Abstract
Available antiulcer medications reveal partial efficacy and numerous adverse reactions. Tetramethylpyrazine (TMP) was known for its potential antioxidant, anti-inflammatory and angiogenic properties. The aim of current study was to investigate the potential gastroprotective effect of TMP against indomethacin-induced gastric ulcer in rats with possible underlying mechanisms. TMP was tested at 3 doses (15, 30 & 60 mg/kg/d po) three days before indomethacin challenge (25 mg/kg ip). Gastric tissue was evaluated morphologically and histopathologically. Oxidative statuses were assessed via glutathione content (GSH), malondialdhyde (MDA) and catalase (CAT) activity, while TNFα and IL-6 were measured as inflammatory mediators. Gastric PGE2 was investigated in addition to vascular endothelial growth factor (VEGF). TMP was effective (at 30 and 60 mg/kg/d) in promoting mucus secretion and preventing histopathologic changes induced by indomethacin. Mechanistically, TMP significantly enhanced GSH content and CAT activity while reducing lipid peroxidation as expressed by MDA concentration. Moreover, TMP effectively reduced TNFα, IL-6 and intracellular adhesion molecule (ICAM-1) concentrations. On the other hand, TMP enhanced both COX-1 and PGE2 and encouraged angiogenesis via increasing VEGF expression. In conclusion, TMP possesses a protective effect against indomethacin-induced gastric ulcer. This could be explained – at least partly – by its antioxidant, anti-inflammatory and angiogenic effects.
Keywords: Tetramethylpyrazine, Ulcer, Oxidative stress, Inflammation, Angiogenesis
1. Introduction
Gastric ulcer appears as a result of discrepancy between hostile and shielding factors in the stomach (Shaker et al., 2010). The key hostile factors comprise high gastric acid secretion, ethanol consumption, irregular motility, Helicobacter pylori infection and non-steroidal anti-inflammatory drugs (NSAIDs). On contrary, the major shielding factors include: prostaglandin synthesis, mucus secretion, production of bicarbonate as well as normal tissue blood supply (Sowndhararajan and Kang 2013). In Saudi Arabia, the rate of H. pylori infection is high among Saudi population suffering from gastric ulcer (BinSaeed, 2009, Saber et al., 2015, Saquib et al., 2017). Moreover, NSAIDs are highly consumed in Saudi Arabia. A survey questionnaire was distributed to 3000 individuals from the major metropolitan areas in the Kingdom, and among 500 responders, 43.33% of them were took painkillers daily (Bahdailah, 2019).
In the middle of the NSAIDs, indomethacin was perceived as the most powerful ulcerogenic to humans (Henry and Robertson, 1993). In the gastric tissue, it was found to induce a range of pro-inflammatory mediators, inhibit the gastroprotective cyclooxygenase-1 (COX-1) and angiogenesis (Yadav et al., 2012). Further, NSAIDs add to gastric mucosal damage through induction of oxidative stress with consequent generation of reactive oxygen species (ROS) (Utsumi et al., 2006). In addition, they block the gastroprotective effects of prostaglandin E2 (PGE 2) that augments mucus and bicarbonate secretions as well as gastric blood supply (Asako et al., 1992). What's more, NSAIDs down-regulates the pro-angiogenic factors such as vascular endothelial growth factor (VEGF), thus delaying ulcer healing (Tarnawski, 2005).
Chinese herbs are the most primeval and still broadly used conventional medicines (Li et al., 2011). Tetramethylpyrazine (TMP) is an isolated purified chemical, recognized as a constituent of Ligusticum wallichii Franchat. This Chinese herb has been largely utilized in the treatment of vascular diseases in CNS and CVS (Chun-sheng et al., 1978, Ke-ji et al., 1983, Jiao et al., 2004). The isolated compound TMP has remedial effects in various diseases. Formerly, TMP has been shown to protect against reserpine-induced gastric lesion in rats, probably by promoting mucous barrier (Wan et al., 1998). In CNS, TMP guarded against brain ischemic injuries (Shao et al., 2017), and enhanced VEGF expression in a rat model of chronic alcoholic encephalopathy (Li et al., 2015). Peripherally, TMP was able to prevent ethanol-induced hepatocellular injury by inhibiting oxidative stress (Lu et al., 2015). Thus, TMP was proven to possess antioxidant, anti-inflammatory and angiogenenic activities in various diseases. Consequently, the current hypothesis is that TMP could protect against gastric ulcer induced by NSAIDs through interfering with mechanisms implicated in their ulcerative potential as oxidative stress, inflammation and angiogenesis. Therefore, the aim of current study was to explore the potential gastroprotective effect of TMP against indomethacin-induced gastric ulcer in rats along with the likely causal mechanisms.
2. Materials and methods
2.1. Drugs and chemicals
Indomethacin, omeprazole and TMP were purchased from Sigma Aldrich (MO, USA) with Cat. # (I8280), (O104) and (183938) respectively. Phosphate buffer, formalin and all other chemicals were of the highest purity grade commercially available.
2.2. Animals
Animal handling was approved - in advance - by the biomedical ethical committee, King Abdulaziz University, Jeddah, Saudi Arabia (ethical approval # 608-18). Male Wister rats were purchased from the animal house of King Fahd Medical Research Center, Jeddah; acclimatized for one week in an air-conditioned atmosphere at (22 ± 2 °C), under a 12 h light/dark cycle and fed ad libitum with free access to water.
2.3. Experimental design
Initial pilot screening has been carried out to determine the optimum time interval to reach the maximum ulcerogenic state after i.p. injection of indomethacin 25 mg/kg (Borra et al., 2011). In fact, rats’ stomachs reached maximum ulcerogenic state after 4 h. Then, 42 adult males Wistar rats weighing 150–170 gm were randomly divided into 7 groups (6 animals each) and fasted 16 h before oral administration of drugs with sawdust exclusion. The first group was the control group and given only regular diet and the vehicle (Na-CMC 0.5% w/v) at a maximum volume of 10 mL/kg. Then, rats were dissected after 3 days of starting the experiment. Second group received only indomethacin at a single dose of 25 mg/kg i.p on the day of dissection. The third group was pretreated with omeprazole 30 mg/kg po for 3 days. On the third day, rats were challenged with indomethacin 25 mg/kg i.p. after one hour of omeprazole last dose. Fourth, fifth and sixth groups were pretreated orally with TMP; 15, 30 and 60 mg/kg, respectively for 3 days. These doses were selected according to the tested dosage range of TMP in previous studies regarding its antioxidant and anti-inflammatory properties (Michel et al., 2017, Wan et al., 1998). On the third day, rats were injected indomethacin 25 mg/kg i.p. after one hour of TMP last dose. Based on the preliminary study, all treatment groups were dissected after 4 h of indomethacin challenge. The seventh group was administered TMP alone 60 mg/kg PO for 3 days and then dissected.
2.4. Morphological and histological examination
Rats’ stomachs were cut through the greater curvature, washed with saline (0.9% NaCl) and photographed using an appropriate digital camera. Then, the stomachs were examined macroscopically for hemorrhagic lesions developing in the glandular mucosa. The length (mm) of each lesion was measured. Ulcer index was determined as follows: Ulcer index = 10/x, where “x” is total mucosal area/total ulcerated area (Sathish et al., 2011).
Representative tissue samples were fixed in formalin saline for preparation of paraffin blocks, and then cut into 4 µm thickness sections by slide microtome. After that, sections were stained with Hematoxylin and Eosin stain for routine histological examination (Bancroft and Gamble, 2008) and examined under light microscopy (Nikon, Eclipse 80i, Japan). The pathological changes were assessed by a qualified histopathologist according to the method of Shah et al. (1997): [1] epithelial cell loss (score: 0–3), [2] hemorrhage (score: 0–4), [3] inflammatory cell infiltration (score: 0–2) and [4] mucosal erosions (score: 0–4).
