Significance
The idea that grasslands can be ancient, particularly in climates that also support forests, is not widely recognized. Consequently, scientists and conservation planners often misinterpret old-growth grasslands to be low-diversity, successional vegetation, from which little is lost through conversion to tillage agriculture or tree plantations. We used a global analysis of herbaceous plant communities to show that after old-growth grasslands are destroyed, the recovery of plant diversity requires hundreds to thousands of years. Such slow rates of recovery underscore the need to replace outdated models of forest succession with models that emphasize the importance of fire, herbivory, and long periods of time to grassland biodiversity. This study offers evidence that old-growth grasslands, like old-growth forests, should be prioritized for conservation.
Keywords: biodiversity, disturbance, succession, land-use change, meta-analysis
Abstract
Earth’s ancient grasslands and savannas—hereafter old-growth grasslands—have long been viewed by scientists and environmental policymakers as early successional plant communities of low conservation value. Challenging this view, emerging research suggests that old-growth grasslands support substantial biodiversity and are slow to recover if destroyed by human land uses (e.g., tillage agriculture, plantation forestry). But despite growing interest in grassland conservation, there has been no global test of whether old-growth grasslands support greater plant species diversity than secondary grasslands (i.e., herbaceous communities that assemble after destruction of old-growth grasslands). Our synthesis of 31 studies, including 92 timepoints on six continents, found that secondary grasslands supported 37% fewer plant species than old-growth grasslands (log response ratio = −0.46) and that secondary grasslands typically require at least a century, and more often millennia (projected mean 1,400 y), to recover their former richness. Young (<29 y) secondary grasslands were composed of weedy species, and even as their richness increased over decades to centuries, secondary grasslands were still missing characteristic old-growth grassland species (e.g., long-lived perennials). In light of these results, the view that all grasslands are weedy communities, trapped by fire and large herbivores in a state of arrested succession, is untenable. Moving forward, we suggest that ecologists should explicitly consider grassland assembly time and endogenous disturbance regimes in studies of plant community structure and function. We encourage environmental policymakers to prioritize old-growth grassland conservation and work to elevate the status of old-growth grasslands, alongside old-growth forests, in the public consciousness.
Grasslands (broadly defined, including savannas and open-canopy grassy woodlands) occupy 28% of the terrestrial biosphere (1), house a significant proportion of global biodiversity (2), and support the livelihoods of at least a billion people via a multitude of ecosystem services (e.g., provisioning of water and carbon storage; 3). Given the global importance of grasslands, it is critical that we accurately conceptualize grassland ecological dynamics to advance our understanding of plant community responses to environmental change. Hindering such advances, the idea of climate-determined succession (4), one of the dominant ecological paradigms of the past century, underemphasizes two ubiquitous aspects of grassland ecology and evolution: fires and large herbivores (5).
Fire and herbivores shaped the ecology and evolution of Earth’s grasslands for millions of years before the existence of humans (6). Through consumption of aboveground plant biomass, these two agents of endogenous disturbance (7, 8) maintain grasslands in places where the climate and soils are suitable for the development of forests (9). When interpreted through the lens of climatic determinism, with a focus on trees rather than herbaceous plant community dynamics, frequent fire and herbivory can appear to reset, or arrest, ecological succession (10, 11). This successional narrative has contributed to a crisis in grassland conservation: around the world, disturbance-dependent grasslands are widely misclassified as degraded forests (12, 13), overlooked for their conservation value (14), targeted for agricultural conversion (15), and viewed as opportunities for carbon sequestration through tree planting and fire exclusion (i.e., afforestation and woody encroachment) (16, 17).
The old-growth grassland concept (18)—modeled on parallel ideas in forest ecology and conservation (19)—is a direct challenge to the narrative that most of Earth’s grasslands are successional communities that ought to become forests (11, 20). Of primary importance, the concept posits that old-growth grasslands are ecologically distinct from recently formed (secondary) grasslands (18). It is also important to note that fire and megafaunal herbivory—the endogenous disturbances that maintain old-growth grassland diversity (8)—are detrimental to many old-growth forests (e.g., 21). Further, readily visible indicators of old-growth forests, such as trees with large girths, are inapplicable to old-growth grasslands, where signs of antiquity are often underground (18). Indeed, old-growth grasslands are characterized by slow-growing, long-lived herbaceous plants, with a suite of traits, including underground storage organs, bud banks, and rhizomes, which enable resprouting and clonal growth after fire and herbivory (22, 23).
Although old-growth grasslands include some of the most biodiverse terrestrial ecosystems, there has been no global-scale test of whether old-growth grasslands are, in fact, more species-rich than secondary grasslands. Several examples suggest that high species richness is characteristic of old-growth grasslands. The savannas of the South American Cerrado support 4,800 endemic plant and vertebrate species (24). The Shola grasslands of India, home to endangered Asian elephants and Bengal tigers, are rich with herbaceous plants (278 species) (25). The world record for local-scale plant species richness (89 vascular species/m2) is held by a montane grassland in Argentina (26). At the 100–1,000-m2 scales, fire-dependent grasslands of the North American Coastal Plain can be as rich in vascular plant species as tropical forests (14). While illustrative of the potential diversity of grassland plant communities, these examples do not tell us how quickly old-growth grassland plant diversity recovers after intensive land-use change, such as agriculture or afforestation.
