Significance
The attributes allowing individuals to attain positions of social power and dominance are common across many vertebrate social systems: aggression, intimidation, and coercion. These traits may be associated with influence, but may also be socially aversive, and thereby decrease social influence of dominant individuals. Using a social cichlid fish, we show that dominant males are aggressive, socially central, and influence group movement. Yet, dominant males are poor effectors of consensus in a more sophisticated association task compared with passive, socially peripheral subordinate males. These influential, subordinate males possess behavioral traits opposite of those generally associated with dominance, suggesting that the link between social dominance and social influence is context dependent, and behavioral traits of dominant males impede group consensus formation.
Keywords: dominance, influence, social, hierarchy, fish
Abstract
Dominant individuals are often most influential in their social groups, affecting movement, opinion, and performance across species and contexts. Yet, behavioral traits like aggression, intimidation, and coercion, which are associated with and in many cases define dominance, can be socially aversive. The traits that make dominant individuals influential in one context may therefore reduce their influence in other contexts. Here, we examine this association between dominance and influence using the cichlid fish Astatotilapia burtoni, comparing the influence of dominant and subordinate males during normal social interactions and in a more complex group consensus association task. We find that phenotypically dominant males are aggressive, socially central, and that these males have a strong influence over normal group movement, whereas subordinate males are passive, socially peripheral, and have little influence over normal movement. However, subordinate males have the greatest influence in generating group consensus during the association task. Dominant males are spatially distant and have lower signal-to-noise ratios of informative behavior in the association task, potentially interfering with their ability to generate group consensus. In contrast, subordinate males are physically close to other group members, have a high signal-to-noise ratio of informative behavior, and equivalent visual connectedness to their group as dominant males. The behavioral traits that define effective social influence are thus highly context specific and can be dissociated with social dominance. Thus, processes of hierarchical ascension in which the most aggressive, competitive, or coercive individuals rise to positions of dominance may be counterproductive in contexts where group performance is prioritized.
The influence that individuals have in social groups depends on their social relationships and dominance hierarchies within groups. In animals, this effect has been studied in contexts ranging from consensus formation (1) to group membership (2, 3) and movement decisions (4). Socially dominant individuals commonly display behavioral traits like aggression, physical exclusion, and coercion, and these traits may define socially dominant individuals (5–9). These behavioral attributes may also mediate social influence, so much so that the two social traits, social dominance (an individual’s social rank or position in a hierarchy) and social influence (the likelihood of effecting a behavioral change in other group members), have been considered equivalent (10). In many animal groups, this relationship holds true, and socially dominant individuals have the greatest influence on group behavior (7). This link may be present for many reasons: for example, due to dominant individuals having a high number of affiliative social bonds, making them more influential in group movement decisions (e.g., ref. 1); because dominant individuals are more effective conduits of social fear (11); or because the motivational salience of informational cues is positively influenced by the perceived dominance of the performer (12). Dominance and influence may also be correlated where the higher aggression of dominant individuals increases their ability to control access to resources (5–9). However, the opposite relationship is also plausible: that the traits associated with dominance may reduce influence in tasks requiring coordination or consensus formation because they are socially aversive. This may occur because the bulk of interactions with dominant individuals are aggressive, such that physical repulsion by dominant individuals leads to a reduction in group cohesion (13, 14), or because dominant individuals themselves may constitute a socially aversive stimulus (12) and the valence of interactions with dominants is generally negative. Making predictions about the relationship between social dominance and social influence is therefore not straightforward. The social connections that define dominance may not be the same that define influence, and the relationship between social dominance and social influence may vary across contexts. In this series of experiments, we examine the relationship between social dominance and social influence as a function of the behavioral and social attributes of dominant and subordinate individuals of the cichlid fish Astatotilapia burtoni. Disentangling this relationship contributes to our understanding of the development, evolution, and expression of behavioral traits and their interactions in social contexts (15), as well as the adaptive significance of dominance (16, 17) and social influence generally (18).
Here, we explore how the behavioral traits that define social dominance in the male cichlid fish A. burtoni interact with their influence in normal, routine behavioral interactions and in a more sophisticated association learning context. We explicitly test the prediction that dominant males have greater social influence than subordinate males. We focus on measuring consensus group movement, which has been explored in the context of social influence in a range of animal taxa (19, 20). While much previous work focuses on the concept of leadership and voluntary following (21–23), we examine social influence more broadly by including repulsive interactions (“pushing”) as well as following, thereby extending the taxonomic and conceptual range of our analysis. Using a definition of social influence that states an individual is influential if its actions result in a behavioral change among other individuals in its group (24) allows inclusion of many forms of social influence that may not be captured by existing definitions of leadership, such as involuntary sharing of information about resources (25), territorial exclusion of con- and heterospecifics (2), and contagion of escape responses through animal collectives (26).
In A. burtoni, there are clear phenotypic signatures of differences in dominance, such that social status is easily identifiable by examining morphology. Dominant males are brightly colored with blue, yellow, and red pigmentation on the body and often display a prominent eye bar during interactions with other group members, whereas subordinate males are cryptically colored (27). Dominant males are reproductively active and defend territories, while subordinate males are nonreproductive and move in shoals (27). These phenotypes are not fixed, and males can begin to switch to dominant behavior and appearance in as little as 20 min when the opportunity for social ascension arises (28–30). Dominant males also have a high frequency of aggressive displays with other males, attacking and chasing subordinates, and courting females (31). The presence of dominant or subordinate males in social groups has different effects on aggression and courtship behavior of other group members (32). Using this species, we allow dominant and subordinate males to learn to associate a colored light with a food reward while in social groups. We then place these informed individuals into new groups of naïve individuals and measure the performance of the new group in the same association task. We compare how the social status of the informed individual (i.e., either an informed dominant or an informed subordinate) affects the time taken for the group to reach consensus and move to the correct conditioned stimulus.