2.5. Mucin assessment
The mixture of the Alcian blue and PAS (Periodic Acid–Schiff) method was utilized for mucin staining, irrespective of the charge nature of the mucin as either acid or neutral mucin (Bancroft and Gamble, 2008). After hydration, sections were placed in acetic acid solution (3%) for 3 min, and then in Alcian Blue Stain (1%, pH 2.5) for 15 min, followed by washing in gently running tap water 1–2 min. Then, slides were placed in periodic acid 0.5%, for 5 min, then in Schiff Reagent for 10 min and washed in lukewarm tap water for 5–10 min. Afterward, slides were stained lightly in hematoxylin stain and quantitative analysis of mucin secretion was assessed by an image analysis software (ImageJ, 1.48a, NIH, USA), as optical density (OD) across six different areas for each rat section.
2.6. Assessment of oxidative stress markers
Reduced glutathione (GSH) was measured by biochemical kit purchased from Biogiagnostics Cat. # CR2510 (Giza, Egypt). The method is built on production of a reduced chromagen (yellow color) which result from reduction of glutathione (GSH) with 5,5′ dithiobis (2-nitrobenzoic acid) (DTNB) (Beutler, 1963). MDA was measured by biochemical kit purchased from Biodiagnostics Cat. # MD 2528 (Giza, Egypt). The end product (thiobarbituric acid reactive product) resulted from thiobarbituric acid (TBA) reaction with malondialdehyde (MDA) in acidic medium at temperature of 95 °C for 30 min (Kei 1978). Enzymatic CAT activity was measured by biochemical kit purchased from Biogiagnostics Cat. # CA 2516 (Giza, Egypt), according to Aebi (1984).
2.7. Assessment of Tumor Necrosis Factor (TNF-α), Interleukin-6 (IL-6) and Intracellular Adhesion Molecule-1 (ICAM-1)
Measurement of TNF-α, IL-6 and ICAM-1 concentrations in stomach homogenate was carried out utilizing enzyme linked immunosorbent assay kits: TNF-α (Cat. #BMS622) and IL-6 (Cat. # BMS625), purchased from Thermo Fisher Scientific, (Austria), while ICAM-1 kit (Cat. # MBS494781) was obtained from MyBioSource (USA). All kits adopted the sandwich technique of ELISA, according to the manufacturers’ instructions.
2.8. Assessment of Cycloxygenase-1 (COX-1) and Prostaglandin E2 (PGE2)
Evaluation of COX-1 activity in gastric tissue homogenate was carried out by COX-1 kit (Cat. # MBS703362) obtained from MyBioSource (USA), based on the sandwich technique. However, the assay of PGE2 was completed using ELISA kit for PGE2 (Cat. # EHPGE2), purchased from Thermo Fisher Scientific, (Austria), based on the competitive technique.
2.9. Immunohistochemical detection of the angiogenic marker VEGF
Immunohistochemical (IHC) staining is used to detect VEGF in tissue slices by labeling the antibody/antigen compound with an enzyme that reacts with a suitable substrate to give a colored product (Buchwalow and Böcker, 2010). Briefly, 4 µm thick paraffin embedded tissue slices were deparaffinized, hydrated and then blocked with bovine serum albumin (BSA, 5%) in tris buffered saline (TBS) for 2 h. The slices were then incubated overnight at 4 °C with the primary rabbit polyclonal antibody to VEGF (Abcam, Anti-VEGF 164 antibody, Catalog #: ab53465), followed by washing and incubation with the secondary antibody. Thereafter, slices were washed with TBS and incubated for 10 min in a solution of 0.02% diaminobenzidine containing 0.01% H2O2. Counter staining was made by hematoxylin, and the slides were visualized under a light microscope. The IHC quantitation of all antibodies staining was completed by image analysis software (ImageJ, 1.48a, NIH, USA), as OD of brown-stained (positive) cells across six different areas for each rat section.
2.10. Measurement of total protein content in tissue homogenates
The measurement of protein content in rat stomachs was accomplished according to the manufacturer instructions using protein assay kit (Cat. #23227), purchased from Thermo-Fisher Scientific, (Austria). Protein Assay is based on bicinchoninic acid (BCA) for the colorimetric recognition and quantitation of total protein (Gornall et al., 1949).
2.11. Statistical analysis
Data are given as mean ± SD. Multiple comparisons were completed by utilizing one-way ANOVA followed by Tukey’s as a post-hoc test. Probability value P < 0.05 was considered as the criterion for significance. All statistical analyses were completed using GraphPad Instat software version 3. Graphs were drawn using GraphPad Prism software version 8 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. Effect of TMP pretreatment on stomach morphology, histopathology and mucus secretion
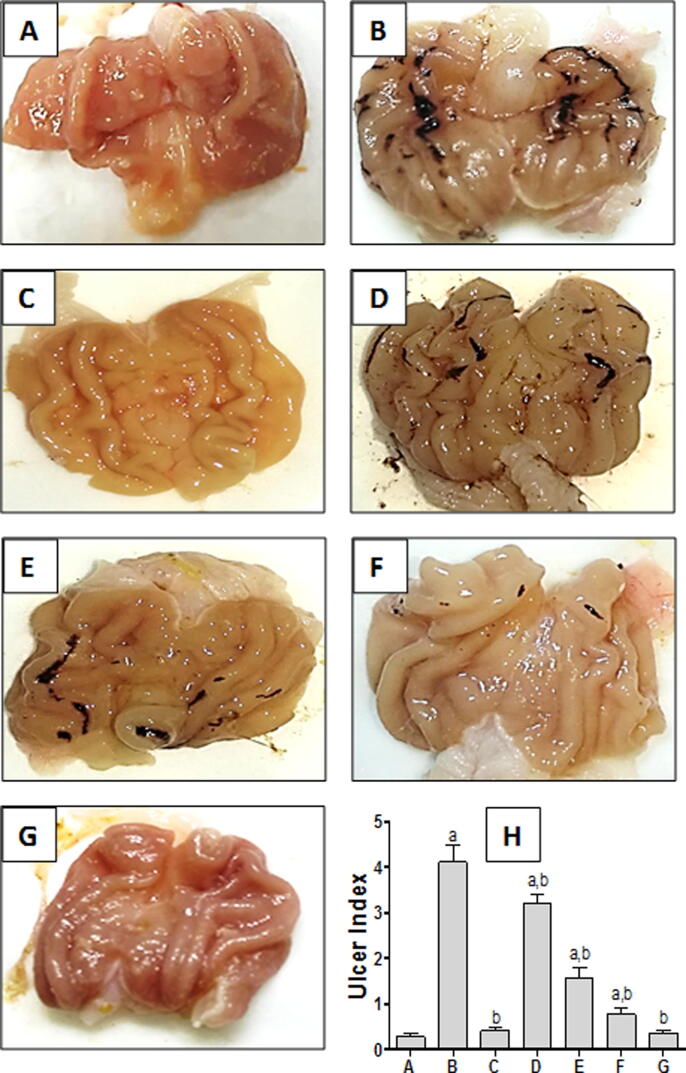
Stomach from control group showed normal mucosa without any injuries (Fig. 1A). However, indomethacin-exposed group showed bloody streaks injuries ranging from 0.5 to 5 mm in length (Fig. 1B), quantified by a significant increase in ulcer index (Fig. 1H). Pretreatment with omeprazole (standard drug) effectively protected the mucosal layer, without any significant difference in ulcer index from the control group (Fig. 1C & H). Rats pretreated with TMP (15 and 30 mg/kg) showed bloody streaks but to a much lesser extent than indomethacin-exposed group (Fig. 1D & E; respectively). On the other hand, pretreatment of rats with TMP (60 mg/kg) revealed superficial blood vessel congestion indicating minor injuries, with significant decrease of ulcer index compared to indomethacin-exposed group (Fig. 1F, H). TMP alone treated group presented normal morphology of stomach tissue (Fig. 1G).
Fig. 1.
