Significance
Roughly two decades ago, troponin T (TnT) of the trimeric thin filament regulatory troponin complex, was shown to contribute directly to the inhibition of skeletal and cardiac muscle contraction, independent of troponins I and C. The molecular basis of this enigmatic role for TnT remains unknown. Hypertrophic and restrictive cardiomyopathies (HCM and RCM) are characterized by impaired relaxation and, thus, an inability of the heart muscle to properly “turn off.” Based on data gleaned from multiple model systems used to investigate HCM and RCM TnT mutations, and recently published thin filament structural models, we propose a mechanism that potentially underlies TnT’s heretofore poorly understood role in muscle relaxation, which, when compromised, may cause disease.
Keywords: troponin T, tropomyosin, Drosophila, cardiomyopathy, diastolic dysfunction
Abstract
Muscle contraction is regulated by the movement of end-to-end-linked troponin−tropomyosin complexes over the thin filament surface, which uncovers or blocks myosin binding sites along F-actin. The N-terminal half of troponin T (TnT), TNT1, independently promotes tropomyosin-based, steric inhibition of acto-myosin associations, in vitro. Recent structural models additionally suggest TNT1 may restrain the uniform, regulatory translocation of tropomyosin. Therefore, TnT potentially contributes to striated muscle relaxation; however, the in vivo functional relevance and molecular basis of this noncanonical role remain unclear. Impaired relaxation is a hallmark of hypertrophic and restrictive cardiomyopathies (HCM and RCM). Investigating the effects of cardiomyopathy-causing mutations could help clarify TNT1’s enigmatic inhibitory property. We tested the hypothesis that coupling of TNT1 with tropomyosin’s end-to-end overlap region helps anchor tropomyosin to an inhibitory position on F-actin, where it deters myosin binding at rest, and that, correspondingly, cross-bridge cycling is defectively suppressed under diastolic/low Ca2+ conditions in the presence of HCM/RCM lesions. The impact of TNT1 mutations on Drosophila cardiac performance, rat myofibrillar and cardiomyocyte properties, and human TNT1’s propensity to inhibit myosin-driven, F-actin−tropomyosin motility were evaluated. Our data collectively demonstrate that removing conserved, charged residues in TNT1’s tropomyosin-binding domain impairs TnT’s contribution to inhibitory tropomyosin positioning and relaxation. Thus, TNT1 may modulate acto-myosin activity by optimizing F-actin−tropomyosin interfacial contacts and by binding to actin, which restrict tropomyosin’s movement to activating configurations. HCM/RCM mutations, therefore, highlight TNT1’s essential role in contractile regulation by diminishing its tropomyosin-anchoring effects, potentially serving as the initial trigger of pathology in our animal models and humans.
Striated muscle contraction is regulated by Ca2+- and myosin-dependent changes in the location of troponin (Tn) and tropomyosin (Tpm) over the surface of the actin-based thin filament (1–4). Tn consists of a Ca2+ binding subunit, TnC, an inhibitory subunit, TnI, and a subunit that connects the complex tightly to Tpm, TnT. Tpm is a semirigid, coiled-coil dimer that binds seven successive actin protomers (5, 6). Tpm molecules link end to end to form continuous strands that track along the winding, long-pitch F-actin helix (5, 7, 8). In resting muscle, when intracellular Ca2+ is low, Tpm is constrained to an inhibitory, “blocking” B-state position over myosin binding sites on F-actin (1–4). Hence, contraction is suppressed. As Ca2+ rises, Ca2+-bound TnC draws a TnI regulatory region away from actin, releasing a steric constraint on Tpm. This enables azimuthal movement of Tpm across F-actin to a “closed” C-state position, which partially uncovers myosin binding sites. Subsequent binding of a small population of myosin cross-bridges further displaces Tpm to an “open” M-state position, completely exposing neighboring binding sites, leading to cooperative, full filament activation.
This three-state model of contractile regulation is strongly supported by biochemical and structural data (3, 4). However, it was developed in the absence of high-resolution structures of thin filament-bound Tn, and therefore the model has been incomplete in important respects. Fortunately, a great deal of Tn’s structure on the thin filament has recently been revealed for the first time, via an incisive cryo-electron microscopy (cryo-EM) study by Yamada et al. (9). Tn’s effects on the shifting position of Tpm along the regulated thin filament are markedly more apparent. Three Tn regions—1) an extended TnI C terminus, 2) the Tn tail (i.e., the TnT N-terminal region), and 3) the Tn core domain (consisting of parts of all three subunits)—all interact directly with F-actin−Tpm so as to influence Tpm position. Furthermore, these regions seemingly have positions on F-actin that, depending upon conditions, may impact myosin binding. To allow contraction, both Tpm and Tn must reposition properly.
Significantly for the current report, information regarding the conformation of the end-to-end overlap of successive Tpms is now available from the above work (9), and also from concurring, independent, in silico studies (10, 11). The overlap domain has a relatively fixed position on F-actin that approximates the Tpm B-state location, whether or not Ca2+ is present (9). Notably, the overlap region includes a TnT helix that closely interacts with both Tpm ends, and also, potentially, with actin. It thus may help anchor the overlap domain on actin and restrain its motion during regulatory repositioning. In light of these findings, the Tn tail, from which this helix derives, takes on particular interest and is the subject of the present study.
The primary switch regulating muscle contraction involves the TnI C terminus and the Tn core domain, which interact with each other and with F-actin−Tpm in a Ca2+-sensitive manner. No matter its primacy, this switch is considered insufficient for contractile activation; actions of myosin cross-bridges are also required. The switch is also insufficient for inhibition, which requires other features of Tn, Tpm, and actin (9, 12–17). In particular, the N-terminal half of TnT, otherwise known as TNT1, may play a role in relaxation, to a degree that is an open subject of investigation. This domain, corresponding to residues 1 to 156 of human cardiac TnT (hcTnT), couples with Tpm through an evolutionarily conserved, highly charged binding element that spans residues 113 to 136 of hcTNT1 (SI Appendix, Fig. S1) (18–20). The α-helical TNT1 tail extends along, binds tightly to, and buttresses the head-to-tail overlap of successive Tpm dimers (9, 11, 18, 21, 22). Additionally, it enhances the affinity of Tpm for F-actin as well as the cooperativity of myosin cross-bridge binding to actin (18, 23–25). Moreover, in the absence of TnI and TnC, both skeletal and cardiac forms of TNT1 bias Tpm toward an inhibitory position that impedes myosin S1 interaction with actin−Tpm−TNT1 filaments (12, 13). These structural and biophysical findings in solution suggest TNT1 may have an inhibitory role in thin filament regulation. However, the functional importance of TNT1 in inhibition of contraction is unclear in intact muscle and in vivo. Is TnT1 ancillary to inhibition, or is it essential for normal relaxation?
Defective contractile regulation is frequently associated with striated muscle pathology. Hypertrophic and restrictive cardiomyopathies (HCM and RCM) are primary disorders of heart muscle (2, 26, 27). HCM is characterized by abnormal thickening and stiffening of the heart walls, cellular and subcellular disarray, and cardiac arrhythmias (26, 27). RCM is typified by restrictive physiology in the presence of normal or reduced diastolic ventricular volumes, normal or reduced systolic volumes, and normal heart wall thickness (27). Impaired relaxation, diastolic dysfunction, and hyperdynamic contractility are observed for both HCM and RCM and have been reported to be the earliest and predominant biomechanical abnormalities that are necessary to drive overt hypertrophic remodeling (26, 28).
Mutations in TNNT2, which encodes hcTnT, account for 15% of all HCM-causing lesions, and ∼75% of these localize to hcTNT1 (18, 19, 29). A number of studies have characterized the molecular-, cellular-, and tissue-level effects of hcTNT1 mutations that flank the Tpm-binding element (2, 18, 19, 29–33). Several missense mutations that affect highly conserved amino acids are also located within this element (SI Appendix, Fig. S1), yet they have received less attention. These include the K124N and R130C HCM and E136K RCM substitutions, which remove or replace invariant charged residues whose sidechains likely participate in, and therefore when mutated may affect, Tpm binding (11, 22). Supporting this, the R130C substitution was shown to weaken hcTNT1’s computationally derived Tpm interaction energy (11), and the K124N and R130C mutations decreased hcTnT’s affinity for Tpm (34). The K124N substitution additionally sensitized myosin ATPase activity in thin filament-regulated acto-myosin assays. The mutations have never been investigated in vivo, however, nor have their effects been assessed across a full range of experimental models, and then considered in light of the new structural advances (9, 11).