We hypothesize that socially dominant informants will have stronger influence on group-level behavior if the traits that define social dominance also define social influence. Alternatively, dominant males may be socially aversive and have low social influence, suggesting that dominance and influence are dissociated. A third hypothesis is that the association between social dominance and social influence is context dependent. For example, dominant males may strongly influence their social partners by chasing and displacing them during normal interactions but may have little influence in a more sophisticated association task, where their aggressive behavior could impair their function as sources of social information.
Results
Phenotypically Dominant Males Are More Central in Social Networks and Have a Strong Influence on Group Movement.
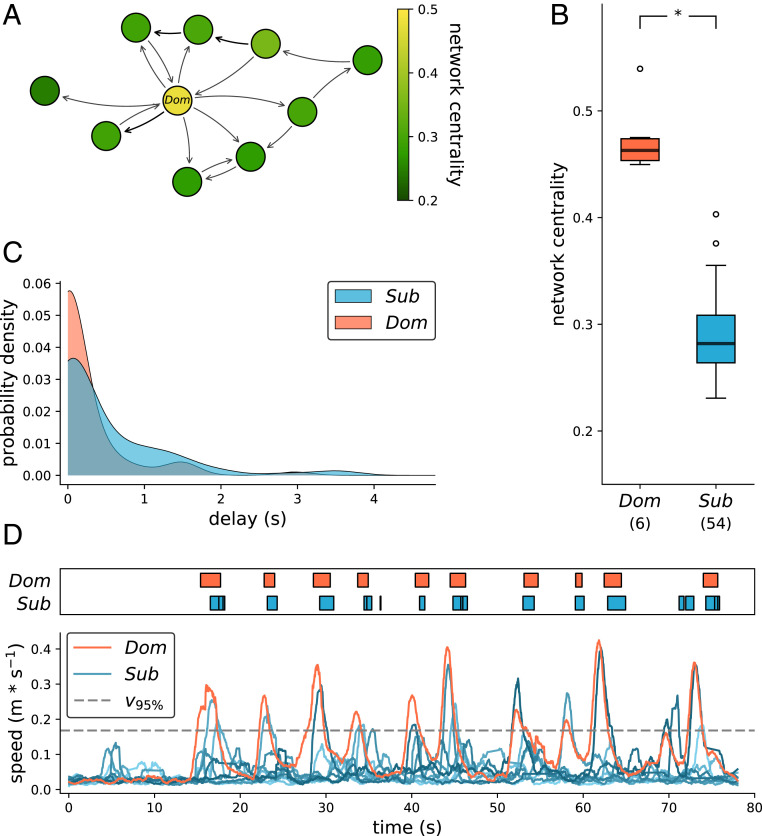
We examined baseline social behavior by placing groups of fish in large holding aquaria and using computer vision-based tracking of interactions to generate social network and behavioral data. Comparing the behavioral interaction network positions of dominant and subordinate males in routine social contexts, we found that dominant males occupy more central social aggression network positions (network randomization test; dominant vs. subordinate centrality, P < 0.001) (Fig. 1 A and B and SI Appendix, Fig. S1). We then tested whether influence over routine group movements differed among dominant and subordinate males by analyzing the onset and duration of social movement events that dominant and subordinate males either initiated or responded to (example in Fig. 1D). To provide an objective definition of motion state changes, these events were defined as two or more group members moving simultaneously faster than the 95th percentile of all speeds, lasting until the speed of all involved individuals falls below this threshold. The first individual to cross the threshold in such an event was considered the event initiator, and the second individual was the responder. We found that dominant males were more frequently the initiators of group movements than subordinate males in these routine conditions (network randomization test, dominant vs. subordinate initiator count, P < 0.001) (Fig. 1C and SI Appendix, Fig. S2).
Fig. 1.
Interaction networks and behavioral analysis from trajectory data. (A) Example of a social network graph created from directed, pairwise initiator–responder counts. Node color denotes network (out-edges Katz) centrality of dominant (Dom) and subordinate (Sub) group members. (B) Aggregation of all individuals from the routine social interaction recordings showing the effect of social status on network centrality (network randomization test, P < 0.001). (C) Onset of speed events across all dominant and subordinate male event initiations (delay = 0) and responses (delay > 0). Dominant individuals were found to be more frequently the initiators of events than subordinates (network randomization test, P < 0.001). (D) Representative examples of speed traces of dominant and subordinate males. Upper represents onset and duration of speed events that exceed a 95% threshold of all speeds (v95%).
Groups with Subordinate Informants Reach Consensus Faster than Those with Dominant Informants.