Macroscopic photographs of rats stomachs; (A) Control group showing normal mucosa; (B) Indomethacin-exposed group with severely hemorrhagic ulcerated mucosal layer; (C)Omeprazole-pretreated group (standard drug) (30 mg/kg)effectively protect mucosal layer; TMP-pretreated groups with dosages 15 and 30 mg/kg, (D and E respectively) viewing bloody streaks less severe than (B); (F and G):TMP pretreated group and TMP-alone group; respectively; both treated with 60 mg/kg), showing minor injuries and normal mucosa, respectively, (H) Ulcer index = 10/x, where “x” is total mucosal area / total ulcerated area. (n = 6) a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
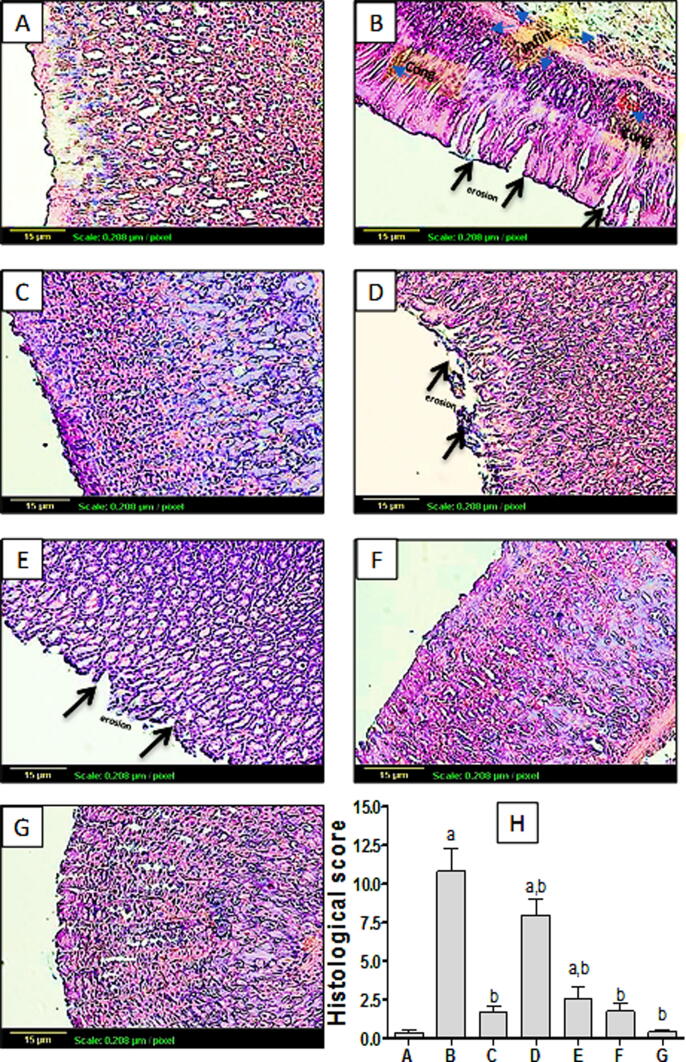
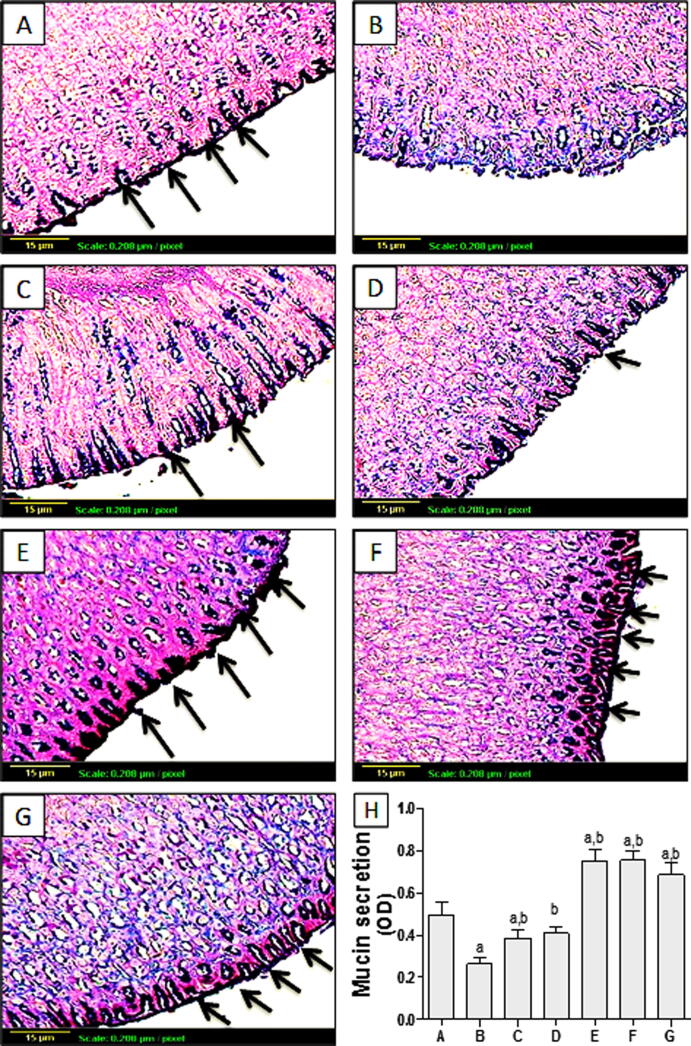
Regarding histopathological examination as shown in Fig. 2A, control group showed normal gastric histological architecture. However, Indomethacin-exposed rats showed reduced mucosal thickness with destructed lining epithelium of mucosal layer associated with focal ulceration and necrosis, as well as congestion of blood vessels, manifested quantitatively by significant elevation of histological score (Fig. 2B, H). Group pretreated with omeprazole (Fig. 2C) showed normal histological structure of gastric tissue. Likewise, pretreatment with TMP at 60 mg/kg dose (Fig. 2F) and TMP-alone treated groups (Fig. 2G) showed no histopathological alterations, with no significant difference in histological score from the control (Fig. 2H). Groups pretreated with TMP 15 and 30 mg/kg (Fig. 2D and E respectively) showed less sever destructed lining epithelium of mucosal layer with little congestion in a dose-related manner, quantified as histological score (Fig. 2H). These effects have been reflected on mucin content. Fig. 3A showed Stomach tissue from control group represented Alcian blue/PAS positive reaction (arrows) which appeared as dark blue or purple color in mucosal layer. Yet, gastric tissue from indomethacin-exposed group showed no reaction in the part of ulceration of the mucosa, with significant reduction of OD compared to the control (Fig. 3B, H). Pretreatment with omeprazole (30 mg/kg) moderately improved mucus production as quantified by significant increase of OD compared to the indomethacin –exposed group (Fig. 3C, H). In addition, TMP pretreated group (15 mg/kg) showed mild improvement of mucus secretion (Fig. 3D). Interestingly, groups pretreated with TMP (30 and 60 mg/kg) as well as TMP-alone treated group demonstrated marked increase of mucus secretion even more than the control group (Fig. 3E, F and G respectively). This effect was further confirmed by significant increases of OD of these groups compared to the control (Fig. 3H).
Fig. 2.
The effect of TMP pretreatment on indomethacin-induced histopathological changes of gastric tissue (×100). Group (A): control group presented normal histological structure of the mucosa, submucosa, muscularis mucosa and serosa, Group (B):indomethacin-exposed group, showed reduced mucosal thickness with destructed lining epithelium of mucosal layer and congested blood vessels (arrow), Group (C):showed normal histological structure of gastric tissue. Likewise, pretreatment with TMP at 60 mg/kg dose (F) and TMP-alone treated groups (G) showed no histopathological alterations. Groups pretreated with TMP 15 and 30 mg/kg (D and E respectively) showed less sever destructed lining epithelium of mucosal layer with little congestion in a dose-related manner, (H) Histological score (n = 6) a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
Fig. 3.