Here, we posit that the K124N, R130C, and E136K hcTnT substitutions diminish hcTNT1’s inhibitory properties by compromising its Tpm-anchoring role in contractile regulation, serving as a trigger for cardiac remodeling. Specifically, we test the hypothesis, based on the aforementioned structural and biochemical results (9, 11–13, 18, 19, 23, 25, 34), that the TNT1−Tpm overlap region is essential for relaxation, and that, correspondingly, suppression of cross-bridge cycling under diastolic/low Ca2+ conditions is defective when these missense mutations are present. First, we developed several Drosophila melanogaster models to scrutinize the mutations’ impact on organ-, cellular-, and myofibrillar-level function. We next ascertained the effects of the equivalent E136K hcTnT variant on activation and, importantly, relaxation properties of rat ventricular myofibrils and cardiomyocytes. Finally, we employed a reductionist in vitro approach, using hcTNT1 peptides, to identify the immediate and completely Ca2+-independent consequences of all three mutations on hcTNT1−Tpm-mediated inhibition of contraction. Our data collectively demonstrate that removing conserved, charged residues in the TNT1−Tpm binding domain impairs TnT’s involvement in inhibitory Tpm positioning, muscle relaxation, and diastole across phyla and across multiple levels of organization. The consistent findings support the functional importance of the recently revealed Tpm overlap−Tn tail region (9, 11) in thin filament-based contractile regulation, and earlier findings that TNT1 biases Tpm to a relatively inhibitory azimuthal location on F-actin (12, 13). We suggest a mechanism whereby TNT1 might strengthen F-actin−Tpm binding and increase energetic demands necessary for Tpm translocation and thin filament activation. Thus, it is plausible that pathological modifications to the N-terminal half of TnT more generally act by perturbing TNT1’s interaction with Tpm (11, 18, 19, 34) and/or F-actin (9) and, in turn, Tpm’s intrinsic inhibitory association with F-actin (8, 12, 13, 15, 35–41).
Results
Overexpression of Mutant TnTs Induces Diastolic Dysfunction and Cardiomyopathy in D. melanogaster.
Drosophila is an outstanding animal model to study striated muscle physiology and pathogenesis. They age and reproduce quickly, are easily maintained, lack genetic redundancy, and offer a wide repertoire of tools that enable extensive genetic manipulation. Additionally, over 75% of human disease genes have fly homologs including those underlying cardiac disorders (42). The adult Drosophila heart, or dorsal vessel, is a tubular structure, which bears resemblance to the early vertebrate embryonic heart, and is made up of ∼80 cardiomyocytes (Fig. 1A). Thin filament conformational transitions accompanying contractile regulation and cardiomyocyte Ca2+ handling are also extremely well conserved between flies and mammals (43, 44), making this a unique system to rapidly explore the effects of Tn mutations on cardiac function.
Fig. 1.
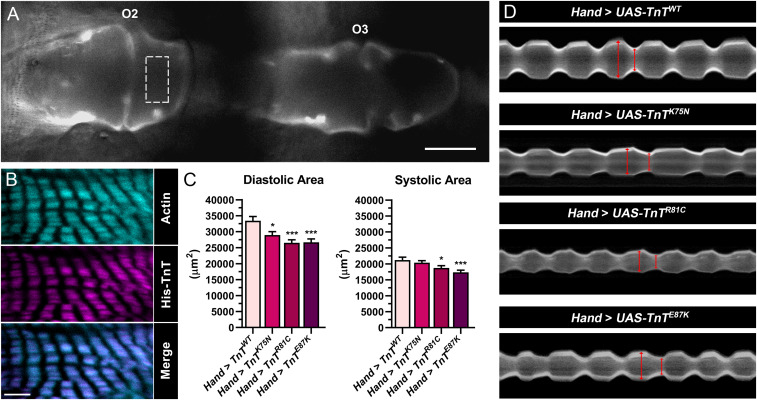
In vivo effects of transgenic overexpression of mutant TnTs in the Drosophila heart. (A) Fluorescent micrograph (40×) of a tdtK-expressing heart tube imaged through the cuticle of a live animal. O2 and O3 denote ostia 2 and 3, which served as positional markers for consistent measurements of chamber areas from multiple flies. Dotted rectangular box depicts the region imaged in B. (Scale bar, 50 µm.) (B) Confocal micrographs of a dissected Hand > TnTWT.His fly heart, taken at 100× magnification, show colocalization of actin and transgenic His-TnT along cardiac thin filaments. (Scale bar, 5 μm.) (C) In vivo analysis of beating hearts from 3-wk-old Hand > TnT flies revealed significantly reduced diastolic chamber areas in all mutants, and reduced systolic chamber areas in the R81C and E87K mutants, relative to control. Data are presented as mean ± SEM (n = 26; *P ≤ 0.05; ***P ≤ 0.001). (D) M-mode kymograms generated from high-speed videos of beating hearts overexpressing WT or mutant TnT and the fluorescent reporter, tdtK. Vertical red lines terminate at opposing edges of the heart wall, with the left line indicating diastolic and the right line indicating the systolic diameter.
We employed the PhiC31 integrase system (45) to generate transgenic lines that permit overexpression of wild-type (WT) Drosophila TnT or the fly equivalent of the K124N, R130C, and E136K cardiomyopathy-inducing TnT variants, specifically in the dorsal vessel. PhiC31 technology ensured the transgenes, preceded by a 5′ upstream activating sequence (UAS), integrated at an identical, predetermined genomic locus, and thus eliminated genetic variability introduced by random insertions. When these lines are crossed with a GAL4 transactivating protein-expressing strain, the progeny inherit both transgenes and ectopically express the TnTs in the same pattern as GAL4 (46), and the results are directly comparable. Initially, to confirm tissue-restricted expression and correct localization of transgenic TnT, Hand4.2-GAL4 Drosophila, which express GAL4 in the heart, were crossed with a line harboring a gene that encodes His-tagged WT TnT, under the control of a UAS regulatory sequence. Anti-His−labeling and confocal microscopy of the progeny (i.e., Hand > TnTWT.His) revealed His-TnT was restricted to, and present throughout, the dorsal vessel. Furthermore, it precisely colocalized with phalloidin-labeled cardiac actin (Fig. 1B). Uniform thin filament incorporation of transgenic TnT, with no resolvable aggregation, was observed.
To test the in vivo effects of the cardiomyopathy-associated TnT variants, males from four transgenic lines, UAS-TnTWT, UAS-TnTK75N, UAS-TnTR81C, and UAS-TnTE87K (numbering according to fly TnT; SI Appendix, Fig. S1), were individually crossed with Hand virgin females that coexpressed tdtK (47); tdtK is a red fluorescent protein that allows visualization of beating hearts directly through the cuticle of live, intact animals (48). Importantly, nontransgenic (Hand x w1118) and WT transgenic (Hand > TnTWT) control hearts behaved indistinguishably from each other (SI Appendix, Fig. S2A), and, therefore, only the latter control was used for subsequent analyses. Female progeny were aged to 3 wk, and their hearts were recorded using fluorescence videography. We measured diastolic and systolic chamber areas spanning regions flanking the second (O2) and third (O3) ostial inflow tracts (Fig. 1A) and observed significant decreases (Fig. 1C) for the mutants relative to control. The reduced diastolic areas are consistent with restrictive cardiac physiology and an inability of the myocardium to relax properly. Corresponding M-mode kymograms that depict mutation-induced, restricted heart wall motion over time are shown in Fig. 1D.