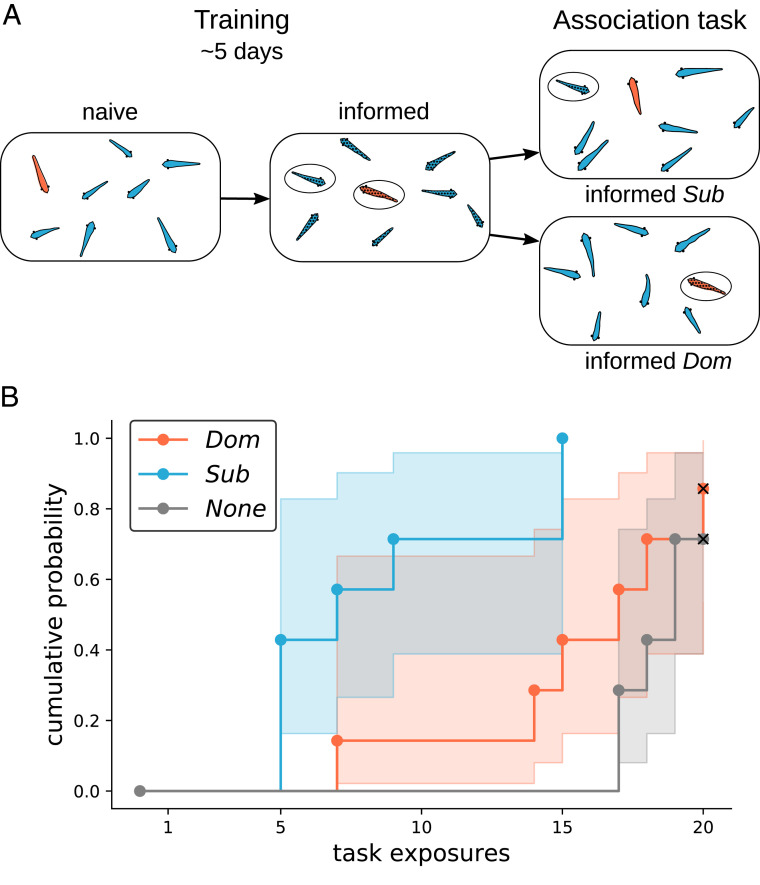
In a separate series of experiments (in the same experimental tanks but using different individuals), we examined whether the social influence displayed by dominant males in routine conditions extended to a more complex task. We trained dominant and subordinate males on an association task and then placed these informed males into groups of individuals naïve to that task. We measured the number of trials (i.e., separate instances of the light association task) in the association task required for groups containing informed dominant or subordinates to reach consensus in moving to the correct conditioned stimulus and compared this time to success with the baseline performance of completely naïve groups (Fig. 2A). We found that groups containing dominant male informants did not reach consensus faster than naïve groups (Cox proportional hazards model, dominant informant vs. naïve, hazard ratio [HR] = 1.82, CI95% = [0.55, 5.99], z = 0.98, P = 0.33) (Fig. 2B), whereas groups containing a subordinate male informant achieved consensus significantly faster than both naïve and dominant male informant groups (subordinate informant vs. naïve, HR = 15.76, CI95% = [3.26, 76.34], z = 3.43, P < 0.001; subordinate informant vs. dominant informant, HR = 8.69, CI95% = [1.97, 38.36], z = 2.85, P < 0.01) (Fig. 2B).
Fig. 2.
Association learning paradigm and group consensus responses. (A) Experimental protocol of group consensus association task. Groups of eight individuals (four males, four females; dominant male indicated in this case by orange coloration) that are naïve to the association task are placed into the arena. Over the course of 20 trials in the association task (4 trials per day for 5 d), these naïve individuals become informed about the correct light stimulus. Within the 5-d training period, all groups showed a behavioral shift from a lack of coordinated movement to a consensus movement toward the conditioned stimulus. After 5 d, all initially naïve groups reached consensus movement toward that correct cue, and subsequently, one dominant male (Dom) and one subordinate male (Sub) were placed into new groups (three males, four females; total group size: eight individuals) that were naïve to the association task. Seven groups each with either a dominant or subordinate informant were then placed in identical training protocols as previously, and the time taken to reach group consensus was measured. We then measured the number of trials taken for seven of eight individuals to move toward the correct conditioned stimulus for two subsequent trials (“group consensus”). (B) The cumulative probability (i.e., the inverse Kaplan–Meier probability) of group consensus over the course of 20 trials. Groups that did not complete the task were right censored in the analysis (indicated by x); shaded areas represent 95% CIs. Groups with a subordinate male informant (Sub) had a faster rate of reaching consensus response than those with a dominant male informant (Dom; Cox proportional hazards model, P < 0.01) or those without an informant (None; P < 0.001).
Dominant Males Have Greater Spatial Separation and Lower Behavioral Signal-to-Noise Ratio.
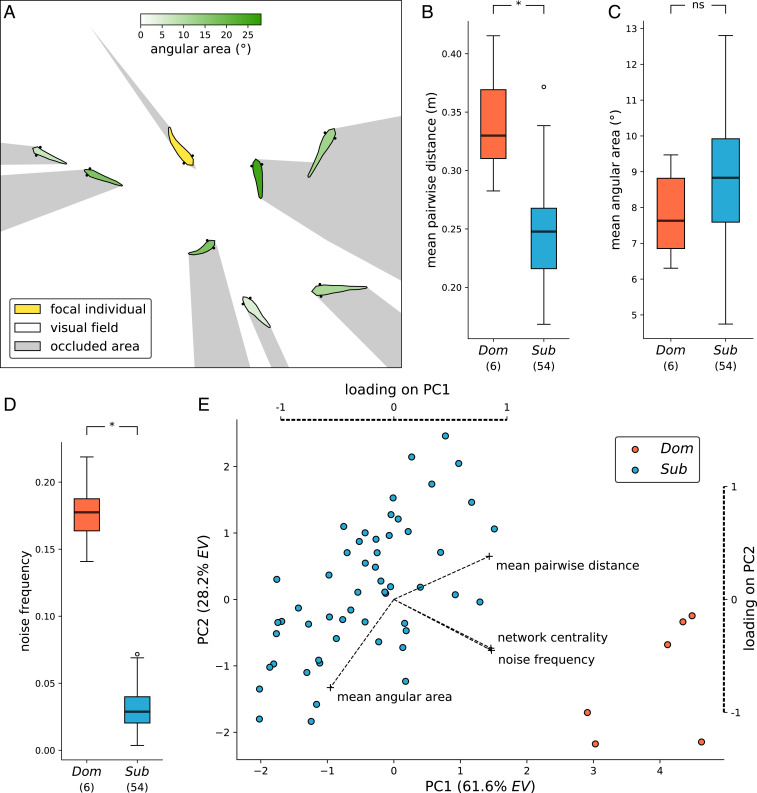
We then explored behavioral and social attributes that may have caused this difference in influence across the two social contexts (routine social interactions and the group association task). We first compared the spatial and visual connectivity of dominant and subordinate males with other group members during routine social interactions. We found that dominant males were more spatially distant from other group members than were subordinate males (network randomization test; dominant vs. subordinate mean pairwise distance, P < 0.001) (Fig. 3B and SI Appendix, Fig. S3). However, there was no difference in the visual connectivity to other group members between dominants and subordinates (network randomization test; dominant vs. subordinate mean angular area subtended on retina, P = 0.196) (Fig. 3C and SI Appendix, Fig. S4).