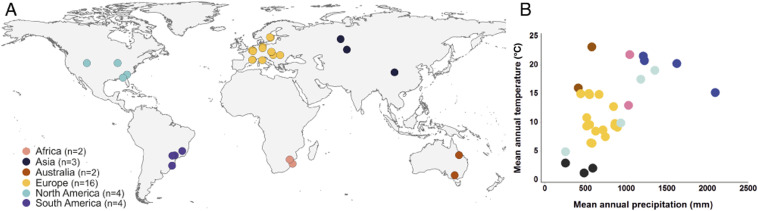
To test the relevance of the old-growth grassland concept to our understanding of global patterns of herbaceous plant diversity, we conducted a meta-analysis of 31 pairs of old-growth grasslands and secondary grasslands on six continents (Fig. 1). Because the application of the term “old growth” to grasslands is recent, we included studies that used a variety of synonymous adjectives, including: ancient, intact, native, natural, pristine, reference, remnant, seminatural, and undisturbed (SI Appendix, Fig. S1). For our analysis, we compared species richness between pairs of old-growth and secondary grasslands using a random-effects model of the log response ratio (lnRR, calculated as loge[secondary grassland richness/old-growth grassland richness]) (27). To determine the rate at which secondary grasslands recover the species richness of old-growth grasslands, we conducted a mixed-effects linear metaregression (27) of 92 secondary grassland ages (range 1–251 y) extracted from the 31 studies. As is standard in meta-analyses (27), we weighted each study by the inverse of the associated variance and evaluated the robustness of our findings through assessments for publication bias and sensitivity (see Methods).
Fig. 1.
Geographic and climatic distribution of paired old-growth grassland and secondary grassland study sites. (A) Locations of the 31 studies included in the meta-analysis. (B) Bivariate plot of mean annual precipitation and mean annual temperature for each study location.
Following the core meta-analysis, we used data from a subset of studies to better understand how variation in species richness and grassland age relate to plant community composition. We first assessed the relationship between total species richness in secondary grasslands and the recovery of old-growth grassland community composition (n = 10 studies). We then assessed the relationship between grassland age and the number of weedy species (including ruderals and exotics; n =11 studies with 29 timepoints). In combination, we expect these analyses of grassland species richness, assembly time, and community composition to validate patterns that many grassland ecologists have recognized in specific ecosystems around the world (e.g., 28 and 29). Through global meta-analysis, we hope to expand recognition of the high species diversity and slow assembly of old-growth grasslands more broadly among ecologists, environmental policymakers, and the public.
Results
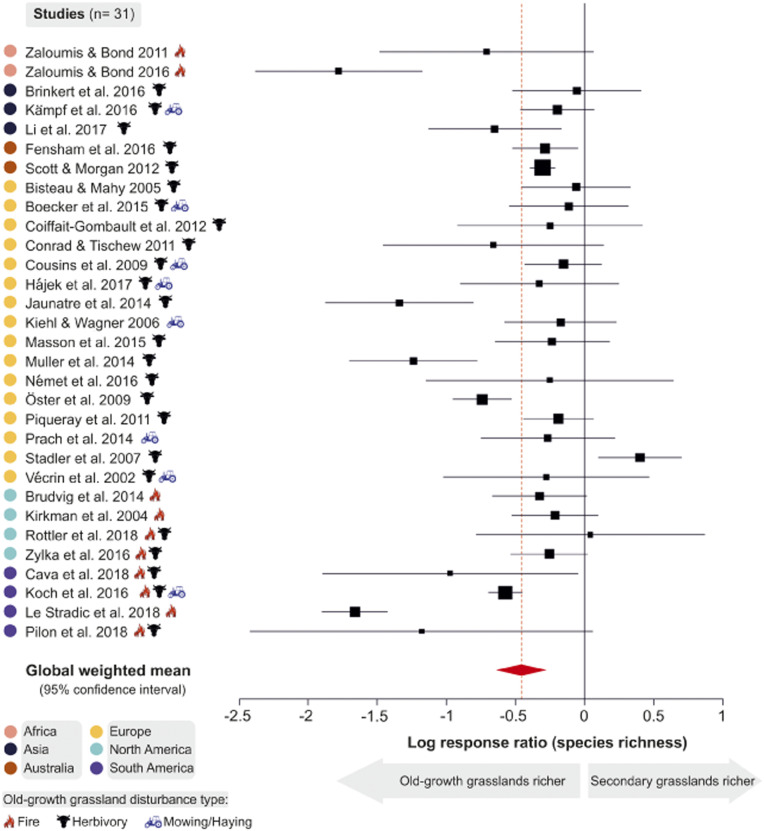
Our results showed that secondary grasslands support 63% of the species richness of old-growth grasslands (95% confidence interval [CI]: 53%, 76%; global weighted mean lnRR: −0.46, 95% CI: −0.64, −0.28; Fig. 2). For individual studies, lnRR ranged from −1.8 to 0.4, with only 2 of 31 studies reporting secondary grasslands to be richer than old-growth grasslands (Fig. 2). The weighted mean lnRR was associated with a high level of between-study heterogeneity (Q = 230, I2 = 90%, P < 0.0001), which is typical of ecological meta-analyses (30). Post hoc assessments suggested that our estimate of the global weighted mean (i.e., lnRR = −0.46) is robust (SI Appendix, Table S1 and Figs. S2–S5). Tests for publication bias (26) were negative (SI Appendix, Table S2 and Figs. S6 and S7).
Fig. 2.
Global comparison of species richness in old-growth grasslands and secondary grasslands. The 31 plant community studies (Left Column) are listed alphabetically by continent and author and are marked by the type of aboveground disturbance that currently maintains old-growth grasslands at each site. For each study, boxes and solid lines display the natural logarithm of the response ratio (loge [secondary grassland richness/old-growth grassland richness]) and 95% CIs, respectively. Box sizes are proportional to the weight of the study (see Methods). Response ratios less than zero indicate that old-growth grasslands are more species-rich than secondary grasslands, whereas values greater than zero indicate secondary grasslands are richer. Displayed as a red diamond and red vertical line, the global weighted mean response ratio (−0.46, I2 = 90%, P = 0.0001) equates to secondary grasslands supporting 63% of the richness of (or 37% fewer species than) old-growth grasslands. (Ends of the diamond indicate the 95% CI: −0.64 to −0.28, equivalent to 53% and 76%.) See Dataset S1 for full study citations.