To conduct a more comprehensive assessment of cardiac dysfunction, we surgically exposed the dorsal vessels of 3-wk-old female control and mutant Hand > TnT flies under oxygenated artificial hemolymph (AHL), and recorded high-speed movies of the spontaneously contracting heart tubes. Videos were analyzed using Semiautomated Optical Heartbeat Analysis (SOHA) software (49, 50). This in situ analysis verified the defects observed in vivo with respect to cardiac restriction for all three mutant lines, Hand > TnTK75N, Hand > TnTR81C, and Hand > TnTE87K vs. Hand > TnTWT (diastolic diameters: 65.6 ± 1.0 μm, 56.8 ± 1.2 μm, and 60.1 ± 0.8 μm vs. 70.7 ± 0.9 μm, respectively; systolic diameters: 43.4 ± 0.9 μm, 38.3 ± 0.9 μm, and 40.8 ± 0.6 μm vs. 48.0 ± 0.9 μm, respectively) (Fig. 2A). The resultant decreased cardiac volumes prompted significantly lower cardiac outputs in the mutants relative to control (94.1 ± 4.1 nL/min, 59.3 ± 3.0 nL/min, and 65.0 ± 5.2 nL/min vs. 131.4 ± 5.2 nL/min, respectively). The mutants additionally exhibited slower rates of cardiac relaxation (162.0 ± 5.0 μm/s, 131.2 ± 6.3 μm/s, and 121.6 ± 7.5 μm/s vs. 167.5 ± 10.7 μm/s, respectively). Similar decreases in relaxation rates were observed when exposed hearts were forced to contract against a viscous load of AHL containing 15% Ficoll (wt/vol) (SI Appendix, Fig. S2B). However, since the impact of mutant TnT expression on systolic and diastolic diameters was similar for each mutant, there was no significant effect on fractional shortening (SI Appendix, Fig. S2C). There were also no differences observed in heart rhythm. Heart period (HP, the time interval from the beginning of systole to the end of the following diastole) between genotypes showed significant differences, as did systolic intervals (SI). However, since they both increased to a similar extent per mutant, we did not observe any significant differences in SI/HP, an index that depicts the proportion of time spent generating active tension during the cardiac cycle, relative to control (SI Appendix, Fig. S2C). Overall, both in vivo and in situ analyses demonstrated that our D. melanogaster models, which express the analogous K124N, R130C, and E136K hcTnT mutations, recapitulate the earliest HCM/RCM organ-level phenotypes, including impaired relaxation and diastolic dysfunction.
Fig. 2.
In situ effects of transgenic overexpression of mutant TnTs on Drosophila cardiac function. (A) In situ analysis of 3-wk-old Hand > TnT hearts resolved cardiac restriction, decreased cardiac outputs, and reduced rates of relaxation in mutants relative to control. Data are presented as mean ± SEM (n = 24 to 36; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001). (B) M-mode kymograms generated from high-speed videos of beating hearts that overexpressed WT or mutant TnT. Vertical red lines terminate at opposing edges of the heart wall, with the left line indicating diastolic and the right line indicating systolic diameter.
Restricted Cardiac Dimensions Result from Ca2+-Dependent and Ca2+-Independent Effects of Mutant TnTs on Drosophila Cardiomyocytes.
The dorsal vessel of Drosophila is composed of a single layer of cardiomyocytes, opposing pairs of which are joined by intercellular junctions to form the heart tube lumen. This arrangement uniquely allows analysis of cardiomyocyte behavior, with single-cell resolution, in the context of a functioning organ. Hence, it renders the fly heart conducive for studying diastolic properties at the individual cellular level without the adverse effects associated with cardiomyocyte isolation (15). To investigate the mechanisms responsible for diastolic dysfunction and, specifically, those underlying shortened cardiomyocytes and reduced resting volumes in the TnT mutants, beating hearts from 3-wk-old Hand > TnT females were exposed and treated sequentially with ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA)/EGTA,acetoxymethyl ester (AM)-, followed by blebbistatin-containing AHL solutions. The cardiac tube diameters, which are directly proportional to cellular length, were measured during diastole and again after each treatment. As shown previously, WT dorsal vessel diastole involves less than full 100% relaxation in this preparation (15, 17, 51). This is evident from the effect of intracellular chelation and complete dissociation of Ca2+ via EGTA/EGTA,AM, which increased the diameter by 2.6% compared to diastole, and from myosin inhibition via blebbistatin that caused another 3.7% increase (Fig. 3). Therefore, prevention of cross-bridge binding in diastole is incomplete, due to a small persistence of Tn−Ca2+, and also because thin filament-based inhibition is, intrinsically, imperfect. If the TNT1 mutations tend to allow unobstructed myosin binding, then these phenomena might increase. This is what was observed. The effects of both agents were significantly exaggerated in the presence of the TNT1 mutations, particularly the effect of blebbistatin (Fig. 3 B and C). After prior relaxation from EGTA/EGTA,AM, cellular lengthening due to blebbistatin was almost twice as large for the mutants as for WT. These observations are consistent with restrictive physiology and diastolic dysfunction that result largely from excessively disinhibited cross-bridge cycling, enhanced basal stress, and incomplete relaxation, at the cardiomyocyte level. Thus, the TnT variants cause Tpm and Tn to block myosin binding inadequately, producing excessive myofibrillar tension and myocyte shortening that restrict proper filling.
Fig. 3.
Ca2+-dependent and Ca2+-independent processes contribute to impaired myocardial relaxation in mutant flies. (A) Small-molecule compounds elicited significant, incremental increases in the diameters of 3-wk-old fly hearts. Incubation with EGTA/EGTA,AM-containing AHL chelated extracellular and intracellular Ca2+, halted contraction, and prompted increases in heart tube diameters from baseline (i.e., diastole). Subsequent incubation with blebbistatin inhibited acto-myosin attachments, leading to further increases in cardiac diameters. (B) Ca2+ chelation prompted a significantly greater increase in the diameter of hearts overexpressing the TnT variants vs. WT. (C) Blebbistatin treatment caused an exaggerated response across the wall of all mutant hearts, relative to control. Data are presented as mean ± SEM (n = 20; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001).
Single Myofibrils Isolated from upE87K Drosophila Show Increased Resting Stiffness.
To explore the effects of mutant TnT on the myocyte’s basic contractile elements, and to examine TNT1’s potentially universal role in contractile inhibition across striated muscle types, we compared the structural and mechanical properties of skeletal, indirect flight muscle (IFM) myofibrils containing TnTE87K to those of WT. IFMs provide ample material for physiological analyses, and their thin filaments demonstrate Ca2+-dependent, Tn−Tpm regulatory switching that is indistinguishable from vertebrates (43). Using CRISPR-Cas9 technology, we created a Drosophila knock-in line with the upE87K missense mutation. As all Drosophila TnT isoforms are encoded by a single gene, upheld (up), and the mutation lies within a constitutive exon, these flies express 100% mutant TnT in every muscle. The E87K substitution (corresponding to human E136K; SI Appendix, Fig. S1) is but two residues proximal to the site of the well-characterized, Drosophila-specific E89K mutation known as up101; up101 is a relatively harsh mutation, which engenders severe defects in fly skeletal muscle (52), and restrictive cardiac pathophysiology (53). These phenotypes result from Tpm mispositioning and poor B-state preservation during muscle relaxation. We postulated that it might be possible to examine IFM myofibrils with the E87K mutation, mechanically, unlike those with up101 which hypercontract and self-destruct, particularly with respect to passive stiffness and adequacy of relaxation.
Similar to up101, upE87K homozygotes are viable and fertile. However, in contrast to the severely damaged fibers that typify up101 IFM, upE87K displayed normal IFM morphology (Fig. 4A) and, hence, were well suited for myofibril isolation. Relative to other insect muscles, individual, fully intact myofibrils are easily purified from the IFM for imaging and, despite their high passive stiffness and resistance to stretch, can be analyzed mechanically to discern relative passive length−tension relationships (54, 55). Whether measured in situ, along intact fibers, or ex vivo following isolation, upE87K myofibrils showed a minor, but significant reduction in sarcomere length relative to control (3.31 ± 0.01 μm, 3.32 ± 0.01 μm vs. 3.37 ± 0.01 μm) (Fig. 4B). To compare mechanical properties under low Ca2+ conditions, individual IFM myofibrils were incrementally stretched from their corresponding resting lengths using a glass microtool, a length-controlled motor, and a calibrated cantilevered force probe (56), and tension was determined (Fig. 4 C and D and SI Appendix, Fig. S3A). We detected significantly higher resting tension in upE87K myofibrils compared to control (2.17 ± 0.46 mN/mm2 vs. 0.37 ± 0.09 mN/mm2) (Fig. 4D). To determine whether the elevated stiffness resulted from uninhibited acto-myosin interactions at rest, we treated both mutant and control myofibrils with the myosin inhibitor, 2,3-butanedione monoxime (BDM) (57). BDM incubation prompted no change for control, but induced a drastic drop in upE87K myofibrillar stiffness (Fig. 4D). Post drug treatment, the tension of the mutant myofibrils was indistinguishable from control (Fig. 4D and SI Appendix, Fig. S3A). These findings are consistent with an upE87K-dependent increase in myofibrillar resting tension due to excessive, unobstructed cross-bridge binding that can be relieved upon treatment with a myosin inhibitor.