The effect of TMP pretreatment on changes of mucus secretion as Alcian blue/PAS reaction in mucosal layer (arrows) (×100). Control group (A) demonstrated strong positive reaction (+++); (B) Indomethacin-exposed group showed negative reaction representing diminished mucin secretion (-); (C):Omeprazole-pretreated group showed moderate reaction (++). Moreover, pretreatment with TMP at dose 15 mg/kg pretreatment illustrated moderate reaction (++) (D). Interestingly, rats pretreated with TMP (30 and 60 mg/kg)(+++) as well as TMP-alone (+++) treated group demonstrated intense reaction even more than the control group (E, F and G respectively), (H) Quantitative ImageJ analysis (n = 6) for mucin secretion expressed as optical densities (OD). a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
3.2. Effect of TMP pretreatment on oxidative stress biomarkers
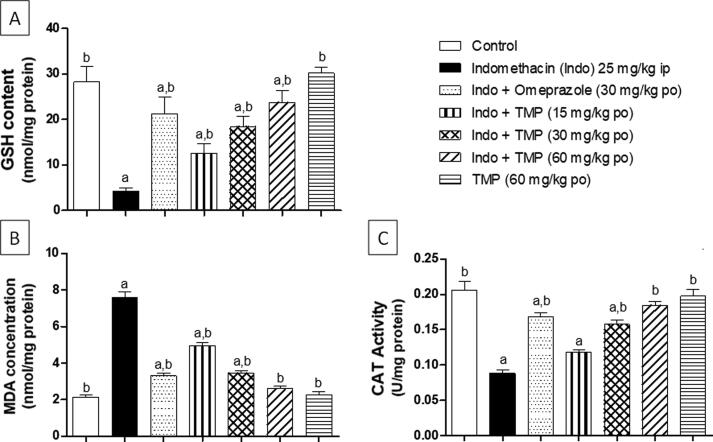
The antioxidant effect of TMP was evaluated via assessment of GSH content, MDA concentration and CAT activity in gastric homogenate. As shown in Fig. 4A, indomethacin exposure significantly (p < 0.001) reduced GSH content by about 85% as matched to the control group. Moreover, pretreatment with omeprazole caused significant (p < 0.001) increase (4-folds) of GSH content, compared to indomethacin-exposed group. Groups pretreated with TMP (15, 30 and 60 mg/kg) demonstrated significant (p < 0.001) rises in GSH content as matched to indomethacin-exposed group by 1.9, 3.3 and 4.5-folds, respectively in a dose-related manner. Regarding MDA concentration, indomethacin exposure caused significant (p < 0.001) increase (2.5-folds) as compared to the control group. On the other hand, pretreatment of rats with omeprazole demonstrated significant (p < 0.001) drop in MDA concentration by 56.6%, compared to indomethacin-exposed animals. Moreover, groups pretreated with TMP 15, 30 and 60 mg/kg showed significant decreases (p < 0.001) in MDA concentration by 34.9%, 54.6 and 65.7% respectively, compared to indomethacin-exposed group. It is worthy noted that animals treated with TMP-alone presented no significant difference, compared to control group (Fig. 4B). As shown in Fig. 4C, indomethacin exposure caused a significant (p < 0.001) reduction of CAT enzyme activity by 57.3% compared to control group. However, group pretreated with omeprazole demonstrated significant (p < 0.001) increase of CAT activity by 81%, compared to indomethacin-exposed rats. Groups pretreated with TMP 30 and 60 mg/kg revealed significant (p < 0.001) increase in CAT activity compared to indomethacin-exposed group in a dose-related manner. However, pretreatment with TMP at dose 15 mg/kg showed no significant difference compared to indomethacin-exposed group. Again, TMP alone-treated group showed no significant difference matched to control group.
Fig. 4.
Effect of pretreatment with TMP on oxidative biomarkers: (A) GSH content, (B) MDA concentration, (C) CAT activity in indomethacin-induced gastric ulcer in rats. Data are presented as mean ± S.D. (n = 6) a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
3.3. Effect of TMP pretreatment on inflammatory biomarkers: TNF-α, IL-6 and ICAM-1
As shown in Table 1, indomethacin-exposure resulted in pro-inflammatory response which was demonstrated by the dramatic (p < 0.001) increase in TNF-α concentration in the gastric tissues by 5.5 folds, compared to the control group. Unfortunately, pretreatment with TMP 15 mg/kg showed no significant difference, compared to indomethacin-exposed group. On the other hand, pretreatment with omeprazole (30 mg/kg) or TMP at doses (30 and 60 mg/kg) displayed an anti-inflammatory effect by significantly (p < 0.001) decreasing TNF-α concentration compared to indomethacin-exposed group by 64.73%, 26.31% and 61.57%respectively. Once more, TMP alone-treated group showed no significant difference compared to the control group. Concerning IL-6, indomethacin exposure caused significant (p < 0.001) increase in its concentration (~1.5-folds) as compared to the control group. Conversely, pretreatment with omeprazole demonstrated significant (p < 0.001) drop in IL-6 concentration by 48.6% as compared to indomethacin-exposed rats. Furthermore, groups pretreated with TMP 15, 30 and 60 mg/kg exhibited significant (p < 0.001) decreases in IL-6 concentration by 20.64%, 39.29 and 59.79% respectively, compared to indomethacin-exposed group. No significant difference from the control group was detected with TMP-alone treatment. A similar pattern of activity was detected with ICAM-1 assessment, where indomethacin caused obvious (5-fold) increase of its concentration, compared to the control group. Again, TMP 15, 30 and 60 mg/kg showed significant dose-related decreases of ICAM-1 concentration, compared to indomethacin-exposed group (Table 1).
Table 1.