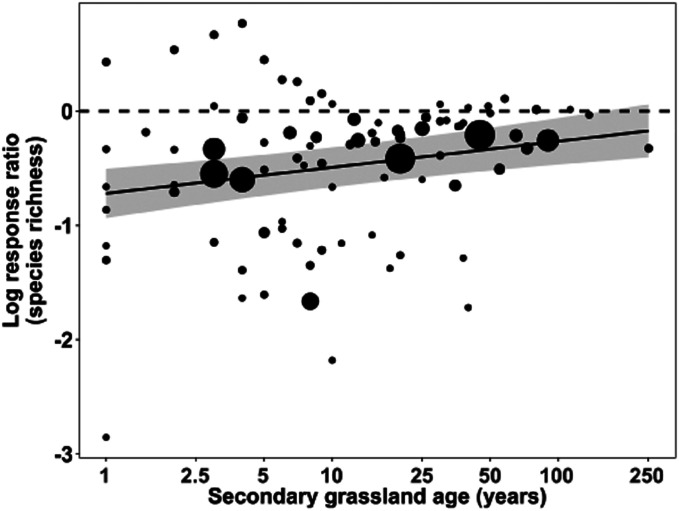
Secondary grassland age was weakly, but positively, related to the recovery of plant species richness (Fig. 3, P = 0.0001, R2 = 0.041). The upper bound of the 95% CI for the metaregression model yielded a minimum global recovery time (to lnRR = 0) for plant species richness of ∼160 y. Extrapolation of the regression equation, which should be interpreted cautiously, projected a mean time of ∼1,400 y for richness to recover. At the last timepoint for which we have data (i.e., 251 y, which is the oldest value permitting interpolation), the regression equation predicted secondary grasslands to recover 84% of the richness of old-growth grasslands (lnRR = −0.17).
Fig. 3.
Relationship between secondary grassland age and the recovery of old-growth grassland species richness. Black circles represent secondary grassland age (n = 92, range: 1–251 y, extracted from n = 31 studies) and are scaled in proportion to their weight (see Methods; note age is represented on a log10 scale). The metaregression model accounts for this weight and the random effect of each study location. The regression equation, lnRR = 0.2279 (log10[secondary grassland age]) − 0.7201 (R2 = 0.041, P = 0.0001), is displayed as a solid black line; gray shading indicates the 95% CI. The horizontal dashed line indicates the response ratio at which secondary and old-growth grassland species richness is equal (lnRR = 0). Response ratios less than zero indicate secondary grasslands that have fewer species compared to old-growth grasslands.
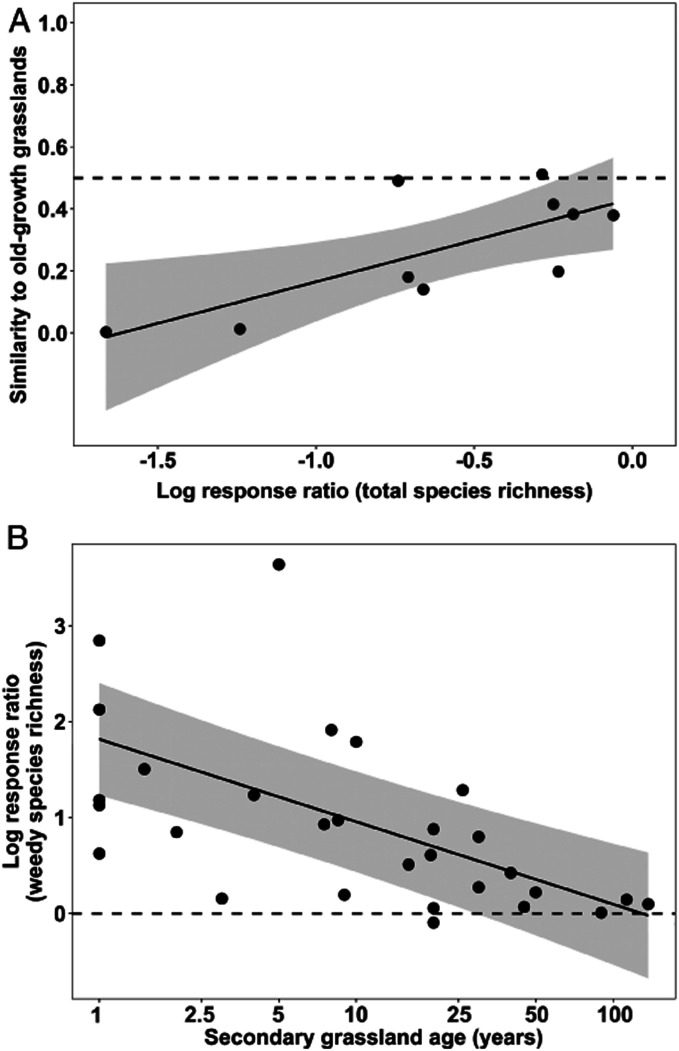
Even as richness increased with time, the communities of plants recolonizing secondary grasslands remained distinct from those of old-growth grasslands. Based on regression of the subset of 10 studies that reported community similarity indices (Fig. 4A), we projected that recovery of species richness (i.e., to lnRR = 0) would equate to just 43% (95% CI: 31%, 56%) compositional similarity between old-growth and secondary grasslands. These persistent differences in community composition can be explained in part by a preponderance of weedy species in secondary grasslands. Regression of 29 timepoints from 11 sites indicated that during the initial 29–130 y of recovery (range based on lower 95% CI and mean regression equation for lnRR = 0; Fig. 4B), secondary grasslands supported more weedy species than did old-growth grasslands.
