 (2008). [Google Scholar]
- 10.Bachelet D., Ferschweiler K., Sheehan T., Strittholt J., Climate change effects on southern California deserts. J. Arid Environ. 127, 17–29 (2016). [Google Scholar]
- 11.Barrows C. W., Sensitivity to climate change for two reptiles at the Mojave–Sonoran Desert interface. J. Arid Environ. 75, 629–635 (2011). [Google Scholar]
- 12.Barger N. N., et al. , Woody plant proliferation in North American drylands: A synthesis of impacts on ecosystem carbon balance. J. Geophys. Res. Biogeosci. 116, G00K07 (2011). [Google Scholar]
- 13.Jasoni R. L., Smith S. D., Arnone J. A., Net ecosystem CO2 exchange in Mojave Desert shrublands during the eighth year of exposure to elevated CO2. Glob. Change Biol. 11, 749–756 (2005). [Google Scholar]
- 14.McCarroll D., Loader N. J., Stable isotopes in tree rings. Quat. Sci. Rev. 23, 771–801 (2004). [Google Scholar]
- 15.Comstock J., Ehleringer J., Photosynthetic responses to slowly decreasing leaf water potentials in Encelia frutescens. Oecologia 61, 241–248 (1984). [DOI] [PubMed] [Google Scholar]
- 16.Ehleringer J. R., Hall A. E., Farquhar G. D., Eds., Stable Isotopes and Plant Carbon-Water Relations (Elsevier, 1993). [Google Scholar]
- 17.Farquhar G. D., Hubick K. T., Condon A. G., Richards R. A., “Carbon isotope fractionation and plant water-use efficiency” in Stable Isotopes in Ecological Research, Rundel P. W., Ehleringer J. R., Nagy K. A., Eds. (Ecological Studies, Springer, 1989), pp. 21–40. [Google Scholar]
- 18.Peñuelas J., Canadell J. G., Ogaya R., Increased water-use efficiency during the 20th century did not translate into enhanced tree growth. Glob. Ecol. Biogeogr. 20, 597–608 (2011). [Google Scholar]
- 19.Lévesque M., Siegwolf R., Saurer M., Eilmann B., Rigling A., Increased water-use efficiency does not lead to enhanced tree growth under xeric and mesic conditions. New Phytol. 203, 94–109 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Dupouey J. -L., Leavitt S., Choisnel E., Jourdain S., Modelling carbon isotope fractionation in tree rings based on effective evapotranspiration and soil water status. Plant Cell Environ. 16, 939–947 (1993). [Google Scholar]
- 21.Silva L. C. R., Anand M., Probing for the influence of atmospheric CO2 and climate change on forest ecosystems across biomes. Glob. Ecol. Biogeogr. 22, 83–92 (2013). [Google Scholar]
- 22.van der Sleen P., et al. , No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 8, 24–28 (2015). [Google Scholar]
- 23.Farquhar G. D., Ehleringer J. R., Hubick K. T., Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503–537 (1989). [Google Scholar]
- 24.Ehleringer J. R., Cerling T. E., Atmospheric CO(2) and the ratio of intercellular to ambient CO2 concentrations in plants. Tree Physiol. 15, 105–111 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Farquhar G. D., O’Leary M. H., Berry J. A., On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 9, 121–137 (1982). [Google Scholar]
- 26.Novick K. A., et al. , The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Chang. 6, 1023–1027 (2016). [Google Scholar]
- 27.Comstock J. P., Ehleringer J. R., Correlating genetic variation in carbon isotopic composition with complex climatic gradients. Proc. Natl. Acad. Sci. U.S.A. 89, 7747–7751 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehleringer J. R., Cooper T. A., Correlations between carbon isotope ratio and microhabitat in desert plants. Oecologia 76, 562–566 (1988). [DOI] [PubMed] [Google Scholar]
- 29.Frank D. C., et al. , Water-use efficiency and transpiration across European forests during the Anthropocene. Nat. Clim. Chang. 5, 579–583 (2015). [Google Scholar]