Fig. 3.
Effects of social status on behavioral parameters. (A) Schematic of visual field computation. (B and C) Aggregated data from all routine social interaction recordings, comparing mean pairwise distance (association connectivity) and mean angular area (visual connectivity) between dominant (Dom) and subordinate (Sub) males with other group members (network randomization tests, P < 0.001 and P = 0.196). (D) Hypothetical noise frequency in the social learning context (rapid, directed swimming) compared by social status (linear model, estimate ± SE = 0.15 ± 0.01, P < 0.001). (E) First two components of a Principal Component Analysis (PCA) on all metrics derived from trajectory and network analyses, comparing dominant and subordinate males in this social parameter space. The first two dimensions explain 61.6 and 28.2% of the variance (explained variance [EV]), respectively. Dashed lines and respective labels indicate the loadings of the used metrics.
Next, we compared the motion signatures of males during routine movement vs. during the association task. We found that dominant males were significantly more likely than subordinate males to swim faster than the 95th percentile of observed speeds. This is comparable with the elevated speed of informed individuals moving toward the unconditioned stimulus during the association task, allowing us to generate a predicted signal-to-noise ratio as the inverse of the social “noise” frequency (rapid, directed swimming during chasing of conspecifics) in routine social contexts. In this analysis, dominant males had significantly higher noise frequencies than subordinate males (linear model; dominant vs. subordinate speed threshold event ratio, estimate ± SE = 0.15 ± 0.01, t58 = 19.0, P < 0.001) (Fig. 3D). Dominants spent 17.74% of time swimming at or above this 95th percentile of speed, whereas subordinates spent only 3.12% of time above this threshold. This resulted in a predicted signal-to-noise ratio of informative behavior for subordinate individuals that was 5.7 times higher for subordinates than dominants. To summarize the differences between dominant and subordinate males in routine social contexts, we used principal component analysis (PCA) to combine trajectory and network metrics in a common social parameter space (Fig. 3E and SI Appendix, Fig. S5A). Further, we performed a clustering analysis of this social parameter space, validating that two distinct clusters of individuals exist (k-means clustering, k = 2, silhouette coefficient analysis) (SI Appendix, Fig. S5B). We found that these two clusters matched exactly the two categories of social status (dominant and subordinate) defined based on male coloration.
Discussion
Here, we demonstrate that dominant male cichlid fish display behavioral traits frequently associated with social dominance and increased social influence, such as high aggression, high social network centrality, and strong influence over patterns of movement (10). Yet, when these dominant males act as potential sources of social information in a group consensus association task, their groups perform poorly. Instead, subordinate males, who occupy peripheral social network positions and have little influence over group movement under normal circumstances, are the most effective in generating group consensus.
The attributes that differentiate dominant and subordinate males, and likely their efficacy as agents of social influence, are social, spatial, and temporal. In our experiments, dominant males occupied more central positions in aggressive interaction networks—a common trait of dominant individuals across species (1). This effect was driven by aggressive interactions of dominant males with other group members, whom they frequently chased and attacked. Group members continually fled from dominant males, and such aggressive behavior was rarely displayed by subordinate males. Consequently, dominant males had greater spatial separation from the rest of the group as they both chased away and were avoided by other group members, whereas subordinate males had higher spatial proximity to other group members (Fig. 3B). This spatial separation likely led to fewer opportunities for processes like local enhancement, which would require the observers to be in physical association with the stimulus and would be prevented if observers were repelled due to the presence of the dominant male at the feeder. Although spatial relationships differed, the visual connectivity to other group members was not different for dominant or subordinate informants (Fig. 3C), likely due to the relatively small arena size allowing unrestricted visual access even at maximum spatial separation. This meant that visual access to social cues was similar for both subordinate and dominant males, making dominant and subordinate equally able to act as visual sources of social information in the association task (33). Nevertheless, dominant males had lower group influence, despite similar opportunity for visual attention (and possibility for observational learning), suggesting that social cues from aggressive dominant males may have had a negative valence compared with cues from passive subordinate males.
Dominant individuals may also have been less reliable sources of social information due to their frequent, aggressive chasing of other group members. In the association task, informed individuals swam quickly and directly toward the correct feeder, inducing a behavioral response in other group members. Such a rapid change in speed is known to be an important social cue influencing collective behavior in zebrafish (34), and path speed and directedness are known to affect both group coherence and information flow in golden shiners (35). However, this mode of swimming—rapid and straight—was rare in subordinate males, which typically moved at slow speeds without abrupt changes and stayed with the group. In contrast, dominant males frequently made rapid accelerations around the arena as they chased other individuals, which subsequently induced accelerated flight responses in subordinates. Thus, the frequent rapid movements of dominant males likely function as behavioral noise against the “signal” of informative social cues of rapid movement toward the correct stimulus in the association task. Thus, dominant males likely had a lower signal-to-noise ratio of socially informative cues, likely contributing to their poor influence on group consensus. This relationship between the signal and noise generated by dominant and subordinate group members is a fruitful avenue for further research.
Dominant males were behaviorally, spatially, and socially differentiable from subordinate males, consistent with many previous studies on this system (Fig. 3E). Under natural conditions, this species forms fission–fusion shoals in which subordinates and females can easily move away from dominant, territorial males, further reducing the latter’s influence over group behavior. However, in many other group-living animals, including closely related cichlid species, dominants and subordinates stay in permanent social contact (36). Further study of the relationship between dominance and influence in closed and open social systems will provide fascinating insight into the ontogeny, costs and benefits, and proximate and ultimate consequences of dominance on group function.