Fig. 4.
Indicators of plant community composition in relation to secondary grassland species richness and age. (A) Relationship between the lnRR of total species richness and the compositional similarity between secondary and old-growth grassland communities. Data are from n = 10 studies that reported similarity indices. The regression equation, similarity = 0.2681(lnRR) + 0.4332 (R2 = 0.536, P = 0.016), is displayed as a solid black line; gray shading indicates the 95% CI. The horizontal dashed line (similarity = 0.5) indicates the level at which secondary grasslands are 50% similar to old-growth grasslands in species composition. At lnRR = 0, secondary and old-growth grasslands are equal in total species richness. (B) Relationship between the lnRR of weedy species richness and age of secondary grasslands. The mixed-effects regression model is based on the n = 11 studies (random effect) that reported weedy species richness for n = 29 timepoints (age as fixed effect). The regression equation, lnRR = −0.8597 (log10[secondary grassland age]) + 1.8164 (R2 = 0.274, P < 0.0001), is displayed as a solid black line; gray shading indicates the 95% CI. At lnRR = 0, secondary and old-growth grasslands are equal in weedy species richness.
Discussion
By demonstrating that secondary grasslands support just 63%, and are missing 37%, of the herbaceous plant species richness of old-growth grasslands (Fig. 2), this meta-analysis provides support for the applicability of the old-growth grassland concept at the global scale (18). Evidence of the slow assembly of old-growth grasslands (Fig. 3) underscores recent calls to move away from the view of most grasslands as a successional stage (11), toward recognition that endogenous disturbances can sustain species-diverse grasslands for very long periods of time in climatic zones that can also support forests (5, 9, 10, 17, 22). Compared to old-growth forests, which are widely recognized and intensively studied (31, 32), we still know relatively little about old-growth grasslands. We hope that these results will motivate future studies, analogous to research on secondary forests (31), to better understand the recovery rates of secondary grasslands with different land-use histories (SI Appendix, Fig. S8) and to compare grasslands of the tropics to those of temperate latitudes (SI Appendix, Fig. S9) (22).
In our analysis, we focused on one aspect of plant diversity—species richness—a community metric available from the modest number of 31 grassland studies that included both old-growth and secondary grasslands. Because species richness figures prominently in the application of community ecology to questions of global change (e.g., 33), we suggest that recognizing old-growth grasslands as distinct from secondary grasslands will improve our understanding of the relationships among plant diversity, community assembly time, and ecosystem functioning (18, 34). Doing so would pull together disparate, but clearly related, lines of research, such as those framed around agricultural legacies (29), fire exclusion (17), declines of native megafauna (35), woody encroachment (36), and nutrient pollution (37), to help clarify the distinct ecological consequences of human activities for old-growth versus secondary grasslands.
Our results show that old-growth grasslands, once destroyed, require at least a century, and more typically millennia, to recover their plant species richness (Fig. 3); full recovery of plant community composition will take even longer (Fig. 4A). To be clear, the recovery of species richness is not the same as the recovery of community composition; two communities can have the same number of species, while the identity of those species can be quite different (38). For the subset of sites that provided compositional data, recovery of species richness equated to just 43% similarity in community composition between secondary and old-growth grasslands (Fig. 4A). Thus, our estimate of the time required for secondary grasslands to attain the richness of old-growth grasslands (160–1400 y; Fig. 3) is certainly less than the time required for secondary grasslands to recover the community composition (i.e., the full suite of species and abundances) of old-growth grasslands. This echoes research on secondary tropical forests, where tree species richness typically rebounds within 50 y, but recovery of the composition of old-growth forests requires many centuries or longer (39).
One reason that richness is thought to recover more rapidly than community composition is because weedy species are quick to colonize secondary grasslands (e.g., 40). Consistent with this idea, and based on the 11 studies that reported data on weedy plants (including ruderal and nonnative species), we found that compared to old-growth grasslands, young secondary grasslands supported more weedy species. Evidence that elevated numbers of weedy species persisted for 29–130 y in secondary grasslands (Fig. 4B) underscores recent calls for grassland experiments to consider compositional changes over longer periods of time (i.e., >10 y (41)). From our analysis, it appears that while weedy species partially compensated for reduced species richness in secondary grasslands, certain species characteristic of old-growth grasslands remained missing even after many decades to centuries (Figs. 3 and 4 A and B).
Why are certain old-growth grassland species missing in secondary grasslands? A plausible explanation is that aboveground disturbances (i.e., fire and herbivory), which select for persistence in old-growth species, are fundamentally different from the anthropogenic disturbances, like tillage agriculture, that destroy underground organs and select for secondary grassland species with high colonization ability (18). To explore this possibility, we revisited the results and discussions of the 31 studies included in the meta-analysis and found that the missing species most frequently described by authors (Dataset S1) were native perennial grasses (typically with C4 photosynthesis) and native perennial forbs (often species with underground storage organs). Missing species were also described as fire-promoting and shade-intolerant, with high capacity to resprout. Authors further described missing species as being stress-tolerant with poor colonization ability, producing seeds dispersed by gravity or ants, forming limited seed banks, and relying on clonal growth or asexual reproduction. In addition, authors noted missing species that were of conservation concern in specific regions. These included: medicinally important species in Africa; annual hemiparasites in Asia; composites (Asteraceae) and legumes (Fabaceae) in North and South America; perennial sedges and orchids and threatened International Union for Conservation of Nature Red List species in Europe; woody subshrubs (underground trees) in South America; and endemic grasses in Australia (Dataset S1). These descriptions match the key functional types of old-growth grassland species that are thought to be most vulnerable to anthropogenic environmental change (22).