Effect of TMP pretreatment with on inflammatory biomarkers: TNF-α, IL-6 and ICAM-1 in indomethacin-induced gastric ulcer in rats.
| Group | TNF-α (ng/mg protein} |
IL-6 (pg/mg protein) |
ICAM-1 (pg/mg protein) |
|---|---|---|---|
| Control | 0.29 ± 0.02 | 38.69 ± 1.89 | 2.02 ± 0.32 |
| Indomethacin (INDO) (25 mg/kg) | 1.9a ± 0.19 | 102.62a ± 15.55 | 10.57a ± 1.59 |
| Omeprazole (30 mg/kg) + INDO | 0.67a,b ± 0.09 | 52.74a,b ± 4.74 | 5.47a,b ± 0.64 |
| TMP (15 mg/kg) + INDO | 1.96a ± 0.13 | 81.43a,b ± 7.30 | 7.67a,b ± 0.93 |
| TMP (30 mg/kg) + INDO | 1.40a,b ± 0.11 | 62.30a,b ± 7.95 | 6.04a,b ± 0.72 |
| TMP (60 mg/kg) + INDO | 0.73a.b ± 0.10 | 41.26b ± 4.50 | 3.87a,b ± 0.35 |
| TMP alone (60 mg/kg) | 0.31b ± 0.03 | 35.45b ± 3.89 | 2.14b ± 0.42 |
Data are presented as mean ± S.D (n = 6). Indomethacin-exposed rats were given only indomethacin at a dose of 25 mg/kg intraperitoneally while all other pretreatments either with omeprazole (30 mg/kg) or TMP 15, 30 and 60 mg/kg were given orally one hour before indomethacin administration. a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
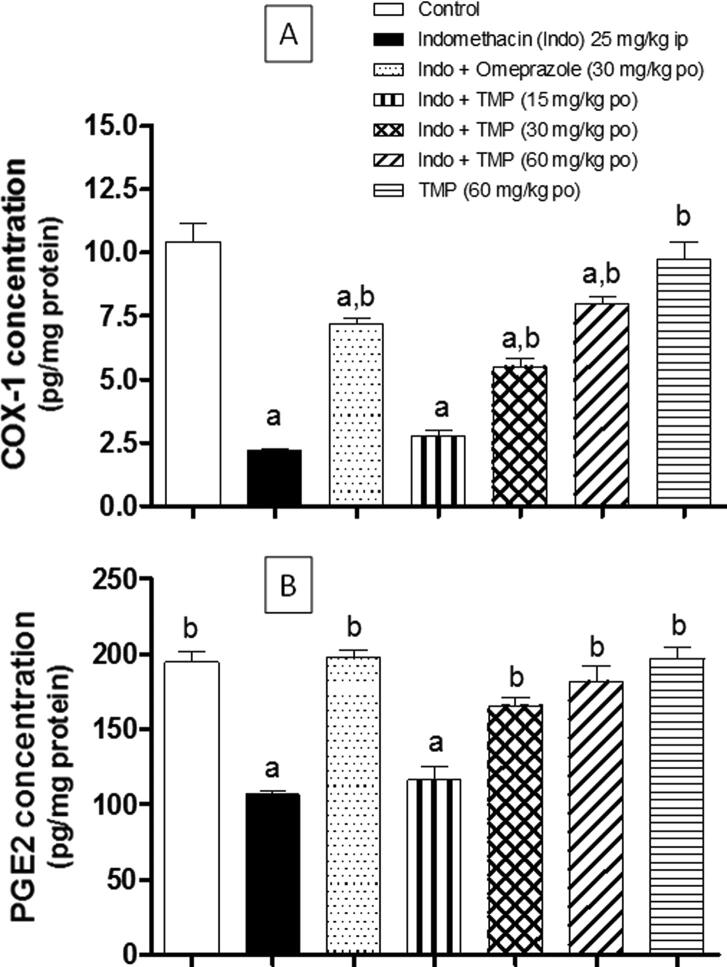
3.4. Effect of TMP pretreatment on COX-1 and PGE2 concentrations
As shown in Fig. 5A, COX-1 concentration in gastric tissue homogenate was decreased significantly (p < 0.001) by 79.1% due to indomethacin-exposure, compared to the control group. However, group pretreated with omeprazole demonstrated significant (p < 0.001) rise of COX-1 concentration by >3 folds, compared to indomethacin-exposed group. Additionally, TMP pretreatment with doses (30 and 60 mg/kg) produced significant (p < 0.001) increases in COX-1 concentration in a dose-related manner, compared to indomethacin-exposed group. Such effects on COX-1 concentration was reflected on the synthesis of PGE2 in gastric tissue homogenate. As shown in Fig. 5B, PGE2 concentration was decreased significantly (p < 0.001) because of indomethacin-exposure, compared to the control group. Again, TMP pretreatment with doses (30 and 60 mg/kg) significantly (p < 0.001) raised PGE2 concentration, compared to indomethacin-exposed group. On the other hand, pretreatment with TMP at dose 15 mg/kg presented no significant difference compared to indomethacin-exposed group. Rats treated with TMP alone demonstrated no significant difference in both COX-1 and PGE2, compared to the control group.
Fig. 5.
Effect of pretreatment with TMP on: (A) COX-1, (B) PGE2 concentrations in indomethacin-induced gastric ulcer in rats. Data are presented as mean ± S.D. (n = 6) a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
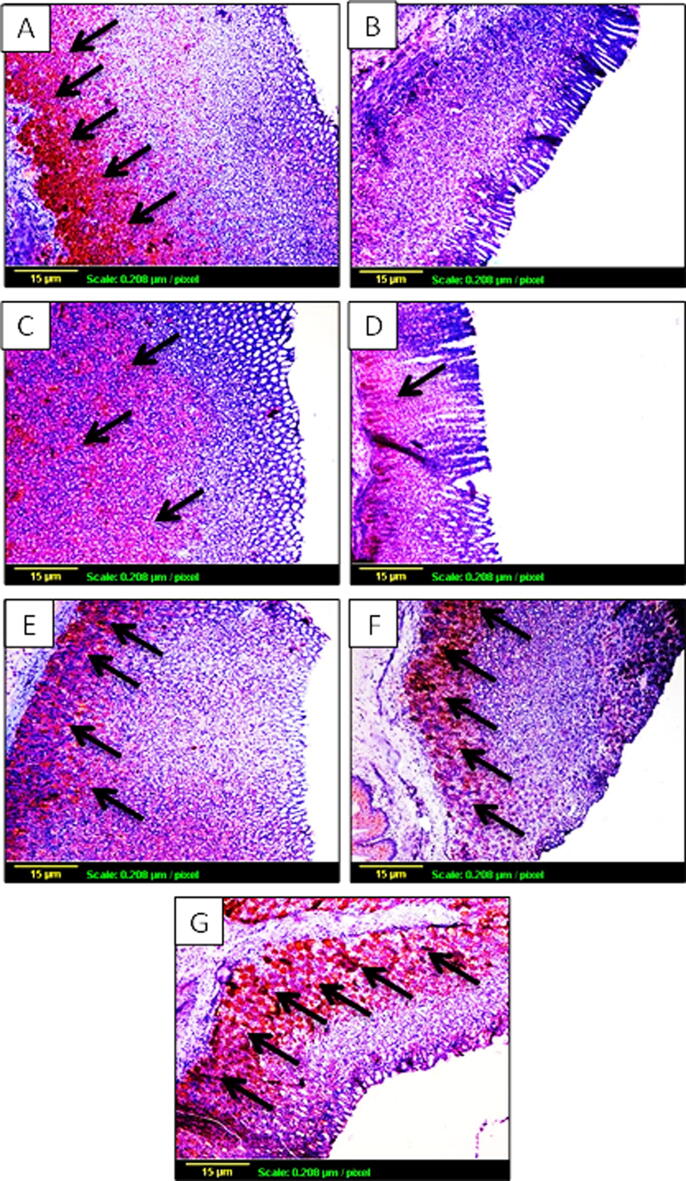
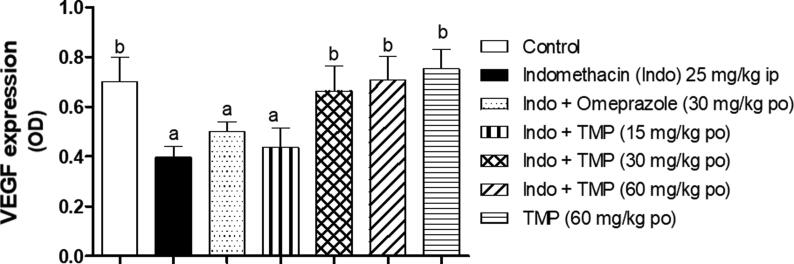
3.5. Effect of TMP pretreatment on VEGF expression
Expression of VEGF as an angiogenic marker using IHC staining was used to determine the degree of ulceration healing in stomach tissues. As shown in Fig. 6A, the degree of brown staining reflects the level of VEGF expression and hence, the angiogenesis status in the tissue. There is an obvious decrease in brown staining in indomethacin-exposed group, compared to control group (Fig. 6B) indicating a decrease in VEGF expression. Furthermore, Pretreatment with omeprazole (30 mg/kg) moderately improved VEGF expression as indicated by arrows (Fig. 6C). On the other hand, TMP pretreated group (15 mg/kg) showed mild increase of VEGF expression (Fig. 6D), while groups pretreated with TMP (30 and 60 mg/kg) as well as TMP-alone treated group demonstrated marked increase of brown coloration even more than the control group which indicate an improvement of VEGF expression in a dose related manner (Fig. 6E, F and G respectively). To confirm the effect of TMP on VEGF expression objectively, quantitative image analysis was carried out using ImageJ software (NIH, USA) and expressed as optical density (OD). As shown in Fig. 7, VEGF expression was decreased significantly (p < 0.001) by about 43% as a result of indomethacin-exposure compared to the control group. It is worth mentioning that groups pretreated with omeprazole and TMP 15 mg/kg failed to demonstrate any significant elevation of VEGF expression, compared to indomethacin-exposed group. Interestingly, TMP pretreated with doses (30 and 60 mg/kg) and TMP-alone groups showed significant (p < 0.001) increases in VEGF expression by 67%,78% and 98% respectively, in a dose related manner compared to indomethacin-exposed group.
Fig. 6.
The effect of TMP pretreatment on expression of vascular endothelial growth factor (VEGF) by immunohistochemical staining (×100). The amount of brown staining reflects the level of VEGF expression and hence, the angiogenesis status in the tissue. (A): Control group; (B): Indomethacin-exposed group; (C): omeprazole-pretreated group; TMP-pretreated groups at doses 15 and 30 mg/kg (D and E respectively); (F): TMP-pretreated group at dose 60 mg/kg; (G): TMP-alone treated group.