The aggressive behavioral traits of dominant males defined their social position, as well as their influence over normal group behavior through repellent interactions. Rather than classical, voluntary leadership, this influence was manifested through aversive interactions in which other group members were driven away from dominant males. However, these same behaviors lowered their influence in a more sophisticated group task. In this sense, the influence of dominant male fish in our experiments was detrimental to group function, similar to aggressive group members reducing task performance in human teams (37, 38), and even comparable with the phenomenon of “toxic leadership” in organizations (39, 40), where aggressive and competitive behavior of dominant group members can lower group performance. We cannot differentiate whether this effect was due to differences in spatial relationships between dominant and subordinate males or if the valence of social cues may have differed between subordinate and dominant males, such that the same behavior had different influence depending on the social position of the performer. Examining patterns of neural activity (27) in group members when observing subordinate and dominant demonstrators would allow analysis of the perceived valence of otherwise equivalent behavioral cues across levels of social hierarchy.
Overall, we find that the association between social dominance and social influence is nuanced and context specific and that the traits of socially dominant individuals that make them influential in some contexts may be the same that reduce their influence in other contexts. Our findings in fish demonstrate that selection for behavioral traits that increase individual competitive ability and access to resources may concomitantly reduce group performance, especially where consensus in movement or opinion is required. More broadly, our findings suggest that social processes of hierarchical ascension in which the most aggressive, competitive, or coercive individuals rise to positions of dominance may be counterproductive in contexts where group function is the priority.
Materials and Methods
Captive A. burtoni descended from a wild-caught stock population (41) were maintained in stable community tanks (26 °C, pH 7.5 to 8.0, 12:12-h light:dark cycle) until transfer to the experimental arenas (205 L, 108 × 54.6 × 42.7 cm, 17- to 20-cm water depth, 26 °C, pH 7.5 to 8.0, 12:12-h light:dark cycle). Groups consisted of both males and females between 40- and 70-mm standard length (SL), although only males were used as informants since they have clear phenotypic indicators of social dominance, whereas females, although likely having social dominance hierarchies, have no reliable visual indicators of dominance status. In analyses of routine group interactions, we used six groups of 10 fish (n = 60 individuals). In the association tasks, we used eight groups of eight fish during initial training (n = 64 individuals), followed by eight further groups each with either a subordinate informant (n = 56 additional individuals) or a dominant informant (n = 56 additional individuals). These informants were taken from the initial group of eight naïve individuals, which had now successfully responded to the association tasks. One of these group sets was abandoned due to technical failure and was not included in the final dataset, leaving seven groups used in analyses of the association task (n = 154 individuals in association trials). All work was conducted in compliance with the Institutional Animal Care and Use Committee at The University of Texas at Austin.
Experimental Paradigm.
The behavioral task measured the number of trials taken to reach group consensus in a simple association task using a food reward and colored light-emitting diodes. Experiments were conducted in experimental arenas with two Eheim automatic fish feeders mounted on opposite ends of each tank. The motor control pins of the feeders were rewired and externally controlled by a digital switch connected to an Arduino Uno microcontroller that also controlled one diffuse red, green, blue light-emitting diode (RGB LED) mounted directly under each feeder (code available: https://github.com/jordanlabmpi/). Four times a day (0830, 1130, 1430, 1730 h) for 5 consecutive days, the association stimulus would be remotely triggered using the Arduino microcontroller, which randomly assigned both LEDs to simultaneously display one of two colors (RGB 255,60,0 [yellow-orange] or 0,255,255 [cyan]) for 3 s, followed by 3 s of no stimulus; the Arduino would then trigger the autofeeder associated with the LED that displayed the yellow-orange stimulus, providing a portion of Tetramin flake food. Neither of these color stimuli elicit an innate response in the focal animals due, for example, to inherent color preferences (42), allowing their use as conditioned stimuli in an association learning paradigm. However, the color of the rewarded stimulus affected the time taken for the association to be achieved, and we therefore kept the rewarded stimulus color consistent throughout all trials and randomized the location of colors to prevent spatial learning. A networked Logitech HD 1080p webcam was mounted above each tank and automatically scheduled to record for 1 min before and after each training event using iSpy open-source security camera software.
Association Task.
Using the protocol described above, groups of eight A. burtoni (four males, four females) underwent the training four times a day for 5 d. Animals remained in the experimental tanks described above for the entire 5 d and were not disturbed or moved between association trials. Behavior and interactions were recorded for 20 min prior to, during, and 10 min following the stimulus onset. Group behavioral response to the task during all trials was scored as the proportion of individuals in the group that responded to the light stimulus by swimming toward it in the 3 s of LED stimulus prior to the 3-s pause and subsequent delivery of food. A successful group response was defined as seven or more of eight group members swimming directly toward the positive stimulus in less than 1 s of stimulus onset, in two or more consecutive trials. The second successful trial was used as the value in subsequent analyses (e.g., if a group responded successfully in trials 11 and 12, we recorded a successful response for that group at trial 12).
Within the 5-d training period, all groups showed a behavioral shift from a lack of coordinated movement to a consensus movement toward the conditioned stimulus. After 5 d, all initially naïve groups reached consensus movement toward that correct cue, and subsequently, one dominant male (“dominant”) and one subordinate male (“subordinate”) were placed into new groups (three males, four females; total group size: eight individuals) that were naïve to the association task (Fig. 2A). For groups with dominant males, all three other males were smaller than the dominant, while for groups with subordinate males, at least one male was larger than the subordinate. We did not observe any dominance shifts (i.e., a dominant becoming a subordinate in a new group or vice versa) in these group transitions. Seven groups each with either a dominant or subordinate informant were then placed in identical training protocols as previously, and the time taken to group consensus was measured.