Fig. 4.
Effects of the hcTnT RCM mutation on IFM myofibrillar properties. (A) Fluorescence (10×) and confocal micrographs (63×, 100×) show no gross structural differences in IFMs (Top) or myofibrils (Middle and Bottom) between control and upE87K Drosophila. Top (10×) depicts hemithoraces that were flash-frozen and sagittally bisected to reveal IFM fiber morphology. Preparations were stained with Alexa-568 phalloidin to label actin. Z discs were distinguished by the expression of GFP-tagged Zasp52, a protein that binds to α-actinin, thereby restricting GFP fluorescence to Z discs. Dotted rectangular boxes highlight the regions imaged below. Sarcomeres were visualized along myofibrils of the hemithoraces, in situ, by confocal microscopy (Middle, 63×). Myofibrils were also isolated for ex vivo imaging (Bottom, 100×). (Scale bars, 5 μm.) (B) upE87K sarcomeres, measured from hemithoraces (in situ) or along isolated myofibrils (ex vivo), were significantly shorter than control (n = 220 to 240). Hence, myofibril removal and isolation from IFMs did not affect mutant sarcomere length. (C) Representative image of an IFM myofibril held between a glass microtool and cantilevered force probe for mechanical assessment. (D) upE87K IFM myofibrils exhibited significantly higher resting tension relative to control, which was restored to WT values postincubation with BDM. Control myofibrils showed no difference in stiffness pre- and post-BDM incubation (n = 7 to 16). Data are presented as mean ± SEM (ns > 0.05; *P ≤ 0.05; ***P ≤ 0.001; ****P ≤ 0.0001).
Ex Vivo Incorporation of Mutant Cardiac TnT Increases Resting Stiffness and Impairs Relaxation of Mammalian Ventricular Myofibrils.
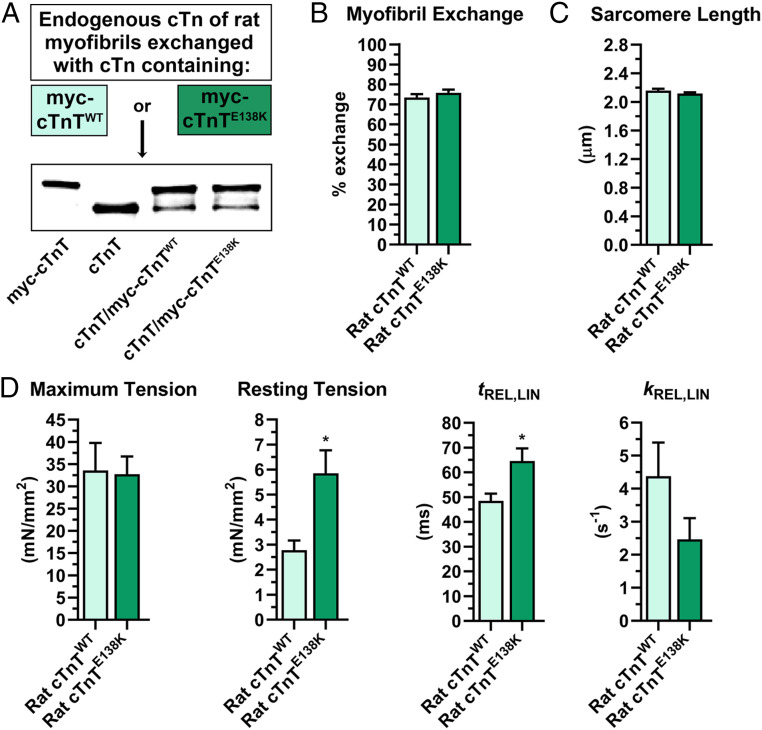
To verify the pathological effects of the TNNT2 RCM mutation in a vertebrate model, we cloned, expressed, and purified WT and mutant rat myc-tagged cTnT. The recombinant proteins were reconstituted into functional, trimeric Tn complexes. The endogenous Tn of myofibrils isolated from the left ventricles of adult rats was then replaced with exogenous Tn containing either WT or E138K rat cTnT (corresponding to human E136K; SI Appendix, Fig. S1). The extent of exchange was similar for both mutant and WT cTnT-containing Tns (75.9 ± 1.8% vs. 73.5 ± 2.5%, respectively) (Fig. 5 A and B), and the variant had no effect on sarcomere length (2.1 ± 0.1 μm vs. 2.2 ± 0.1 μm) (Fig. 5C). A rapid solution switching approach was then used to study the kinetics of myofibrillar force development and relaxation following abrupt changes in Ca2+ (58, 59). Substituting endogenous Tn with myc-cTnTE138K−Tn significantly increased the resting tension of myofibrils relative to those containing myc-cTnTWT−Tn (5.86 ± 0.92 mN/mm2 vs. 2.78 ± 0.39 mN/mm2), without altering maximum tension (32.75 ± 3.98 mN/mm2 vs. 33.59 ± 6.19 mN/mm2) or the rate constant of tension generation (kACT: 2.59 ± 0.19 s−1 vs. 2.60 ± 0.17 s−1) (Fig. 5D and SI Appendix, Fig. S3B and Table S2). Moreover, myofibrils with myc-cTnTE138K−Tn had a significantly longer initial, linear phase of relaxation, compared to those harboring myc-cTnTWT−Tn (tREL,LIN: 64.59 ± 5.08 s vs. 48.48 ± 2.93 s) (Fig. 5D and SI Appendix, Fig. S3B), which reflects a slower rate of cross-bridge detachment (59). There were no significant differences in the rate constants of the initial or exponential phases of relaxation in myofibrils with myc-cTnTE138K−Tn vs. myc-cTnTWT−Tn (kREL,LIN: 2.47 ± 0.64 s−1 vs. 4.38 ± 1.02 s−1; kREL,EXP: 33.95 ± 2.56 s−1 vs. 29.28 ± 1.50 s−1), or in the rate constant of tension redevelopment (kTR: 3.36 ± 0.23 s−1 vs. 3.24 ± 0.35 s−1) (Fig. 5D and SI Appendix, Table S2). Of note is that rat ventricular cardiomyocytes, expressing mutant cTnTE138K via adenovirus-mediated gene transfer, exhibited reduced sarcomere lengths, higher contraction velocities, and prolonged relaxation times, akin to what we observed in the fly models (SI Appendix, Fig. S4). Therefore, the fly skeletal and vertebrate cardiac myofibril data illustrate that the TnT variant leads to disinhibition of thin filaments at rest and impairs relaxation. These results support our hypothesis that HCM/RCM charge-altering mutations in hcTNT1’s Tpm-binding element may jeopardize its important role in contractile inhibition and relaxation.
Fig. 5.
Effects of the hcTnT RCM mutation on rat cardiac myofibrillar properties. (A) Myofibrils isolated from rat ventricles were incubated with myc-cTnTWT− or myc-cTnTE138K−containing Tn to replace endogenous Tn complexes. Western blotting was performed with an anti-TnT antibody to quantify the extent of Tn exchange. The first two lanes of the blot show mobility differences between purified recombinant rat myc-cTnT and endogenous rat cTnT, followed by rat myofibrillar cTnT composition after exchange with either myc-cTnTWT− or myc-cTnTE138K−containing Tn. (B) Tn exchange was quantified by dividing the signal intensity of the myc-tagged protein by the total intensity of cTnT (myc-cTnT + cTnT), which confirmed a similar extent of exchange for both myc-cTnTWT− and myc-cTnTE138K−containing Tn, within myofibrils (n = 4 biological replicates, 9 or 10 technical replicates each). (C) No difference was observed in sarcomere lengths along myofibrils exchanged with either myc-cTnTWT− or myc-cTnTE138K−containing Tn. (D) Analysis of mechanical properties of rat ventricular myofibrils exchanged with myc-cTnTWT− vs. myc-cTnTE138K−containing Tn revealed no difference in maximum tension generated. Significant differences were observed in myofibrillar resting tension and in the duration of the linear phase of relaxation (tREL,LIN). However, the rate constant of this phase (kREL,LIN) was not affected (n = 4; six to eight myofibrils/replicate). Data are presented as mean ± SEM (*P ≤ 0.05).