Fig. 7.
Quantitative image analysis for VEGF immunohistochemical staining, expressed as optical density (OD) across 6 different fields for each rat stomach section, Data are presented as mean ± S.D. (n = 6) a and b: statistically significant from the corresponding control and indomethacin group, respectively, at P < 0.05 using one-way analysis of variance (ANOVA) followed by Tukey’s as a post-hoc test.
4. Discussion
The prevention or treatment of peptic ulcers is a major challenge that faces health care authorities these days. Ten percent of the worldwide population is affected by this disease (Sharmila et al, 2013). The prevalence of H. pylori infection and peptic ulcer is high in Saudi Arabia as it is diagnosed in around 73% of the studied Saudi patients in the Western region of the Kingdom (Saber et al., 2015). On the other hand, a survey study done in the major metropolitan areas in the Kingdom including 3000 individuals showed that NSAIDs are highly consumed as daily pain killers (Bahdailah, 2019). Moreover, the current gastric ulcer treatment has numerous adverse reactions as well as partial efficacy against gastric illnesses (de Lira Mota et al., 2009) . Thus, the invention of safe and effective anti-ulcer drugs of plant origin is a medicinal research goal nowadays.
Tetramethylpyrazine (TMP) is an isolated purified chemical from a Chinese herb “Ligusticium Wallichii” that has been largely utilized in the treatment of vascular diseases. It guards against brain ischemic injuries and endorses cell proliferation and differentiation stimulated by ischemia (Chun-sheng et al., 1978). Moreover, TMP has been shown to protect against reserpine-induced gastric lesion in rats, probably in promoting mucous barrier (Wan et al., 1998). However, there is a shortage of data about the potential protective effects of TMP against experimentally induced gastric lesions as well as the possible underlying mechanisms. Therefore, this study was carried out to explore the possible gastroprotective effect of TMP versus indomethacin-induced stomach ulceration in rats as well as the causal mechanisms regarding oxidative stress, inflammation and angiogenesis.
Many investigational animal models have been used to induce gastric ulcer. Alcohol (ethanol/HCl) solution - when administrated orally - causes acute tissue injury and damage, accompanied by blood flow impairment and neutrophil efflux (Adinortey et al., 2013). Inversely, NSAID-induced ulcer model is considered one of the most popular gastric ulcer models (Suleyman et al., 2010). This model is based on the imbalance between anti-inflammatory and pro-inflammatory mediators at the site of injury as decreased prostaglandin E2 and increased secretion of IL-6 as well as TNF-α by epithelial cell (Kirchner et al., 1997, Bindu et al., 2013). It is worth mentioning that NSAIDs are extensively used for many indications as pain and inflammation in rheumatic disorders and osteoarthritis (Pal et al., 2010). They also can be used for prevention and treatment of ischemic heart disease (Gladding et al., 2008). Therefore, NSAIDs-induced ulcer model has been utilized in the current study.
Initially, TMP doses 15, 30 and 60 mg/kg were determined based on the tested dosage range of TMP in preceding researches, in which TMP was tested at doses up to 150 mg/kg orally in rats. Moreover, these doses are far below the LD50 of TMP which is 800 mg/kg via the oral route in mice (Wan et al., 1998, Chen et al., 2017, Michel et al., 2017). In addition, omeprazole was used as a reference drug for the test process. Clinically, omeprazole is broadly used as protective drug from gastric ulceration and many studies concerning gastroprotective actions have used omeprazole as a reference drug (Ketuly et al., 2013, Sidahmed et al., 2013).
In the current study, indomethacin exposure resulted in gross morphological injuries as well as histopathological changes and reduction of mucus amount within the gastric tissues of rat stomachs. On the other hand, TMP pretreatment at doses (15 and 30 mg/kg) showed lesser injuries relative to indomethacin-exposed group. While pretreatment with TMP highest dose demonstrated minor injuries with no histopathological alterations. Interestingly, TMP showed increase mucus secretion at all tested doses and these findings were in harmony with earlier study of Wan et al. (1998), in which TMP pretreatment showed significant increase of gastric mucus production.
Remarkably, Gastric ulcer occurs because of increased concentration of ROS such as hydrogen peroxide, hydroxyl radicals and superoxide anions. These (ROS) sequentially bring about oxidative stress in gastric tissue that has a crucial role in gastric bleeding and development of ulcer (Repetto and Llesuy, 2002). Those noxious effects of ROS can be counteracted by intracellular antioxidant enzymes, such as catalase, defending against ROS-made side effects. On the other hand, GSH can stop tissue injury by neutralizing ROS (Al Batran et al., 2013). Furthermore, oxidative stress can lead to an upsurge in lipid peroxidation and hence, increase its product MDA which is commonly used as a marker for lipid peroxidation (Dursun et al., 2009). In present study, indomethacin induced oxidative stress as evidenced by decreased endogenous antioxidants like GSH content and CAT activity in gastric tissue accompanied by considerable rise in lipid peroxidation level which expressed as MDA concentration. These results were congruous with previous studies which presented that indomethacin plays a vital role in production of ROS and associated gastric mucosal apoptosis (Dursun et al., 2009, Kim et al., 2011). On contrary, pretreatment with TMP was found to be effective in alleviating-ROS mediated adverse effects in a dose-related manner. These findings agree with previous results which showed that TMP indirectly suppress oxidative stress via upregulating antioxidant enzyme activities (Li et al., 2011) and direct scavenging properties of TMP (Yang et al., 2011). It is worthy noted that omeprazole itself has a significant antioxidant activity in-vitro by being a powerful scavenger of hypochlorous acid at acidic pH values that mimic intragastric clinical conditions (Lapenna et al., 1996). In addition, omeprazole has been found to prevent oxidative damage of gastric tissue, via significantly blocking stress-induced generation of hydroxyl radical (Biswas et al., 2003)
Alongside its direct harmful effect, oxidative stress induced by indomethacin could stimulate inflammatory response, encouraging the production of pro-inflammatory mediators including TNF-α and IL-6 (Rahman, 2002). Furthermore, indomethacin-induced oxidative stress can cause mitochondrial respiration uncoupling and hence, results in inflammation and manufacture of pro-inflammatory cytokines such as TNF-α and IL-6. These pro-inflammatory cytokines are responsible for induction of adhesion molecules like ICAM-1 which plays a major role in initiation and progression of the injury and inflammation of gastric tissue (Bindu et al., 2013). In fact, ICAM-1 expression has been found to provide adhesion between endothelial cells and leukocytes following injury (Bella et al., 1998). In the current study, TMP pretreatment significantly reduced the concentration of TNF-α, IL-6 and ICAM-1, compared to indomethacin-exposed rats and eventually decreased inflammation. These results were consistent with previous study in which TMP was found to reduce the production of inflammatory cytokines (Li et al., 2009).
As evidenced in the current study, gastric damage which occur as a side effect of NSAIDs consumption is mainly due to COX-1 enzyme inhibition, which leads to inhibition of PGE2 synthesis and then, weakness or loss of gastric protection. This prostaglandin (PGE2) plays an important role in production of mucus and increasing gastric blood flow which consequently leads to the gastro-protective actin (Takeuchi and Amagase, 2018). Again, indomethacin obviously decreased the gastric PGE2, the action that was partially prevented by TMP pretreatment. To our knowledge; the present study exposed for the first time a promotive effect of TMP on gastro-protective PGE2 versus indomethacin-induced gastric ulcers in rats. Additionally, Gaudio et al., (2006) reported that PGE2 has a healing-promoting effect which is associated with angiogenesis through stimulation of VEGF production in the fibroblasts, leading to stimulation of cell propagation. Furthermore, angiogenesis is crucial component for the tissue repair process. It reconstructs microvasculature that plays an important part in the ulcer healing process. COX-derived angiogenic growth factors such as VEGF and prostaglandin PGE2 are dynamically involved in these biological actions (Tarnawski et al., 2001). In this study, rats pretreated with TMP (15, 30 and 60 mg/kg) showed noticeable expression of VEGF. This could eventually promote healing process through endothelial cell production, survival and enhancing blood supply. These findings were in accordance with previous report of Chen et al. (2017) which approved that TMP has a promotive effect on VEGF expression in rat model of collagen-induced arthritis. Indeed, VEGF is a growth factor which has an important role in mucosal protection through its action on viability of endothelial cells and permeability of blood vessels (Ferrara, 2000). It should be pointed out that VEGF expression is augmented during healing process of the gastric ulcer, telling that VEGF might has an important role in mucosal repair (Szabo et al., 2000).