Deep Learning-Based Automated Tracking and Analysis of Behavior.
We trained an implementation of a Mask and Region-based Convolution Neural Network (Mask R-CNN) (43, 44) on a small set of 34 manually labeled images. Given the simplicity of this highly specific segmentation task and using standard image augmentations such as rotation, horizontal, or vertical flipping, this allowed the accurate detection and segmentation of individual fish in each of the video frames. In terms of raw data, Mask R-CNN predictions resulted in binary pixel masks for each frame and individual, respectively. These masks were then skeletonized into 1-pixel midlines along each mask’s long axis using morphological image transformations. Subsequently, this allowed the estimation of fish spine poses (45) as seven equidistantly spaced points along these midlines. The second spine point represents an individual’s head position, and the vector pointing from the second to the first spine point is its orientation. These positional data were then used to automatically reconstruct continuous fish trajectories using a simple, distance-based identity assignment approach. Accuracy and high detection frequency were visually verified with a Python-based graphical user interface (45) developed within the laboratory that was also used to manually correct false identity assignments and losses. Mask R-CNN predictions resulted in a mean coverage of 96.3% throughout all analyzed videos and automatic trajectory assignment in an average of 14 losses per individual; 1.6% of all detections were false positives or poorly segmented, resulting in a mean coverage of 94.8% in the manually corrected trajectories. Movie S1 shows a visualization of the tracking pipeline. All code is available at https://github.com/jordanlabmpi/social-influence.
Behavioral, Visual, and Spatial Connectivity Analyses.
In order to examine baseline differences in the behavior of dominant and subordinate males in social contexts, we placed six additional groups of 10 individuals in identical tanks as described above and filmed their behavior for 5 min in the absence of external stimuli (“routine social context”). We calculated the behavioral, visual, and spatial interactions between all fish of each group. To estimate the number of behavioral interactions that dominant and subordinate males had with other group members, trajectory data were used to determine events with elevated swimming speed (above the 95th percentile of the speed distribution). The first two individuals passing this threshold in such events were treated as event initiator and responder, and a delay time between the two individuals was calculated (Fig. 1 C and D). All other group members passing the threshold while either the initiator or the responder was still at elevated speed were considered to be part of the same event but not counted as direct responders. Each separate event lasted until the speed of all initiators or responders fell below the threshold again.
From these dyadic initiator–responder events, we created behavioral interaction networks using the “network” package (46) in Python. Here, the count of the directed, pairwise events between each pair of network nodes defines the weight of the respective edge (directed from initiator to responder). This allowed the calculation of out-edges Katz centrality (47) as a measure of behavioral influence in standard conditions (Fig. 1). Related to this, we also calculated the initiator count for each fish as the number of events in which an individual was the initiator. Additionally, the ratio of the total time spent in these events above the speed threshold to the full duration of the recording was calculated for each fish, constituting individual hypothetical noise frequency in the social training context (fast, directed movement in the absence of LED stimulus).
Spatial connectivity between group members was calculated as their mean pairwise distances. We then computed the visual connectivity as the mean angular area subtended by each individual on the retinas of all other group members, utilizing the contours of the Mask R-CNN detection results as occluding objects in a ray-casting approach (Fig. 3A). Casting rays from both eyes of a focal fish toward these contours (including the focal individual), we modeled the nearly complete field of view known from other freshwater fishes (48). These measures generated three connectivity scores for each dominant and subordinate group member: a behavioral (“interaction”) connectivity, spatial (“association”) connectivity, and visual connectivity. Finally, we conducted a PCA (Fig. 3E and SI Appendix, Fig. S5A) on the speed threshold event ratio (noise frequency) and connectivity scores. After determining the appropriate number of clusters using silhouette analysis (49), we performed a clustering analysis of the principal components (50) (k-means clustering, k = 2) (SI Appendix, Fig. S5B) to assess the overall consistency of metrics derived from trajectory and network analyses with the phenotypic indicators of dominance that we used to identify dominant from subordinate individuals.
Data Analysis and Statistics.
For time-to-consensus movement analysis, we conducted a Kaplan–Meier Survival Analysis (51) with the “survival” package in R (52) using the first of two consecutive trials in which seven or more individuals responded to the stimulus onset as time to criterion and right censoring groups that did not complete the social learning task. We then fit a Cox proportional hazards model with the social status of the informed individual (none, subordinate, dominant) as the single covariate. This allowed the comparison of the survival estimates between the groups that were initially naïve to the stimulus during the training regime and the groups with both dominant and subordinate informants. Further, we validated the proportional hazard assumption for each of the groups using the same package in R.
For comparisons of the baseline behavioral traits of dominant and subordinate fish, we performed either network randomization tests in Python or linear models in R. In the case of network centrality, the mean angular area subtended on the retina, and in the mean pairwise distance, network randomization tests were necessary because these metrics, by definition, are nonindependent for individuals of the same group and social network. Further, the initiator count is also based on dyadic interactions and should be considered nonindependent within the networks. Therefore, we preformed network randomization (i.e., node randomization by assigning the dominance status to an individual that was randomly drawn from the group, n = 1,000) for each of the six “routine social context” groups to construct null models in which social dominance is detached from the respective response variable (53). For each randomization of the six networks and for each response variable, an estimate was calculated as the mean difference of the respective metric between the assigned dominant individual and the mean of the remaining, assigned subordinate individuals. These estimates can be considered as null distributions for the test statistics and were used to calculate two-tailed P values for the actually observed differences between dominant and subordinate individuals. Accordingly, the null hypotheses that the observed differences were drawn from the respective null models were rejected when the corresponding P value was smaller than, or equal to, the significance level α = 0.05. SI Appendix, Figs. S1–S3 show visualizations of the network randomization tests.