TNNT2 Cardiomyopathy Mutations Impair Human Cardiac TNT1’s Inhibitory Properties In Vitro.
To examine the inhibitory properties of the K124N, R130C, and E136K hcTnT variants in a fully defined reconstituted system, we employed a four-element in vitro motility (IVM) assay. The translocation of fluorescently labeled rabbit skeletal F-actin, over beds of immobilized rabbit skeletal myosin, was recorded and filament sliding velocity was assessed under different experimental conditions. We first examined the effects of Tpm and Tpm−hcTNT1WT on actin filament sliding velocity over a range of myosin concentrations (12.5 μg/mL to 100 μg/mL) (Fig. 6A). The addition of bovine cardiac Tpm decreased F-actin velocities, by 50% or more at the lower myosin concentrations (i.e., 12.5 μg/mL and 25 μg/mL) assayed (Fig. 6A). With greater myosin concentration on the IVM surface, the heads cooperatively overcame part of this inhibition. However, even at 75 μg/mL myosin, when maximum sliding velocity was reached, F-actin was propelled at a velocity of 3.6 ± 0.2 μm/s, while F-actin−Tpm sliding velocity was 3.2 ± 0.2 μm/s (Fig. 6A). These observations support the notion that, under subsaturating myosin conditions, Tpm can be inhibitory because its default azimuthal location along F-actin is dictated by numerous, highly favorable, interfacial electrostatic interactions that position Tpm such that it adversely affects acto-myosin cycling (8, 12, 13, 15, 35–41). Addition of hcTNT1WT to F-actin−Tpm drastically reduced filament sliding velocities across all myosin concentrations (Fig. 6A). At 75 μg/mL myosin, the average sliding speed decreased roughly 50% relative to F-actin to 1.9 ± 0.3 μm/s (Fig. 6A). The inhibitory effect was much larger than that resulting from Tpm alone and is consistent with earlier studies showing an hcTNT1WT-mediated stabilization of Tpm in the B state (12). Two-way ANOVA confirmed that, as expected, myosin concentration (P < 0.0001) and the addition of Tpm or Tpm−hcTNT1WT (i.e., filament type, P < 0.0001) significantly affected F-actin sliding velocities (Fig. 6A). To test whether the TNNT2 mutations affect hcTNT1’s inhibitory action, we repeated the assay with hcTNT1K124N-, hcTNT1R130C-, and hcTNT1E136K-containing filaments. All three mutant filaments exhibited higher myosin-propelled sliding velocities relative to controls at nearly every myosin concentration assayed (Fig. 6 B–D). Two-way ANOVA again confirmed significant effects of myosin concentration, and each mutant Tpm−hcTNT1 relative to Tpm−hcTNT1WT, on F-actin sliding velocities, with nonsignificant interaction effects (Fig. 6 B–D). Thus, the rate of change of velocity for each mutant filament vs. WT, over the various beds of myosin tested, did not differ. Moreover, at a subsaturating myosin concentration, a relatively greater proportion of filaments with the HCM hcTNT1K124N and RCM hcTNT1E136K peptides were motile (SI Appendix, Fig. S5). The increase in sliding velocities and higher proportion of motile filaments that contain mutant hcTNT1 peptides confirm less complete hcTNT1-mediated thin filament inhibition. These results are in accord with our findings overall, in Drosophila hearts and myofibrils, and rat myofibrils and cardiomyocytes.
Fig. 6.
In vitro effects of mutant hcTNT1 peptides on F-actin−Tpm sliding velocity. (A) IVM assays were performed over a range of myosin concentrations. The addition of Tpm to F-actin (dark gray line) reduced sliding velocities relative to F-actin alone (light gray line), at all myosin concentrations. This inhibitory effect was more pronounced upon addition of Tpm−hcTNT1WT (black line). Myosin concentration and the filament type (i.e., F-actin−Tpm ± hcTNT1WT) had significant effects on F-actin sliding velocity as determined by two-way ANOVA. (B–D) Filaments containing the three mutant hcTNT1 peptides (blue lines) showed higher sliding velocities relative to internal F-actin−Tpm−hcTNT1WT controls (black lines), at all myosin concentrations tested. Two-way ANOVA confirmed significant effects of filament type (WT vs. mutant hcTNT1-containing filaments) as well as myosin concentration, on F-actin−Tpm sliding velocity. Note, motility of the internal F-actin−Tpm−hcTNT1WT controls, from each experiment, did not significantly differ (SI Appendix, Fig. S8). Data are presented as mean ± SEM (n = 2 to 7 replicates; 20 to 30 filaments/replicate).
Discussion
Newly revealed structural details of the thin filament offer the prospect that investigations of its protein components can provide novel insight into how missense mutations provoke disease, and also into the more general mechanism of contractile regulation. Since its discovery more than half a century ago, Tn’s ability to shut off muscle contraction has been attributed to TnI (1, 3, 60–62). Therefore, it was unexpected, in 2002, when two groups separately demonstrated that the skeletal and cardiac TNT1 fragments could reduce F-actin−Tpm-activated myosin S1 ATPase activity and S1-actin binding, and prompt inhibitory conformational states of Tpm (12, 13). A likely candidate segment responsible for these effects is the Tpm-binding region of TNT1, which includes several charged residues and has high sequence identity/similarity (SI Appendix, Fig. S1), suggesting it is critical to TnT function in all striated muscle. Here we explored the importance and mechanism of TNT1-mediated suppression of contractile activity by investigating how its disruption, due to naturally occurring K124N, R130C, and E136K hcTnT mutations, influences relaxation across a host of distinct model systems.
We began by generating fly models to evaluate the effects of the TnT variants on whole heart function. Transgenic TnT incorporated uniformly along Drosophila cardiac thin filaments (Fig. 1), and the mutant proteins engendered restricted heart tubes, reduced cardiac outputs, and slower relaxation rates (Figs. 1 and 2), consistent with diastolic dysfunction and impaired relaxation that precede hypertrophy in humans (26, 28). Measurements of heart diameters following sequential treatment with EGTA/EGTA,AM and blebbistatin revealed that the mutants’ cardiac restriction resulted from both Ca2+-dependent and Ca2+-independent processes (Fig. 3). The former potentially include altered Ca2+ handling and/or elevated Ca2+ sensitivity. The latter likely involve poor Tpm-based obstruction of myosin binding sites, leading to a greater number of freely cycling cross-bridges during both diastole and systole. Elevated Ca2+ sensitivity of reconstituted thin filaments containing the K124N and R130C hcTnT HCM mutations was previously reported (34). However, at the cardiomyocyte level, a greater increase in resting cell length was observed upon blebbistatin-mediated inhibition of myosin-dependent tension, in the effective absence of free intracellular Ca2+. Therefore, we propose that TNT1 normally serves as an indispensable anchor that helps restrain Tpm azimuthal motion, and the predominant cause underlying diastolic dysfunction is defective anchoring of the Tpm overlap domain in the inhibitory position recently described (9). Defective Tpm anchoring in a myosin-blocking position could also affect Ca2+ sensitivity: myosin binding enhances TnC’s affinity for Ca2+ (1), and, therefore, weakening of the thin filament B state could drive both Ca2+-dependent and Ca2+-independent effects of the TnT mutations, and represent the ultimate cause of disease.