In conclusion, TMP possesses a protective effect against indomethacin-induced gastric ulcer in rats. This could be explained – at least partly – by its antioxidant, anti-inflammatory and angiogenic effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
-
•
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. G: 683-140-1439. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
-
•
The authors are grateful to Prof. Ashraf B. Abdel-Naim – Professor of Pharmacology & Toxicology, Faculty of Pharmacy, King Abdulaziz University – for generous offering of ELISA kits (ICAM-1 and COX-1).
-
•
The authors are thankful to Prof. Adel B. Khlossy – Professor of Pathology, Faculty of Veterinary Medicine, Cairo University – for histopathological examination and analysis
Footnotes
Peer review under responsibility of King Saud University.
References
- Adinortey M.B., Ansah C., Galyuon I., Nyarko A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013 [Google Scholar]
- Aebi H. vol. 105. Academic Press; 1984. [13] Catalase in vitro; pp. 121–126. (Methods in Enzymology). [Google Scholar]
- Asako H., Kubes P., Wallace J., Gaginella T., Wolf R.E., Granger D.N. Indomethacin-induced leukocyte adhesion in mesenteric venules: role of lipoxygenase products. Am. J. Physiol.-Gastrointest. Liver Physiol. 1992;262(5):G903–G908. doi: 10.1152/ajpgi.1992.262.5.G903. [DOI] [PubMed] [Google Scholar]
- Bahdailah A.A. Pattern use of non-steroidal anti-inflammatory drugs among Saudi community: patients’ perspective. Pharmacol. Toxicol. Biomed. Rep. 2019;5(2) [Google Scholar]
- Bancroft J.D., Gamble M., editors. Theory and Practice of Histological Techniques. Elsevier Health Sciences; 2008. [Google Scholar]
- Al Batran R., Al-Bayaty F., Al-Obaidi M.M.J., Abdualkader A.M., Hadi H.A., Ali H.M., Abdulla M.A. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS ONE. 2013;8(5):e64751. doi: 10.1371/journal.pone.0064751. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bella J., Kolatkar P.R., Marlor C.W., Greve J.M., Rossmann M.G. The structure of the two amino-terminal domains of human ICAM-1 suggests how it functions as a rhinovirus receptor and as an LFA-1 integrin ligand. Proc. Natl. Acad. Sci. 1998;95(8):4140–4145. doi: 10.1073/pnas.95.8.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Bindu S., Mazumder S., Dey S., Pal C., Goyal M., Alam A., Iqbal M.S., Sarkar S., Siddiqui A.A., Banerjee C., Bandyopadhyay U. Nonsteroidal anti-inflammatory drug induces proinflammatory damage in gastric mucosa through NF-κB activation and neutrophil infiltration: Anti-inflammatory role of heme oxygenase-1 against nonsteroidal anti-inflammatory drug. Free Radical Biol. Med. 2013;65:456–467. doi: 10.1016/j.freeradbiomed.2013.07.027. [DOI] [PubMed] [Google Scholar]
- BinSaeed A. Glimpse of the epidemiological research on Helicobacter Pylori in Saudi Arabia. Saudi J. Gastroenterol. 2009;15(2):85. doi: 10.4103/1319-3767.48963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas K., Bandyopadhyay U., Chattopadhyay I., Varadaraj A., Ali E., Banerjee R.K. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biol. Chem. 2003;278(13):10993–11001. doi: 10.1074/jbc.M210328200. [DOI] [PubMed] [Google Scholar]
- Borra S.K., Lagisetty R.K., Mallela G.R. Anti-ulcer effect of Aloe vera in non-steroidal anti-inflammatory drug induced peptic ulcers in rats. Afr. J. Pharm. Pharmacol. 2011;5(16):1867–1871. [Google Scholar]
- Buchwalow I.B., Böcker W. Immunohistochemistry. Basics Methods. 2010;1:1–149. [Google Scholar]
- Chen L., Liu T., Wang Q., Liu J. Anti-inflammatory effect of combined tetramethylpyrazine, resveratrol and curcumin in vivo. BMC Complement. Altern. Med. 2017;17(1):233. doi: 10.1186/s12906-017-1739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun-sheng L., Hsiao-meng Y., Yun-hsiang H., Chun P., Chi-fen S. Radix Salviae Miltiorrhizae and Rhizoma Ligustici Wallichii in Coronary Heart Disease. Chin. Med. J. 1978;4(1):43–46. [PubMed] [Google Scholar]
- Dursun H., Bilici M., Albayrak F., Ozturk C., Saglam M.B., Alp H.H., Suleyman H. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009;9(1):36. doi: 10.1186/1471-230X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog. Horm. Res. 2000;55:15–16. [PubMed] [Google Scholar]
- Gaudio E., Barbaro B., Alvaro D., Glaser S., Francis H., Ueno Y., Meininger C.J., Franchitto A., Onori P., Marzioni M., Taffetani S. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130(4):1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- Gladding Patrick A., Webster Mark W.I., Farrell Helen B., Zeng Irene S.L., Park Robert, Ruijne Nicola. The antiplatelet effect of six non-steroidal anti-inflammatory drugs and their pharmacodynamic interaction with aspirin in healthy volunteers. Am. J. Cardiol. 2008;101(7):1060–1063. doi: 10.1016/j.amjcard.2007.11.054. [DOI] [PubMed] [Google Scholar]
- Gornall A.G., Bardawill C.J., David M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- Henry D., Robertson J. Nonsteroidal anti-inflammatory drugs and peptic ulcer hospitalization rates in New South Wales. Gastroenterology. 1993;104(4):1083–1091. doi: 10.1016/0016-5085(93)90277-j. [DOI] [PubMed] [Google Scholar]
- Jiao F., Cong K., Li Y. Observation on ischemic cerebrovascular disease (113 cases) treated by injection of ligustrazine. J. Pract. Tradition. Chin. Med. 2004;20:174–175. [Google Scholar]
- Ke-ji C., Zhen-huai Q., Wei-Liang W., Mu-Ying Q. Tetramethylpyrazine in the treatment of cardiovascular and cerebrovascular diseases. Planta Med. 1983;47(02):89. [PubMed] [Google Scholar]
- Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Ketuly K.A., Hadi A.H.A., Golbabapour S., Hajrezaie M., Hassandarvish P., Ali H.M., Majid N.A., Abdulla M.A. Acute toxicity and gastroprotection studies with a newly synthesized steroid. PLoS ONE. 2013;8(3):e59296. doi: 10.1371/journal.pone.0059296. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim J.H., Kim B.W., Kwon H.J., Nam S.W. Curative effect of selenium against indomethacin-induced gastric ulcers in rats. J. Microbiol. Biotechnol. 2011;21(4):400–404. [PubMed] [Google Scholar]
- Kirchner T., Aparicio B., Argentieri D.C., Lau C.Y., Ritchie D.M. Effects of tepoxalin, a dual inhibitor of cyclooxygenase/5-lipoxygenase, on events associated with NSAID-induced gastrointestinal inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 1997;56(6):417–423. doi: 10.1016/s0952-3278(97)90593-7. [DOI] [PubMed] [Google Scholar]
- Lapenna D., de Gioia S., Ciofani G., Festi D., Cuccurullo F. Antioxidant properties of omeprazole. FEBS Lett. 1996;382(1–2):189–192. doi: 10.1016/0014-5793(96)00155-x. [DOI] [PubMed] [Google Scholar]