- 30.Yi K., et al. , Linking variation in intrinsic water-use efficiency to isohydricity:A comparison at multiple spatiotemporal scales. New Phytol. 221, 195–208 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Li D., et al. , Climate, intrinsic water-use efficiency and tree growth over the past 150 years in humid subtropical China. PLoS One 12, e0172045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donohue R. J., Roderick M. L., McVicar T. R., Farquhar G. D., Impact of CO2 fertilization on maximum foliage cover across the globe’s warm, arid environments. Geophys. Res. Lett. 40, 3031–3035 (2013). [Google Scholar]
- 33.Nowak R. S., Ellsworth D. S., Smith S. D., Functional responses of plants to elevated atmospheric CO2: Do photosynthetic and productivity data from FACE experiments support early predictions? New Phytol. 162, 253–280 (2004). [Google Scholar]
- 34.Smith S. D., et al. , Elevated CO2 increases productivity and invasive species success in an arid ecosystem. Nature 408, 79–82 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Naumburg E., et al. , Photosynthetic responses of Mojave Desert shrubs to free air CO2 enrichment are greatest during wet years. Glob. Change Biol. 9, 276–285 (2003). [Google Scholar]
- 36.Hamerlynck E. P., et al. , Photosynthetic responses of Larrea tridentata to a step-increase in atmospheric CO2 at the Nevada Desert FACE Facility. J. Arid Environ. 44, 425–436 (2000). [Google Scholar]
- 37.Ellsworth D. S., et al. , Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob. Change Biol. 10, 2121–2138 (2004). [Google Scholar]
- 38.Housman D. C., et al. , Increases in desert shrub productivity under elevated carbon dioxide vary with water availability. Ecosystems (N. Y.) 9, 374–385 (2006). [Google Scholar]
- 39.Condon A. G., Richards R. A., Broad sense heritability and genotype × environment interaction for carbon isotope discrimination in field-grown wheat. Aust. J. Agric. Res. 43, 921–934 (1992). [Google Scholar]
- 40.Schuster W. S. F., Phillips S. L., Sandquist D. R., Ehleringer J. R., Heritability of carbon isotope discrimination in Gutierrezia microcephala (Asteraceae). Am. J. Bot. 79, 216–221 (1992). [Google Scholar]
- 41.Zhang J., Marshall J. D., Jaquish B. C., Genetic differentiation in carbon isotope discrimination and gas exchange in Pseudotsuga menziesii: A common-garden experiment. Oecologia 93, 80–87 (1993). [DOI] [PubMed] [Google Scholar]
- 42.Sandquist D. R., Ehleringer J. R., Carbon isotope discrimination differences within and between contrasting populations of Encelia farinosa raised under common-environment conditions. Oecologia 134, 463–470 (2003). [DOI] [PubMed] [Google Scholar]
- 43.Ehleringer J. R., Variation in leaf carbon isotope discrimination in Encelia farinosa: Implications for growth, competition, and drought survival. Oecologia 95, 340–346 (1993). [DOI] [PubMed] [Google Scholar]
- 44.Ehleringer J. R., Intraspecific competitive effects on water relations, growth and reproduction in Encelia farinosa. Oecologia 63, 153–158 (1984). [DOI] [PubMed] [Google Scholar]
- 45.Schuster W. S. F., Sandquist D. R., Phillips S. L., Ehleringer J. R., Comparisons of carbon isotope discrimination in populations of aridland plant species differing in lifespan. Oecologia 91, 332–337 (1992). [DOI] [PubMed] [Google Scholar]
- 46.Donovan L. A., Ehleringer J. R., Potential for selection on plants for water-use efficiency as estimated by carbon isotope discrimination. Am. J. Bot. 81, 927–935 (1994). [Google Scholar]
- 47.McAdam S. A. M., Brodribb T. J., The evolution of mechanisms driving the stomatal response to vapor pressure deficit. Plant Physiol. 167, 833–843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderegg W. R., Diffenbaugh N. S., Observed and projected climate trends and hotspots across the National Ecological Observatory Network regions. Front. Ecol. Environ. 13, 547–552 (2015). [Google Scholar]
- 49.Seager R., Vecchi G. A., Greenhouse warming and the 21st century hydroclimate of southwestern North America. Proc. Natl. Acad. Sci. U.S.A. 107, 21277–21282 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stahlschmidt Z. R., DeNardo D. F., Holland J. N., Kotler B. P., Kruse-Peeples M., Tolerance mechanisms in North American deserts: Biological and societal approaches to climate change. J. Arid Environ. 75, 681–687 (2011). [Google Scholar]