By contrast, the noise frequency (ratio of time spent in above speed threshold events to trial duration) is not dependent between individuals of a group. Here, we fit a linear regression model with social status as predictor and noise frequency as response variable. Further, we tested the model’s assumptions of normality of residuals with the Shapiro–Wilk test and homoscedasticity with the Breusch–Pagan test (54).
Supplementary Material
Acknowledgments
We thank members of the laboratories of H.A.H. and A.J. for many fruitful discussions. This work was supported by the National Science Foundation Bio/computational Evolution in Action Consortium (BEACON) Center for the Study of Evolution in Action (H.A.H. and A.J.), Dr. Dan Bolnick and the Howard Hughes Medical Institute (A.J.), Deutsche Forschungsgemeinschaft Cluster of Excellence 2117 “Centre for the Advanced Study of Collective Behavior” Grant 422037984 (to P.N., O.D., and A.J.), a University of Texas (UT) at Austin Undergraduate Research Fellowship (to L.K.G.), a UT Austin Global Research Fellowship (to M.R.-S.), UT Austin Graduate School Bruton and Summer Fellowships (to M.R.-S.), a Department of Integrative Biology Doctoral Dissertation Improvement grant (to M.R.-S.), and NSF Grant IOS1354942 (to H.A.H.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: The data that support the findings of this study are available on Dryad (data for statistical analyses, linear models and survival analysis, DOI https://doi.org/10.5061/dryad.qz612jmbz) and GitHub (raw data and analysis scripts, tracking, networks and visualization, https://github.com/jordanlabmpi/social-influence).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2000158117/-/DCSupplemental.
Data Availability.
The code and raw data that support the findings of this study are available at https://github.com/jordanlabmpi/social-influence and data used in analyses deposited at Dryad DOI https://doi.org/10.5061/dryad.qz612jmbz.
References
- 1.King A. J., Douglas C. M., Huchard E., Isaac N. J., Cowlishaw G., Dominance and affiliation mediate despotism in a social primate. Curr. Biol. 18, 1833–1838 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Jordan L. A. et al., Group structure in a restricted entry system is mediated by both resident and joiner preferences. Behav. Ecol. Sociobiol. 64, 1099–1106 (2010). [Google Scholar]
- 3.Jordan L. A., Wong M. Y. L., Balshine S. S., The effects of familiarity and social hierarchy on group membership decisions in a social fish. Biol. Lett. 6, 301–303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy M., Ákos Z., Biro D., Vicsek T., Hierarchical group dynamics in pigeon flocks. Nature 464, 890–893 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Janson C., Aggresive competition and individual food consumption in wild brown capuchin monkeys (Cebus apella). Behav. Ecol. Sociobiol. 18, 125–138 (1985). [Google Scholar]
- 6.Di Bitetti M. S., Janson C. H., Social foraging and the finder’s share in capuchin monkeys, Cebus apella. Anim. Behav. 62, 47–56 (2001). [Google Scholar]
- 7.Drews C., The concept and definition of dominance in animal behaviour. Behaviour 125, 283–313 (1993). [Google Scholar]
- 8.Barta Z., Giraldeau L.-A., The effect of dominance hierarchy on the use of alternative foraging tactics: A phenotype-limited producing-scrounging game. Behav. Ecol. Sociobiol. 42, 217–223 (1998). [Google Scholar]
- 9.Krause J., James R., Franks D. W., Croft D. P., Animal Social Networks, (Oxford University Press, 2015). [Google Scholar]
- 10.Rowell T. E., The concept of social dominance. Behav. Biol. 11, 131–154 (1974). [DOI] [PubMed] [Google Scholar]
- 11.Jones C. E., Monfils M.-H., Dominance status predicts social fear transmission in laboratory rats. Anim. Cogn. 19, 1051–1069 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terburg D., Aarts H., van Honk J., Memory and attention for social threat: Anxious hypercoding-avoidance and submissive gaze aversion. Emotion 12, 666–672 (2012). [DOI] [PubMed] [Google Scholar]
- 13.McCort W. D., Graves H. B., Social dominance relationships and spacing behavior of swine. Behav. Processes 7, 169–178 (1982). [DOI] [PubMed] [Google Scholar]
- 14.Fero K., Moore P. A., Social spacing of crayfish in natural habitats: What role does dominance play? Behav. Ecol. Sociobiol. 62, 1119–1125 (2008). [Google Scholar]
- 15.Ioannou C. C., Ramnarine I. W., Torney C. J., High-predation habitats affect the social dynamics of collective exploration in a shoaling fish. Sci. Adv. 3, e1602682 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chagnon N. A., Life histories, blood revenge, and warfare in a tribal population. Science 239, 985–992 (1988). [DOI] [PubMed] [Google Scholar]
- 17.von Rueden C., Gurven M., Kaplan H., Why do men seek status? Fitness payoffs to dominance and prestige. Proc. Biol. Sci. 278, 2223–2232 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger J., Rosenholtz S. J., Zelditch M. Jr., Status organizing processes. Annu. Rev. Sociol. 6, 479–508 (1980). [Google Scholar]
- 19.Strandburg-Peshkin A., Farine D. R., Crofoot M. C., Couzin I. D., Habitat and social factors shape individual decisions and emergent group structure during baboon collective movement. eLife 6, e19505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harcourt J. L., Ang T. Z., Sweetman G., Johnstone R. A., Manica A., Social feedback and the emergence of leaders and followers. Curr. Biol. 19, 248–252 (2009). [DOI] [PubMed] [Google Scholar]
- 21.Webster M. M., Experience and motivation shape leader–follower interactions in fish shoals. Behav. Ecol. 28, 77–84 (2017). [Google Scholar]
- 22.Krause J., Hoare D., Krause S., Hemelrijk C. K., Rubenstein D. I., Leadership in fish shoals. Fish. 1, 82–89 (2000). [Google Scholar]