In addition to functional traits (e.g., persistence–colonization trade-offs; 42 and 43), a multitude of ecological mechanisms likely contribute to the lower richness and slow recovery of secondary grasslands relative to old-growth grasslands. Indeed, a central focus of grassland restoration ecology is to identify these mechanisms and overcome the limitations to old-growth grassland community assembly (44). In some ecosystems, landscape effects, such as spatial isolation (45) or limited habitat connectivity (46), restrict the arrival of plant propagules. In others, site-level conditions, such as severely altered soil conditions (47) and species interactions (e.g., priority effects and plant–soil feedbacks; 48), limit the establishment of old-growth grassland species. Also important, but often overlooked in grassland restoration studies (49), is the role of vegetation-disturbance feedbacks (e.g., 50 and 51). Plant communities determine the quantity and quality of biomass available for fire and herbivores to consume, and in turn, fire and herbivores influence grassland community composition via selection on plant traits (52). Given their differences in species composition (Fig. 4 A and B), we should expect pairs of old-growth and secondary grasslands to also differ in aspects of their disturbance regime, such as frequency, seasonality, and intensity, even if they experience the same disturbance type (i.e., fire, grazing, or haying). In sum, altered disturbance regimes, reinforced by vegetation-disturbance feedbacks (53), should be considered among the probable mechanisms for reduced species richness and slow compositional recovery of secondary grassland communities (8).
Conclusion
In light of the high diversity of old-growth grasslands (Fig. 2) and the many documented challenges to their restoration (8), we encourage environmental policymakers to give old-growth grasslands equal consideration as old-growth forests (32) in efforts to conserve Earth’s biodiversity. We are particularly concerned that recent research and emerging land-use policies, both meant to promote tree planting for carbon sequestration, are a threat to undervalued grassland biodiversity and ecosystem services (16, 17). Fundamental to these afforestation efforts has been the assumption that old-growth grasslands that occur where climate-vegetation models suggest forest as the potential vegetation must be degraded. Our analysis shows that the reality on the ground is much more complicated. Indeed, most of the species-rich old-growth grasslands in this analysis occur in climates that can support forests (Fig. 1 and ref. 5). We urge conservation initiatives to safeguard against the conversion of old-growth grasslands for tree planting or tillage agriculture, to maintain biodiverse grasslands with frequent fires and megafaunal herbivores, and to emphasize the recovery of grassland plant communities in efforts to restore Earth’s biodiversity.
Materials and Methods
Literature Search and Screening.
To identify studies that compared species richness in old-growth grasslands and secondary grasslands, we conducted a literature search of peer-reviewed journal articles in the Web of Science database (27, 54). This initial Web of Science search yielded a total of 8,336 articles. We examined the titles of these 8,336 articles for relevance and retained 745 articles. We then screened the abstract (and methods in some cases) of these 745 articles to arrive at a shortlist of 99 articles for detailed examination. We then examined in detail the full texts of these 99 articles, which resulted in a final set of 31 articles that met our eligibility criteria (described below) for inclusion in the analyses (SI Appendix, Fig. S1) (55).
For the initial search, we used the advanced search function in Web of Science to identify articles published from 1 January 1900 to 14 November 2018 that fit the following topic search (TS) criteria (i.e., terms found in titles, abstracts, and keywords):
TS = (savanna* OR grassland* OR woodland* OR pine OR pinus OR eucalypt* OR cerrado OR prairie OR veld* OR steppe) AND TS = (herb* OR grass* OR forb* OR understor*) AND TS = (richness OR diversity) AND TS = (“old growth” OR secondary OR succession* OR remnant* OR “old field” OR restor* OR reference OR abandon* OR “post agric*” OR “woody encroach*” OR mine OR mining OR degrad* OR pasture OR plantation OR afforest*).
We subsequently scrutinized the articles to ensure that they met the following criteria. 1) Study sites were grasslands, broadly defined to include savannas and open-canopy grassy woodlands (22). As such, the studies in our analysis encompass herbaceous-dominated ecosystems with scattered trees that are often called “forests” or “woodlands” (12). 2) Studies included old-growth grasslands (18) that were either clearly described in the article or that we were able to verify through correspondence with the authors. Because there is a wide range of synonymous terminology for old-growth grasslands in the literature, we screened studies to ensure that there were no major human-induced structural or functional alterations to the historical herbaceous plant communities and that current ecosystem management closely resembled historical, endogenous disturbance regimes (8). As such, we included study sites that supported large herbivores (domestic livestock and/or native megafauna), were burned with prescribed fire or wildfire, or where other regular aboveground disturbance (i.e., mowing or haying) served as a surrogate for fire and herbivory (18). 3) Studies included secondary grasslands (11) on sites previously occupied by old-growth grasslands that had been destroyed by tillage agriculture, tree plantations, or other intensive land uses. 4) Studies reported data for herbaceous plant species richness for both old-growth and secondary grasslands. 5) Study plots were not treated with nutrient additions (e.g., nitrogen or phosphorous fertilizers). 6) Studies were conducted in a unique location; where multiple papers provided data for the same study location, we excluded all but the most complete (i.e., best replicated, longest duration) paper for that location. 7) We only included studies on “actively restored” grasslands (i.e., restoration treatments such as sowing seed mixtures, soil, or hay transfer) if the study included a control treatment of “passive restoration” (i.e., secondary grassland communities assembling without propagule additions). For such studies, we only extracted data from the passively restored secondary grasslands and the paired old-growth grasslands.