- Li H., Yang X., Shi W., Ma Z., Feng G.K., Yin Y.L., Fan Y.X., Jiang J. Protective Effects of tetramethylpyrazine on cerebrovascular regulations in rats with chronic alcoholic encephalopathy. Biomed. Environ. Sci. 2015;28(9):691–695. doi: 10.3967/bes2015.098. [DOI] [PubMed] [Google Scholar]
- Li N., Zhu Y., Deng X., Gao Y., Zhu Y., He M. Protective effects and mechanism of tetramethylpyrazine against lens opacification induced by sodium selenite in rats. Exp. Eye Res. 2011;93(1):98–102. doi: 10.1016/j.exer.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Li X.Y., He J.L., Liu H.T., Li W.M., Yu C. Tetramethylpyrazine suppresses interleukin-8 expression in LPS-stimulated human umbilical vein endothelial cell by blocking ERK, p38 and nulear factor-κB signaling pathways. J. Ethnopharmacol. 2009;125(1):83–89. doi: 10.1016/j.jep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Lu C., Jiang Y., Zhang F., Shao J., Wu L., Wu X., Lian N., Chen L., Jin H., Chen Q., Lu Y. Tetramethylpyrazine prevents ethanol-induced hepatocyte injury via activation of nuclear factor erythroid 2-related factor 2. Life Sci. 2015;141:119–127. doi: 10.1016/j.lfs.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Michel H.E., Tadros M.G., Esmat A., Khalifa A.E., Abdel-Tawab A.M. Tetramethylpyrazine ameliorates rotenone-induced Parkinson’s disease in rats: involvement of its anti-inflammatory and anti-apoptotic actions. Mol. Neurobiol. 2017;54(7):4866–4878. doi: 10.1007/s12035-016-0028-7. [DOI] [PubMed] [Google Scholar]
- Pal C., Bindu S., Dey S., Alam A., Goyal M., Iqbal M.S., Maity P., Adhikari S.S., Bandyopadhyay U. Gallic acid prevents nonsteroidal anti-inflammatory drug-induced gastropathy in rat by blocking oxidative stress and apoptosis. Free Radical Biol. Med. 2010;49(2):258–267. doi: 10.1016/j.freeradbiomed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Rahman I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem. Pharmacol. 2002;64(5–6):935–942. doi: 10.1016/s0006-2952(02)01153-x. [DOI] [PubMed] [Google Scholar]
- Repetto M.G., Llesuy S.F. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med. Biol. Res. 2002;35(5):523–534. doi: 10.1590/s0100-879x2002000500003. [DOI] [PubMed] [Google Scholar]
- Saber T., Ghonaim M.M., Yousef A.R., Khalifa A., Al Qurashi H., Shaqhan M., Samaha M. Association of helicobacter pylori caga gene with gastric cancer and peptic ulcer in Saudi patients. J. Microbiol. Biotechnol. 2015;25(7):1146–1153. doi: 10.4014/jmb.1501.01099. [DOI] [PubMed] [Google Scholar]
- de Lira Mota K.S., Dias G.E.N., Pinto M.E.F., Luiz-Ferreira Â., Souza-Brito A.R.M., Hiruma-Lima C.A., Barbosa-Filho J.M., Batista L.M. Flavonoids with gastroprotective activity. Molecules. 2009;14(3):979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saquib N., Saquib J., Alhadlag A., Albakour M.A., Aljumah B., Sughayyir M., Alhomidan Z., Alminderej O., Aljaser M., Al-Mazrou A. Chronic disease prevalence among elderly Saudi men. Int. J. Health Sci. 2017;11(5):11–16. [PMC free article] [PubMed] [Google Scholar]
- Sathish R., Sahu A., Natarajan K. Antiulcer and antioxidant activity of ethanolic extract of Passiflora foetida L. Indian J. Pharmacol. 2011;43:336–339. doi: 10.4103/0253-7613.81501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker E., Mahmoud H., Mnaa S. Anti-inflammatory and anti-ulcer activity of the extract from Alhagi maurorum (camelthorn) Food Chem. Toxicol. 2010;48(10):2785–2790. doi: 10.1016/j.fct.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Shah A.H., Khan Z.A., Baig M.A., Qureshi S., Al-Bekairi A.M. Gastroprotective effects of pretreatment with Zizyphus sativa fruits against toxic damage in rats. Fitoterapia (Milano) 1997;68(3):226–234. [Google Scholar]
- Shao Z., Wang L., Liu S., Wang X. Tetramethylpyrazine protects neurons from oxygen-glucose deprivation-induced death. Med. Sci. Monit.: Int. Med. J. Exp. Clin. Res. 2017;23:5277–5282. doi: 10.12659/MSM.904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharmila S., Devi P.V., Divyapriya S. Gastroprotective effect of aqueous extract of Aegle marmelos unripe fruit in albino rats. Int. J. Med. Pharma. Sci. 2013;3(1):21–26. [Google Scholar]
- Sidahmed H.M.A., Azizan A.H.S., Mohan S., Abdulla M.A., Abdelwahab S.I., Taha M.M.E., Hadi A.H.A., Ketuly K.A., Hashim N.M., Loke M.F., Vadivelu J. Gastroprotective effect of desmosdumotin C isolated from mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-helicobacter pylo. BMC Complement. Altern. Med. 2013;13(1):183. doi: 10.1186/1472-6882-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sowndhararajan K., Kang S.C. Protective effect of ethyl acetate fraction of acacia ferruginea DC against ethanol-induced gastric ulcer in rats. J. Ethnopharmacol. 2013;148(1):175–181. doi: 10.1016/j.jep.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Suleyman H., Albayrak A., Bilici M., Cadirci E., Halici Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation. 2010;33(4):224–234. doi: 10.1007/s10753-009-9176-5. [DOI] [PubMed] [Google Scholar]
- Szabo S., Khomenko T., Gombos Z., Deng X.M., Jadus M.R., Yoshida M. Transcription factors and growth factors in ulcer healing. Aliment. Pharmacol. Therapeut.: Ulcer Heal. Prostagland. 2000;14:33–43. doi: 10.1046/j.1365-2036.2000.014s1033.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Amagase K. Roles of cyclooxygenase, prostaglandin E2 and EP receptors in mucosal protection and ulcer healing in the gastrointestinal tract. Curr. Pharm. Des. 2018;24(18):2002–2011. doi: 10.2174/1381612824666180629111227. [DOI] [PubMed] [Google Scholar]
- Tarnawski A., Szabo I.L., Husain S.S., Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J. Physiol.-Paris. 2001;95(1–6):337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Utsumi H., Yasukawa K., Soeda T., Yamada K.I., Shigemi R., Yao T., Tsuneyoshi M. Noninvasive mapping of reactive oxygen species by in vivo electron spin resonance spectroscopy in indomethacin-induced gastric ulcers in rats. J. Pharmacol. Exp. Ther. 2006;317(1):228–235. doi: 10.1124/jpet.105.095166. [DOI] [PubMed] [Google Scholar]
- Wan J.L., Wang C.L., Chang Q.D. Effect of tetramethylpyrazine on reserpine-induced gastric lesion in rats. Dig. Dis. Sci. 1998;43(8):1652–1656. doi: 10.1023/a:1018846628019. [DOI] [PubMed] [Google Scholar]
- Yadav S.K., Adhikary B., Chand S., Maity B., Bandyopadhyay S.K., Chattopadhyay S. Molecular mechanism of indomethacin-induced gastropathy. Free Radical Biol. Med. 2012;52(7):1175–1187. doi: 10.1016/j.freeradbiomed.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Yang Q.H., Liang Y., Xu Q., Zhang Y., Xiao L., Si L.Y. Protective effect of tetramethylpyrazine isolated from Ligusticum chuanxiong on nephropathy in rats with streptozotocin-induced diabetes. Phytomedicine. 2011;18(13):1148–1152. doi: 10.1016/j.phymed.2011.05.003. [DOI] [PubMed] [Google Scholar]