To further investigate the E136K mutation, including its effect on TNT1’s inhibitory role in skeletal muscle, we generated upE87K knock-in Drosophila. These flies, which are reminiscent of up101 (E89K) flies that exhibit severe diastolic dysfunction and IFM hypercontraction due to an impaired thin filament B state (53), enabled mechanical evaluation of individual myofibrils containing 100% mutant TnT. Mutant IFM myofibrils displayed a higher level of resting tension that was restored to WT levels by the myosin inhibitor, BDM (Fig. 4). These results corroborated those obtained following cardiac-restricted overexpression of UAS-TnTE87K. We then established the generality of the results in a vertebrate model system, by demonstrating elevated resting tension in isolated rat ventricular myofibrils with 75% of endogenous Tn replaced by Tn containing the rat equivalent of the same, RCM-causing variant. Furthermore, relaxation kinetics revealed a significant increase in the linear relaxation interval in myofibrils after Tn exchange, suggesting a slower rate of cross-bridge detachment and force cessation (Fig. 5) (59). Likewise, expression of the variant in rat ventricular cardiomyocytes increased relaxation times and reduced sarcomere lengths (SI Appendix, Fig. S4). These findings imply disinhibited thin filaments that permit excessive acto-myosin interactions, thereby prolonging the time required for force termination, elevating myofibril stiffness, and abnormally shortening cardiomyocytes at rest.
To study the effects of the TNT1 variant peptides on acto-myosin interactions in a fully defined, reconstituted system, we conducted IVM assays using hcTNT1 peptides. In concordance with previous work, Tpm is inhibitory in this context, and WT hcTNT1 exaggerates this inhibition (12, 13). Both sliding speeds and the number of motile filaments were affected, particularly under subsaturating myosin conditions (Fig. 6A and SI Appendix, Fig. S5). Therefore, in addition to structural evidence that implies TNT1 can restrict Ca2+-dependent motion of the Tpm end-to-end overlap domain along Tn-regulated thin filaments (9), we verified earlier IVM data that suggest hcTNT1WT alone can likewise temper myosin-based shifts in Tpm and, ergo, enhance Tpm’s inhibitory effects (12, 13). Filaments reconstituted with each of the three mutant hcTNT1 peptides were, nevertheless, propelled at considerably higher velocities compared to F-actin−Tpm−hcTNT1WT (Fig. 6 B–D). These observations indicate inefficient blocking of acto-myosin activity due to less effective inhibitory Tpm positioning and, thus, impaired hcTNT1-mediated thin filament regulation by the HCM/RCM variants.
Cryo-EM and computationally driven docking protocols have produced contemporary models of the N-terminal, α-helical TNT1 tail complexed with overlapping Tpms, along thin filaments and in isolation (9, 11). Taking into account these structures, previous findings, and our current results, we offer a plausible mechanism for TNT1’s role in anchoring the Tpm overlap region and, hence, its contribution to contractile inhibition, as well as the molecular origins of cardiomyopathy. Numerous electrostatic contacts between successive F-actin protomers and Tpm, along its entire length including the end-to-end overlap, seemingly establish an energetically favorable configuration where Tpm impedes acto-myosin associations and inherently promotes relaxation (8, 12, 13, 15, 35–41). TNT1 binds to the Tpm overlap domain via a series of conserved amino acids (11, 18–20). This high-affinity interaction is predicted to occur through integrated hydrophobic networks and multiple, closely packed salt bridges that involve several invariant residues (11). When bound, TNT1 enhances the affinity and the interaction energetics of Tpm for F-actin (11, 18, 21–25). We submit that TNT1 binding to Tpm could, in turn, optimize the accessibility of charged amino acid sidechains of the Tpm end-to-end overlap, and its surrounding regions, to their oppositely charged binding partners along adjacent actin protomers (16). Precise TNT1−Tpm coupling may thereby, at least in part, account for the enhanced affinity of Tpm for F-actin (18, 23–25). Additionally, putative interactions directly between TNT1 and actin could also fortify F-actin−Tpm binding. The augmented F-actin−Tpm attractive forces may, consequently, stabilize Tpm’s intrinsic inhibitory positioning and heighten the energetic demands required to uniformly displace Tpm for activation. Earlier studies have shown HCM-causing mutations that remove or replace charged residues located throughout and adjacent to TNT1's conserved Tpm-binding element can reduce the affinity of TNT1 for Tpm (18, 34) and of Tpm for F-actin (18). These findings are consistent with an interface between TNT1 and Tpm that can be disrupted by amino acid substitutions (11). Perturbations which weaken TNT1−Tpm interaction may dampen TNT1’s effect on F-actin−Tpm contacts, or disrupt TNT1−actin associations and, thus, ease restrictions on Tpm motion that compromise normal inhibition of cross-bridge binding at rest. Additionally, by buttressing the Tpm−Tpm overlap, TNT1 increases cooperative activation (12, 24), and likely inactivation, of the thin filament. Mutations that reduce TNT1−Tpm binding may influence the latter by diminishing the cooperative propagation of inhibitory positioning of Tpm to the next Tpm along the actin filament, resulting in a greater number of exposed myosin binding sites at rest, and impaired relaxation associated with early cardiac remodeling and disease. In sum, we propose a molecular basis for the noncanonical, regulatory role of TnT, acting to reinforce inhibitory positioning of the end-to-end overlap domain of Tpm. This additional layer of control complements TnI-mediated inhibition and is necessary for normal muscle relaxation, and its disruption potentially accounts for the most proximal, disease-inducing effects of specific TNNT2 mutations.
Materials and Methods
Detailed information regarding materials and methods is available in SI Appendix.
Construction of UAS-TnT Transgenes and Transgenic Drosophila.
A full-length fly TnT (up) construct, RE18550, was obtained from the Drosophila Genomics Resource Center and cloned into the pUASTattB vector. HCM/RCM mutations, K75N, R81C, E87K, were introduced via the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, #200523). Transgenic lines were generated using the PhiC31 integrase system (14, 45). To create homozygous upE87K flies, a point mutation was introduced into the TnT-encoding up gene via CRISPR-Cas9 technology, following published protocols (63).
Fly Husbandry.
Flies were raised at 25 °C on standard medium. Heart-restricted transgene expression was achieved by the GAL4-UAS bipartite expression system (46), using the Hand4.2-GAL4 cardiac driver line.
Drosophila In Vivo Cardiac Analysis.
Adult hearts coexpressing transgenic UAS-TnT constructs and the tandem-tomato (tdtK) fluorescent protein (47, 48) were visualized through the cuticle of live flies. Twenty-six 3-wk-old animals were anesthetized, glued to a coverslip, and their cardiac tubes recorded at 250 frames per second using a Hamamatsu Orca Flash 4.0 CMOS camera (Hamamatsu Photonics K.K.) on a Zeiss Imager.M1 microscope (Carl Zeiss Microscopy) with a 25× air lens. Chamber areas were determined by outlining the region of the dorsal vessel spanning Ostia 2 and 3 in Fiji (https://imagej.net/Fiji).
Drosophila In Situ Cardiac Analysis.
The heart tubes of 24 to 36 3-wk-old, female Hand > TnT flies were surgically exposed under oxygenated AHL at 25 °C (64). Imaging of beating, semiintact preparations, and analysis of cardiac performance using SOHA software, were performed as described previously (49, 50).
Measurement of EGTA/EGTA,AM-Induced and Blebbistatin-Induced Changes in Cardiac Dimensions.
Semiintact preparations of beating hearts from twenty 3-wk-old, female Hand > TnT flies were filmed and assessed prior to and following incubations with EGTA/EGTA,AM-, and, subsequently, blebbistatin-containing AHL, as delineated previously (15, 17, 51). EGTA is a high-affinity Ca2+ chelator, EGTA,AM is a cell-permeant form of EGTA, and blebbistatin is a small-molecule myosin inhibitor.
Drosophila IFM Myofibril Isolation and Mechanics.
IFM myofibrils were isolated using a modified version of a published protocol (65). Myofibrils were pelleted at 850 × g at 4 °C for 5 min and resuspended in 300 µL of bath solution (66). A drop of suspended myofibrils was plated on a 15 °C chamber of an inverted microscope, and 1 mL of bath solution was added. Myofibrils were lifted horizontally between a glass microtool, controlled by a length-controlled motor (Mad City Labs), and a calibrated cantilevered force probe (compliance of 12.26 µm/µN) (56). Then, 7 to 16 myofibrils were stretched incrementally to determine resting tension at different sarcomere lengths at pCa 9. Tension was also assessed in the presence of 50 mmol/L BDM. Data were collected and analyzed using customized LabView software.
Ex Vivo Exchange of Myc-cTnT within Rat Myofibrils.