Data Extraction.
Response variables.
Total species richness.
We extracted the mean total species richness per unit area for the old-growth grasslands and secondary grasslands in each study. For studies that only presented richness in figures (n = 20), we calculated the mean richness using the image analysis software ImageJ (56). For studies (n = 2) that reported median richness but not mean, we used the median, since these studies had either nonnormal data (as verified from figures) or a large (>25) sample size (57). In cases where studies appeared to have measured richness, but did not report richness, we contacted the authors (n = 4).
Weedy species richness.
For each study, we determined whether the authors presented data on weedy species. We included any group of species that the authors identified using one, or a combination of, the following terms: ruderals (including annuals, perennials, or both), weedy species, arable weeds, alien species, exotic species, or invasive species. Using the same approaches as for total species richness (described above), we were able to extract weedy species richness from 11 studies, yielding 29 timepoints.
Compositional similarity.
Ten out of the thirty-one studies reported the similarity of old-growth and secondary grassland plant communities as Jaccard’s (n = 4), Bray-Curtis (n = 3), and Sorensen’s (n = 3) indices. For studies that reported dissimilarity we converted the index to similarity (i.e., 1-dissimilarity). Because these indices range from 0 to 1, we analyzed them without further transformation (58).
Predictor and moderator variables.
Location.
We used the latitude and longitude of the sites reported in the methods of each study to map study locations (Fig. 1A) using Q-GIS v 2.10.1 (59).
Precipitation and temperature.
We obtained mean annual precipitation and temperature for each location from the WorldClim2 database (60). In cases where the author-reported precipitation deviated more by more than 100 mm (n = 4 cases) from the WorldClim2 data, we used the author-reported values. We plotted mean annual precipitation versus mean annual temperature (Fig. 1B) with Minitab (Minitab, Inc., Pennsylvania State University).
Type of secondary grassland.
We used authors’ descriptions of land-use history to classify secondary grasslands into one or more of the following categories for supplemental analyses: tree plantations/woody encroachment; tillage agriculture; soil excavation; planted pasture; or other (SI Appendix, Table S3 and Fig. S8).
Age of secondary grassland.
We determined the assembly time (in years) for each secondary grassland based on author-reported time since the last grassland-damaging (i.e., exogenous (8)) disturbance or time since land abandonment. If secondary grasslands were classified by a range of ages, we used the mean of the range. For open-ended classes, we approximated the value to get a conservative estimate of age (e.g., for Öster et al. (61), we considered the class “<10 y” to be 5 y and “>50 y” to be 55 y). In the one study with multiple sites of different ages (i.e., Brudvig et al. (62), with sites of age 90 y, 69 y, 58 y) we calculated a weighted mean age (weighted by sample size). For studies (n = 3) that provided a large number (>30) of data points across a range of secondary grassland ages, we extracted a subset of discrete timepoints to represent the range. For the 2 (of the 92 secondary grassland timepoints) that were sampled after one growing season, we coded their age as 1 y rather than as a fraction of a year, to avoid giving them undue weight in our log(time) regression (Fig. 3).
Statistical Analyses.
Effect size.
To compare the differences between old-growth and secondary grassland species richness, we calculated the lnRR as the effect size (63). The lnRR has been widely used in ecology (64) and has several desirable properties. A major advantage of lnRR over other effect-size metrics is that it does not require variance data for computation (63). For our calculations, lnRR = loge (Ȳs/Ȳo) where Ȳs = species richness for secondary grasslands, and Ȳo = species richness for old-growth grasslands. Thus, values of lnRR < 0 indicated old-growth grasslands have greater richness than secondary grasslands, whereas lnRR > 0 indicated secondary grasslands are richer than old-growth grasslands, and lnRR = 0 indicated both grassland classes are equally rich in species. For studies that provided more than one data point by time, we calculated the composite effect size per study (65).
Variance and weights.
We calculated the variance of lnRR (VlnRR) based upon reported sample sizes (66) using VlnRR = (Ns + No)/(Ns × No), where Ns is the sample size for secondary grasslands, and No is the sample size for old-growth grasslands. By calculating VlnRR in this manner, we were able to standardize the variance estimates across studies and obtain estimates for those studies that did not report variance, or that had pseudoreplicated or otherwise poorly described study designs (67, 68) (Dataset S1). We weighted the lnRR for each study by the inverse variance, such that studies with higher variance were given lower weights (27). We used OpenMEE (69) to perform the meta-analysis (Fig. 2), including tests for heterogeneity, publication bias, and sensitivity. We considered meta-analysis results to be statistically significant (at α = 0.05) if the 95% CI of the overall mean lnRR did not include zero.
Handling nonindependent observations.
Several studies reported multiple (nonindependent) data points (n = 18), unbalanced study designs, or both. We calculated the variance of the composite lnRR for studies with unequal sample sizes (and thus unequal variances) as (1/n)2 (Vi + 2 (rij √Vi √Vj)), where n = number of observations in the study, and rij = correlation coefficient (covariance) between the pair of effect sizes under consideration (27, 65). If a study had equal sample sizes (and thus equal variances), the above formula was simplified to: (V/n) × Variance Inflation Factor (VIF) (27), where VIF = 1 + (n − 1) × r. This formula thus takes into account the nonindependence of multiple observations reported by a single study through the covariance factor. The possible values of “r” range from 0 to 1 (assuming the correlation is positive), but we cannot know the true value of r in the selected studies. To apply r = 0 would be to assume that observations are independent and result in an underestimation of the variance. Conversely, to apply r = 1 would assume perfect (100%) correlation and certainly overestimate the variance (65). We chose to apply r = 0.5 as a plausible value for our analysis (65) and verified that other plausible values (i.e., r = 0.25 and 0.75) yielded similar results (SI Appendix, Table S1 and Figs. S2 and S3).