- 51.Williams A. P., et al. , Large contribution from anthropogenic warming to an emerging North American megadrought. Science 368, 314–318 (2020). [DOI] [PubMed] [Google Scholar]
- 52.Ehleringer J. R., Sandquist D. R., A tale of ENSO, PDO, and increasing aridity impacts on drought-deciduous shrubs in the Death Valley region. Oecologia 187, 879–895 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Cayan D. R., et al. , Future dryness in the southwest US and the hydrology of the early 21st century drought. Proc. Natl. Acad. Sci. U.S.A. 107, 21271–21276 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams A. P., et al. , Forest responses to increasing aridity and warmth in the southwestern United States. Proc. Natl. Acad. Sci. U.S.A. 107, 21289–21294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Udall B., Overpeck J., The twenty-first century Colorado River hot drought and implications for the future. Water Resour. Res. 53, 2404–2418 (2017). [Google Scholar]
- 56.Keeling R. F., et al. , Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis. Proc. Natl. Acad. Sci. U.S.A. 114, 10361–10366 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marshall J. D., Monserud R. A., Homeostatic gas-exchange parameters inferred from 13C/12C in tree rings of conifers. Oecologia 105, 13–21 (1996). [DOI] [PubMed] [Google Scholar]
- 58.Voelker S. L., et al. , A dynamic leaf gas-exchange strategy is conserved in woody plants under changing ambient CO2 : Evidence from carbon isotope discrimination in paleo and CO2 enrichment studies. Glob. Change Biol. 22, 889–902 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Keenan T. F., et al. , Increase in forest water-use efficiency as atmospheric carbon dioxide concentrations rise. Nature 499, 324–327 (2013). [DOI] [PubMed] [Google Scholar]
- 60.Battipaglia G., et al. , Elevated CO2 increases tree-level intrinsic water use efficiency: Insights from carbon and oxygen isotope analyses in tree rings across three forest FACE sites. New Phytol. 197, 544–554 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Saurer M., Siegwolf R. T. W., Schweingruber F. H., Carbon isotope discrimination indicates improving water-use efficiency of trees in northern Eurasia over the last 100 years. Glob. Change Biol. 10, 2109–2120 (2004). [Google Scholar]
- 62.Giammarchi F., Cherubini P., Pretzsch H., Tonon G., The increase of atmospheric CO2 affects growth potential and intrinsic water-use efficiency of Norway spruce forests: Insights from a multi-stable isotope analysis in tree rings of two alpine chronosequences. Trees (Berl.) 31, 503–515 (2017). [Google Scholar]
- 63.Gedalof Z., Berg A. A., Tree ring evidence for limited direct CO2 fertilization of forests over the 20th century. Global Biogeochem. Cycles 24, GB3027 (2010). [Google Scholar]
- 64.Suess H. E., Radiocarbon concentration in modern wood. Science 122, 415–417 (1955).13246648 [Google Scholar]
- 65.Keeling C. D., The suess effect: 13carbon-14carbon interrelations. Environ. Int. 2, 229–300 (1979). [Google Scholar]
- 66.White J. W. C., Vaughn B. H., Michel S. E., Data from “Stable isotopic composition of atmospheric carbon dioxide (13C and 18O), 1990–2014.” NOAA ESRL. ftp://aftp.cmdl.noaa.gov/data/trace_gases/co2c13/flask/. Accessed 15 January 2020.
- 67.Dlugokencky E. J., Mund J. W., Crotwell A. M., Crotwell M. J., Thoning K. W., Data from “Atmospheric carbon dioxide dry air mole fractions, 1968–018.” NOAA ESRL. https://www.esrl.noaa.gov/gmd/ccgg/arc/?id=132. Accessed 15 January 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data that are reported in this manuscript are provided in SI Appendix, Table S4, including data on climate, CO2 concentration and δ13C, population and sample sizes, and population mean δ13C values, ci/ca ratios, and iWUE values.