Rat myc-tagged WT Tnnt2 and the RCM mutant (E138K) were cloned, expressed, purified, and reconstituted as Tn complexes (67). Myofibrils isolated from Sprague–Dawley rat ventricles (68) were resuspended in a solution containing 15 µM recombinant Tn complex and incubated overnight at 4 °C to replace endogenous Tn (69, 70). The extent of exchange was quantified via Western blot analysis.
Vertebrate Myofibril Mechanics.
A small bundle of Tn-exchanged ventricular myofibrils was mounted between two microtools, one of which was attached to a calibrated cantilevered force probe (8.67 μm/μN). Myofibrils were activated and relaxed using standard procedures of rapid solution switching to investigate contraction and relaxation kinetics (58, 59, 71). Data were collected and analyzed using customized LabView software.
Immunohistochemistry and Fluorescence Imaging of Drosophila Muscle.
Confocal and fluorescence microscopy of heart tubes and IFMs were performed as described previously (14, 15). GFP-Zasp52−expressing flies were generated via standard crosses. In situ and isolated IFM myofibrils were stained with Alexa-568 phalloidin (1:100 in phosphate-buffered saline), mounted on a glass slide, and imaged at 63× and 100×, respectively, with a Leica TCS SPE RGBV confocal microscope (Leica Microsystems). The average distance between adjacent GFP-tagged Z discs, from stained hemithoraces and isolated myofibrils imaged exclusively at 100×, was measured using Fiji (n = 220 to 240 sarcomeres).
hcTNT1 Expression and Purification.
The TNNT2 sequence encoding residues 1 to 156 of hcTNT1 was cloned into a pET15b expression vector. K124N, R130C, and E136K substitutions were introduced using site-directed mutagenesis (described above). Peptides were expressed and purified as outlined in Hinkle et al. (25).
In Vitro Motility.
Sliding speeds of Alexa-568-phalloidin-labeled rabbit skeletal F-actin (10 nmol/L), and filaments decorated with 300 nmol/L of both bovine cardiac Tpm and WT or mutant hcTNT1, over purified, full-length rabbit skeletal myosin (72) were measured via IVM assays (73); 20 to 30 filaments per replicate were manually tracked and measured over a range of myosin concentrations (12.5 μg/mL to 100 μg/mL) at 30 °C, pH 7.2, and an ionic strength of 37 mmol/L, in two to seven replicate experiments using the Fiji plugin, MTrackJ.
Statistical Analysis.
Statistical analyses were performed using GraphPad Prism software (v8.1.3). Details of analyses, and raw data (as scatter plots), are provided in SI Appendix, Figs. S6 and S7.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants R01HL124091 (A.C.), R01HL063774 (L.S.T.), 2K12 HD057022-11 (K.C.W.), R01HL114940 (B.J.B.), and R01HL108917 and R01HL137259 (B.O.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2001692117/-/DCSupplemental.
Data Availability.
All data, associated protocols, methods, and sources of materials are available in the main text or SI Appendix.
References
- 1.Tobacman L. S., Thin filament-mediated regulation of cardiac contraction. Annu. Rev. Physiol. 58, 447–481 (1996). [DOI] [PubMed] [Google Scholar]
- 2.Tardiff J. C., Thin filament mutations: Developing an integrative approach to a complex disorder. Circ. Res. 108, 765–782 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geeves M. A., “Thin filament regulation” in Comprehensive Biophysics, Egelman E. H., Goldman Y. E., Ostap E. M., Eds. (Academic, Oxford, United Kingdom, 2012), Vol. 4, pp. 251–267. [Google Scholar]
- 4.Lehman W., Thin filament structure and the steric blocking model. Compr. Physiol. 6, 1043–1069 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Hitchcock-DeGregori S. E., Barua B., “Tropomyosin structure, function, and interactions: A dynamic regulator” in Fibrous Proteins: Structures and Mechanisms, Parry D. A. D., Squire J., Eds. (Springer, 2017), pp. 253–284. [DOI] [PubMed] [Google Scholar]
- 6.Li X. E., Lehman W., Fischer S., The relationship between curvature, flexibility and persistence length in the tropomyosin coiled-coil. J. Struct. Biol. 170, 313–318 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hitchcock-DeGregori S. E., Tropomyosin: Function follows structure. Adv. Exp. Med. Biol. 644, 60–72 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Holmes K. C., Lehman W., Gestalt-binding of tropomyosin to actin filaments. J. Muscle Res. Cell Motil. 29, 213–219 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Yamada Y., Namba K., Fujii T., Cardiac muscle thin filament structures reveal calcium regulatory mechanism. Nat. Commun. 11, 153 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orzechowski M., Li X. E., Fischer S., Lehman W., An atomic model of the tropomyosin cable on F-actin. Biophys. J. 107, 694–699 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavadai E., Rynkiewicz M. J., Ghosh A., Lehman W., Docking troponin T onto the tropomyosin overlapping domain of thin filaments. Biophys. J. 118, 325–336 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobacman L. S. et al., The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J. Biol. Chem. 277, 27636–27642 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Maytum R., Geeves M. A., Lehrer S. S., A modulatory role for the troponin T tail domain in thin filament regulation. J. Biol. Chem. 277, 29774–29780 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Viswanathan M. C., Blice-Baum A. C., Schmidt W., Foster D. B., Cammarato A., Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance. Front. Physiol. 6, 116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viswanathan M. C. et al., Distortion of the actin A-Triad results in contractile disinhibition and cardiomyopathy. Cell Rep. 20, 2612–2625 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt W., Cammarato A., The actin “A‐Triad”s’ role in contractile regulation in health and disease. J. Physiol., 10.1113/JP276741 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viswanathan M. C. et al., A role for actin flexibility in thin filament-mediated contractile regulation and myopathy. Nat. Commun. 11, 2417 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palm T., Graboski S., Hitchcock-DeGregori S. E., Greenfield N. J., Disease-causing mutations in cardiac troponin T: Identification of a critical tropomyosin-binding region. Biophys. J. 81, 2827–2837 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinkle A., Tobacman L. S., Folding and function of the troponin tail domain. Effects of cardiomyopathic troponin T mutations. J. Biol. Chem. 278, 506–513 (2003). [DOI] [PubMed] [Google Scholar]
- 20.Jin J. P., Chong S. M., Localization of the two tropomyosin-binding sites of troponin T. Arch. Biochem. Biophys. 500, 144–150 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson P., Amphlett G. W., Perry S. V., The primary structure of troponin T and the interaction with tropomyosin. Biochem. J. 151, 85–97 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearlstone J. R., Smillie L. B., The binding site of skeletal alpha-tropomyosin on troponin-T. Can. J. Biochem. 55, 1032–1038 (1977). [DOI] [PubMed] [Google Scholar]
- 23.Hill L. E., Mehegan J. P., Butters C. A., Tobacman L. S., Analysis of troponin-tropomyosin binding to actin. Troponin does not promote interactions between tropomyosin molecules. J. Biol. Chem. 267, 16106–16113 (1992). [PubMed] [Google Scholar]
- 24.Schaertl S., Lehrer S. S., Geeves M. A., Separation and characterization of the two functional regions of troponin involved in muscle thin filament regulation. Biochemistry 34, 15890–15894 (1995). [DOI] [PubMed] [Google Scholar]
- 25.Hinkle A., Goranson A., Butters C. A., Tobacman L. S., Roles for the troponin tail domain in thin filament assembly and regulation. A deletional study of cardiac troponin T. J. Biol. Chem. 274, 7157–7164 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Garfinkel A. C., Seidman J. G., Seidman C. E., Genetic pathogenesis of hypertrophic and dilated cardiomyopathy. Heart Fail. Clin. 14, 139–146 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Masarone D. et al., Epidemiology and clinical aspects of genetic cardiomyopathies. Heart Fail. Clin. 14, 119–128 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Green E. M. et al., A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351, 617–621 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thierfelder L. et al., Alpha-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: A disease of the sarcomere. Cell 77, 701–712 (1994). [DOI] [PubMed] [Google Scholar]
- 30.Szczesna D. et al., Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. J. Biol. Chem. 275, 624–630 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Miller T. et al., Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79N) mutation. J. Biol. Chem. 276, 3743–3755 (2001). [DOI] [PubMed] [Google Scholar]