Between-study heterogeneity.
We calculated the weighted overall mean effect size (lnRR) for n = 31 studies using a random-effects model, with between-study variance estimated using the restriction maximum likelihood (REML) approach (70). We also used REML to calculate effects by subgroups using moderator variables (as in SI Appendix, Fig. S8). To test if there was significant heterogeneity associated with the effect sizes, we computed the Q statistic (a measure of between-study variance) and tested this against a X2 distribution (with n-1 degrees of freedom [dof], n = number of studies) (27). Because the Q statistic has low power and is not intuitive by itself (27, 65), we also report heterogeneity with the I2 statistic (approximately equal to 100 × (Q − dof/Q)), which yields a more intuitive measure of heterogeneity, from 0 to 100% (65).
Sensitivity analyses.
We performed several sensitivity analyses to verify that the meta-analysis results were robust. 1) To determine if using the pseudoreplicated (within-study) sample sizes affected our conclusions, we performed a post hoc sensitivity analysis by repeating our meta-analysis without the two studies with highest sample sizes (and consequently highest weights) (SI Appendix, Table S1 and Fig. S5). Given the overall result did not change with exclusion of these highly weighted studies, this analysis supported our use of study sample sizes to estimate variances (71, 72). 2) To assess the sensitively of our results to our assumption of covariance of r = 0.5, we repeated the analysis using other plausible covariance values (r = 0.25, r = 0.75) and verified that results did not deviate substantially with changes in r (as in 73; SI Appendix, Table S1 and Figs. S2 and S3). 3) Given the high between-study heterogeneity obtained from the weighted model and to further assess the sensitivity of the calculated overall mean lnRR to the weighting of studies, we calculated an unweighted mean lnRR (27) (SI Appendix, Fig. S4).
Metaregression of species richness and time.
We tested for the effect of secondary grassland age on lnRR using a metaregression mixed-effects model that treated each study as a random effect (i.e., REML method; 27). To understand the proportion of variance explained by the regression model (27), we calculated R2 as QM/(QM + QE). We performed the metaregression using the metafor package (70) in R (v 3.6.3) (74). We created all of the regression analyses figures using the ggplot2 package (75) in R (v 3.6.3) (74).
Regression analyses of compositional similarity and weedy species.
To understand the relationship between the lnRR of total species richness and compositional similarity of secondary grasslands to old-growth grasslands, we performed fixed-effect linear regression in the R base package stats (v 3.6.3) (74) for the n = 10 studies that reported compositional similarity data. To analyze how lnRR of weedy species richness changes with secondary grassland age, we constructed a linear mixed-effects model to predict weedy species lnRR (with secondary grassland age as the fixed effect and study as the random effect) using the nlme package (76) in R (v 3.6.3) (74) for n = 11 studies (and 29 timepoints). For both of these analyses, we chose not to weight the data points (as would be done in metaregression) given that these studies represent a relatively small subset of the full meta-analysis dataset.
Exploratory models of unexplained variance in lnRR.
Given the high between-study heterogeneity (I2 = 90%, Fig. 2), we explored whether unexplained variance in lnRR was attributable to variables that were not part of our core hypotheses (as in 77). We constructed linear mixed-effects models, with study as a random effect, to predict lnRR based on: continent, latitude, secondary grassland age, MAP, MAT, type of secondary grassland, and sample area (SI Appendix, Table S3).
We generated a starting model using the nlme package (76) in R (v 3.6.3) (74). We then used a step backward selection method, based on Akaike Information Criteria (AIC), to identify the best model (MASS package (78)). The marginal R2 value associated with each model was calculated separately by using the PIECEWISESEM package (79). To visualize the relationships between lnRR and the continuous and categorical predictor variables retained in the top models (ΔAIC < 2 compared to the best model; SI Appendix, Table S3), we presented results by secondary grassland type (SI Appendix, Fig. S8) and conducted a metaregression for latitude (SI Appendix, Fig. S9).
Publication Bias.
To assess publication bias, we first performed nonparametric correlation tests (Spearman’s rho and Kendall’s tau) between the standardized effect sizes and the composite variance as a substitute (27) (SI Appendix, Table S2). A significant positive or negative correlation would indicate publication bias (27). Second, we performed a Cumulative Meta-Analysis (CMA) to assess publication bias (SI Appendix, Fig. S6) with publications sorted by year and using a random-effects model (80). The CMA recalculates the cumulative effect size after adding studies, one by one (80). In the end, if the effect sizes do not converge with the calculated effect size, this would suggest bias (27). Last, to assess publication bias, we calculated the Rosenberg’s fail-safe number—i.e., the number of studies with the same weight as the average of the current set of studies that would be needed to render the results nonsignificant at α = 0.05 (81); results are considered unbiased if the number is high (>5 × n + 10) (65). We chose this metric because it uses a weighted approach, whereas alternative metrics (e.g., Rosenthal’s and Orwin’s) use an unweighted approach (27). To test if variation in sampling area affected lnRR, we conducted a regression between plot size in each study and lnRR (SI Appendix, Fig. S7).
Data Availability Statement.
All data used for the analyses are provided in Dataset S1.
Supplementary Material
Acknowledgments
We thank D. Boecker, R. Fensham, N. Pilon, and C. Rottler for providing data from their publications. We appreciate the help of J. Gurevitch for clarification on functions and outputs of the OpenMEE software. A. M. Lawing provided helpful feedback on earlier versions of the manuscript. A.N.N. was supported by the Graduate Excellence Fellowship and the McMillan-Ward Memorial Graduate Fellowship of Texas A&M University. J.W.V. was supported by US Department of Agriculture, National Institute of Food and Agriculture, Sustainable Agricultural Systems Grant 12726253, USDA-NIFA McIntire-Stennis Project 1016880, and the National Science Foundation under award number DEB-1931232.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1922266117/-/DCSupplemental.