- 23.Couzin I. D., Krause J., Franks N. R., Levin S. A., Effective leadership and decision-making in animal groups on the move. Nature 433, 513–516 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Strandburg-Peshkin A., Papageorgiou D., Crofoot M. C., Farine D. R., Inferring influence and leadership in moving animal groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373, 20170006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giraldeau L.-A., Caraco T., Social Foraging Theory, (Princeton University Press, 2018). [Google Scholar]
- 26.Rosenthal S. B., Twomey C. R., Hartnett A. T., Wu H. S., Couzin I. D., Revealing the hidden networks of interaction in mobile animal groups allows prediction of complex behavioral contagion. Proc. Natl. Acad. Sci. U.S.A. 112, 4690–4695 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruska K. P., Fernald R. D., Astatotilapia burtoni: A model system for analyzing the neurobiology of behavior. ACS Chem. Neurosci. 9, 1951–1962 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Hofmann H. A., Gonadotropin-releasing hormone signaling in behavioral plasticity. Curr. Opin. Neurobiol. 16, 343–350 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Maruska K. P., Fernald R. D., Behavioral and physiological plasticity: Rapid changes during social ascent in an African cichlid fish. Horm. Behav. 58, 230–240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huffman L. S., Mitchell M. M., O’Connell L. A., Hofmann H. A., Rising StARs: Behavioral, hormonal, and molecular responses to social challenge and opportunity. Horm. Behav. 61, 631–641 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Fernald Russell, Quantitative behavioural observations of Haplochromis burtoni under semi-natural conditions. Animal Behaviour 25, 643–653 (1977). [Google Scholar]
- 32.Desjardins J. K., Hofmann H. A., Fernald R. D., Social context influences aggressive and courtship behavior in a cichlid fish. PLoS One 7, e32781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chance M. R. A., The Social Structure of Attention, Chance M. R. A., Larsen R. R., Eds. (Wiley, 1976). [Google Scholar]
- 34.Lemasson B. et al., Motion cues tune social influence in shoaling fish. Sci. Rep. 8, 9785 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ioannou C. C., Singh M., Couzin I. D., Potential leaders trade off goal-oriented and socially oriented behavior in mobile animal groups. Am. Nat. 186, 284–293 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Jordan A., Taborsky B., Taborsky M., “Cichlids as a model system for studying social behaviour and evolution” in The Behaviour, Ecology, and Evolution of Cichlid Fishes: A Contemporary Modern Synthesis, Abate M., Noakes D. L. G., Eds. (Fish & Fisheries Series, Springer, Amsterdam, 2020). [Google Scholar]
- 37.Hrdy S. B., Infanticide among animals: A review, classification, and examination of the implications for the reproductive strategies of females. Ethol. Sociobiol. 1, 13–40 (1979). [Google Scholar]
- 38.Glomb T. M., Liao H., Interpersonal aggression in work groups: Social influence, reciprocal, and individual effects. Acad. Manage. J. 46, 486–496 (2003). [Google Scholar]
- 39.Matos K., O’Neill O., Lei X., Toxic leadership and the masculinity contest culture: How “win or die” cultures breed abusive leadership. J. Soc. Issues 74, 500–528 (2018). [Google Scholar]
- 40.Reed G. E., Toxic leadership. Mil. Rev. 84, 67–71 (2004). [Google Scholar]
- 41.Fernald R. D., Hirata N. R., Field study of Haplochromis burtoni: Quantitative behavioural observations. Anim. Behav. 25, 964–975 (1977). [Google Scholar]
- 42.Egger B., Klaefiger Y., Theis A., Salzburger W., A sensory bias has triggered the evolution of egg-spots in cichlid fishes. PLoS One 6, e25601 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He K., Gkioxari G., Dollár P., Girshick R., “Mask r-cnn” in Proceedings of the IEEE International Conference on Computer Vision, (IEEE, New York, 2017), pp. 2961–2969. [Google Scholar]
- 44.GitHub, matterport/Mask_RCNN: Mask R-CNN for object detection and instance segmentation on Keras and TensorFlow. Version 2.1. https://github.com/matterport/Mask_RCNN. Accessed 20 November 2019.
- 45.Francisco F. A., Nührenberg P., Jordan A., High-resolution animal tracking with integration of environmental information in aquatic systems. bioRxiv:2020.02.25.963926 (26 February 2020).
- 46.Hagberg A., Swart P., Chult D. S., Exploring Network Structure, Dynamics, and Function Using NetworkX, (Los Alamos National Lab., Los Alamos, NM, 2008). [Google Scholar]
- 47.Newman M., Networks: An Introduction, (Oxford University Press, 2020). [Google Scholar]
- 48.Pita D., Moore B. A., Tyrrell L. P., Fernández-Juricic E., Vision in two cyprinid fish: Implications for collective behavior. PeerJ 3, e1113 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rousseeuw P. J., Silhouettes: A graphical aid to the interpretation and validation of cluster analysis. J. Comput. Appl. Math. 20, 53–65 (1987). [Google Scholar]
- 50.Pedregosa F. et al., Scikit-learn: Machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011). [Google Scholar]
- 51.Kaplan E. L., Meier P., Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53, 457–481 (1958). [Google Scholar]
- 52.Therneau T., A Package for Survival Analysis in R. R package version 3.2-3. https://CRAN.R-project.org/package=survival (2020).
- 53.Farine D. R., A guide to null models for animal social network analysis. Methods Ecol. Evol. 8, 1309–1320 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeileis A., Hothorn T., Diagnostic Checking in Regression Relationships, (R News, 3rd Ed., 2002), Vol. 2, pp. 7–10, https://CRAN.R-project.org/doc/Rnews/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The code and raw data that support the findings of this study are available at https://github.com/jordanlabmpi/social-influence and data used in analyses deposited at Dryad DOI https://doi.org/10.5061/dryad.qz612jmbz.