- 32.Harada K., Potter J. D., Familial hypertrophic cardiomyopathy mutations from different functional regions of troponin T result in different effects on the pH and Ca2+ sensitivity of cardiac muscle contraction. J. Biol. Chem. 279, 14488–14495 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Manning E. P., Guinto P. J., Tardiff J. C., Correlation of molecular and functional effects of mutations in cardiac troponin T linked to familial hypertrophic cardiomyopathy: An integrative in silico/in vitro approach. J. Biol. Chem. 287, 14515–14523 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangadharan B. et al., Molecular mechanisms and structural features of cardiomyopathy-causing troponin T mutants in the tropomyosin overlap region. Proc. Natl. Acad. Sci. U.S.A. 114, 11115–11120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown J. H. et al., Structure of the mid-region of tropomyosin: Bending and binding sites for actin. Proc. Natl. Acad. Sci. U.S.A. 102, 18878–18883 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li X. E. et al., Tropomyosin position on F-actin revealed by EM reconstruction and computational chemistry. Biophys. J. 100, 1005–1013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barua B., Pamula M. C., Hitchcock-DeGregori S. E., Evolutionarily conserved surface residues constitute actin binding sites of tropomyosin. Proc. Natl. Acad. Sci. U.S.A. 108, 10150–10155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von der Ecken J. et al., Structure of the F-actin-tropomyosin complex. Nature 519, 114–117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barua B., Fagnant P. M., Winkelmann D. A., Trybus K. M., Hitchcock-DeGregori S. E., A periodic pattern of evolutionarily conserved basic and acidic residues constitutes the binding interface of actin-tropomyosin. J. Biol. Chem. 288, 9602–9609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rynkiewicz M. J. et al., Tropomyosin must interact weakly with actin to effectively regulate thin filament function. Biophys. J. 113, 2444–2451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehman W., Orzechowski M., Li X. E., Fischer S., Raunser S., Gestalt-binding of tropomyosin on actin during thin filament activation. J. Muscle Res. Cell Motil. 34, 155–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reiter L. T., Potocki L., Chien S., Gribskov M., Bier E., A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 11, 1114–1125 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cammarato A. et al., Drosophila muscle regulation characterized by electron microscopy and three-dimensional reconstruction of thin filament mutants. Biophys. J. 86, 1618–1624 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Limpitikul W. B., Viswanathan M. C., O’Rourke B., Yue D. T., Cammarato A., L-type calcium channels are a major source of plasmalemmel calcium influx for Drosophila cardiomyocytes. Biophys. J. 116, 152a–153a (2019). [Google Scholar]
- 45.Groth A. C., Fish M., Nusse R., Calos M. P., Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics 166, 1775–1782 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brand A. H., Perrimon N., Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401–415 (1993). [DOI] [PubMed] [Google Scholar]
- 47.Shaner N. C. et al., Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Klassen M. P. et al., Age-dependent diastolic heart failure in an in vivo Drosophila model. elife 6, e20851 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fink M. et al., A new method for detection and quantification of heartbeat parameters in Drosophila, zebrafish, and embryonic mouse hearts. Biotechniques 46, 101–113 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cammarato A., Ocorr S., Ocorr K., Enhanced assessment of contractile dynamics in Drosophila hearts. Biotechniques 58, 77–80 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Kronert W. A. et al., Prolonged cross-bridge binding triggers muscle dysfunction in a Drosophila model of myosin-based hypertrophic cardiomyopathy. eLife 7, e38064 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fyrberg E., Fyrberg C. C., Beall C., Saville D. L., Drosophila melanogaster troponin-T mutations engender three distinct syndromes of myofibrillar abnormalities. J. Mol. Biol. 216, 657–675 (1990). [DOI] [PubMed] [Google Scholar]
- 53.Viswanathan M. C., Kaushik G., Engler A. J., Lehman W., Cammarato A., A Drosophila melanogaster model of diastolic dysfunction and cardiomyopathy based on impaired troponin-T function. Circ. Res. 114, e6–e17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kulke M. et al., Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle. J. Cell Biol. 154, 1045–1057 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hao Y., Bernstein S. I., Pollack G. H., Passive stiffness of Drosophila IFM myofibrils: A novel, high accuracy measurement method. J. Muscle Res. Cell Motil. 25, 359–366 (2004). [DOI] [PubMed] [Google Scholar]
- 56.Colomo F., Piroddi N., Poggesi C., te Kronnie G., Tesi C., Active and passive forces of isolated myofibrils from cardiac and fast skeletal muscle of the frog. J. Physiol. 500, 535–548 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ostap E. M., 2,3-Butanedione monoxime (BDM) as a myosin inhibitor. J. Muscle Res. Cell Motil. 23, 305–308 (2002). [DOI] [PubMed] [Google Scholar]
- 58.Tesi C., Colomo F., Nencini S., Piroddi N., Poggesi C., The effect of inorganic phosphate on force generation in single myofibrils from rabbit skeletal muscle. Biophys. J. 78, 3081–3092 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tesi C., Piroddi N., Colomo F., Poggesi C., Relaxation kinetics following sudden Ca2+ reduction in single myofibrils from skeletal muscle. Biophys. J. 83, 2142–2151 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartshorne D. J., Perry S. V., Davies V., A factor inhibiting the adenosine triphosphatase activity and the superprecipitation of actomyosin. Nature 209, 1352–1353 (1966). [DOI] [PubMed] [Google Scholar]
- 61.Hartshorne D. J., Perry S. V., Schaub M. C., A protein factor inhibiting the magnesium-activated adenosine triphosphatase of desensitized actomyosin. Biochem. J. 104, 907–913 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perry S., Cole H., Head J., Wilson F., “Localization and mode of action of the inhibitory protein component of the troponin complex” in Cold Spring Harbor Symposia on Quantitative Biology, Gordon J., Ed. (Cold Spring Harbor Laboratory Press, 1973), Vol. 37, pp. 251–262. [Google Scholar]
- 63.Gratz S. J., Rubinstein C. D., Harrison M. M., Wildonger J., O’Connor‐Giles K. M., CRISPR‐Cas9 genome editing in Drosophila. Curr. Protoc. Mol. Biol. 111, 31.2.1-31.2.20 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogler G., Ocorr K., Visualizing the beating heart in Drosophila. J. Vis. Exp., 1425 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swank D. M., Mechanical analysis of Drosophila indirect flight and jump muscles. Methods 56, 69–77 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker J. S., Walker L. A., Margulies K., Buttrick P., de Tombe P., Protein kinase A changes calcium sensitivity but not crossbridge kinetics in human cardiac myofibrils. Am. J. Physiol. Heart Circ. Physiol. 301, H138–H146 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Engel P. L. et al., Identification of a region of troponin I important in signaling cross-bridge-dependent activation of cardiac myofilaments. J. Biol. Chem. 282, 183–193 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Jeong M. Y. et al., Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci. Transl. Med. 10, eaao0144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brenner B., Kraft T., Yu L. C., Chalovich J. M., Thin filament activation probed by fluorescence of N-((2-(iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1,3-diazole-labeled troponin I incorporated into skinned fibers of rabbit psoas muscle. Biophys. J. 77, 2677–2691 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.She M., Trimble D., Yu L. C., Chalovich J. M., Factors contributing to troponin exchange in myofibrils and in solution. J. Muscle Res. Cell Motil. 21, 737–745 (2000). [DOI] [PubMed] [Google Scholar]
- 71.Colomo F., Nencini S., Piroddi N., Poggesi C., Tesi C., “Calcium dependence of the apparent rate of force generation in single striated muscle myofibrils activated by rapid solution changes” in Mechanisms of Work Production and Work Absorption in Muscle, Sugi H., Pollack G. H., Eds. (Springer, 1998), pp. 373–382. [DOI] [PubMed] [Google Scholar]
- 72.Margossian S. S., Lowey S., “Preparation of myosin and its subfragments from rabbit skeletal muscle” in Methods in Enzymology, Kaplan N. O., Colowick S. P., Eds. (Academic, 1982), Vol. 85, pp. 55–71. [DOI] [PubMed] [Google Scholar]
- 73.Kron S. J., Spudich J. A., Fluorescent actin filaments move on myosin fixed to a glass surface. Proc. Natl. Acad. Sci. U.S.A. 83, 6272–6276 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, associated protocols, methods, and sources of materials are available in the main text or SI Appendix.