References
- 1.Dinerstein E. et al., An ecoregion-based approach to protecting half the terrestrial realm. Bioscience 67, 534–545 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy B. P., Andersen A. N., Parr C. L., The underestimated biodiversity of tropical grassy biomes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150319 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parr C. L., Lehmann C. E., Bond W. J., Hoffmann W. A., Andersen A. N., Tropical grassy biomes: Misunderstood, neglected, and under threat. Trends Ecol. Evol. 29, 205–213 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Clements F. E., Plant Succession: An Analysis of the Development of Vegetation, (Carnegie Institution, Washington, 1916). [Google Scholar]
- 5.Pausas J. G., Bond W. J., Humboldt and the reinvention of nature. J. Ecol. 107, 1031–1037 (2019). [Google Scholar]
- 6.Strömberg C. A. E., Evolution of grasses and grassland ecosystems. Annu. Rev. Earth Planet. Sci. 39, 517–544 (2011). [Google Scholar]
- 7.McIntyre S., Hobbs R., A framework for conceptualizing human effects on landscapes and its relevance to management and research models. Conserv. Biol. 13, 1282–1292 (1999). [Google Scholar]
- 8.Buisson E. et al., Resilience and restoration of tropical and subtropical grasslands, savannas, and grassy woodlands. Biol. Rev. Camb. Philos. Soc. 94, 590–609 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Staver A. C., Archibald S., Levin S. A., The global extent and determinants of savanna and forest as alternative biome states. Science 334, 230–232 (2011). [DOI] [PubMed] [Google Scholar]
- 10.Fill J. M. et al., Updating models for restoration and management of fiery ecosystems. For. Ecol. Manage. 356, 54–63 (2015). [Google Scholar]
- 11.Veldman J. W., Clarifying the confusion: Old-growth savannahs and tropical ecosystem degradation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371, 20150306 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratnam J. et al., When is a “forest” a savanna, and why does it matter? Glob. Ecol. Biogeogr. 20, 653–660 (2011). [Google Scholar]
- 13.Kumar D. et al., Misinterpretation of Asian savannas as degraded forest can mislead management and conservation policy under climate change. Biol. Conserv. 241, 108293 (2020). [Google Scholar]
- 14.Noss R. F., Forgotten Grasslands of the South: Natural History and Conservation, (Island Press, Washington, 2012). [Google Scholar]
- 15.Searchinger T. D. et al., High carbon and biodiversity costs from converting Africa’s wet savannahs to cropland. Nat. Clim. Change 5, 481–486 (2015). [Google Scholar]
- 16.Veldman J. W. et al., Comment on “The global tree restoration potential”. Science 366, eaay7976 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Abreu R. C. R. et al., The biodiversity cost of carbon sequestration in tropical savanna. Sci. Adv. 3, e1701284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veldman J. W. et al., Toward an old‐growth concept for grasslands, savannas, and woodlands. Front. Ecol. Environ. 13, 154–162 (2015). [Google Scholar]
- 19.Feller J. M., Brown D. E., From old-growth forests to old-growth grasslands: Managing rangelands for structure and function. Ariz. Law Rev. 42, 319–341 (2000). [Google Scholar]
- 20.Holdridge L. R., Life Zone Ecology, (Tropical Science Center, San Jose, Costa Rica, rev. ed., 1967). [Google Scholar]
- 21.Brando P. M. et al., Abrupt increases in Amazonian tree mortality due to drought-fire interactions. Proc. Natl. Acad. Sci. U.S.A. 111, 6347–6352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bond W. J., Parr C. L., Beyond the forest edge: Ecology, diversity and conservation of the grassy biomes. Biol. Conserv. 143, 2395–2404 (2010). [Google Scholar]
- 23.Pausas J. G., Lamont B. B., Paula S., Appezzato-da-Glória B., Fidelis A., Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 217, 1435–1448 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Strassburg B. B. et al., Moment of truth for the Cerrado hotspot. Nat. Ecol. Evol. 1, 99 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Sankaran M., Diversity patterns in savanna grassland communities: Implications for conservation strategies in a biodiversity hotspot. Biodivers. Conserv. 18, 1099–1115 (2009). [Google Scholar]
- 26.Wilson J. B., Peet R. K., Dengler J., Pärtel M., Plant species richness: The world records. J. Veg. Sci. 23, 796–802 (2012). [Google Scholar]
- 27.Koricheva J., Gurevitch J., Mengersen K., Eds., Handbook of Meta-Analysis in Ecology and Evolution, (Princeton University Press, Princeton, 2013). [Google Scholar]
- 28.Stromberg M. R., Griffin J. R., Long-term patterns in coastal California grasslands in relation to cultivation, gophers, and grazing. Ecol. Appl. 6, 1189–1211 (1996). [Google Scholar]
- 29.Isbell F., Tilman D., Reich P. B., Clark A. T., Deficits of biodiversity and productivity linger a century after agricultural abandonment. Nat. Ecol. Evol. 3, 1533–1538 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Senior A. M. et al., Heterogeneity in ecological and evolutionary meta-analyses: Its magnitude and implications. Ecology 97, 3293–3299 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Gibson L. et al., Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Watson J. E. M. et al., The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2, 599–610 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Isbell F. I., Polley H. W., Wilsey B. J., Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecol. Lett. 12, 443–451 (2009). [DOI] [PubMed] [Google Scholar]
- 34.Adler P. B. et al., Productivity is a poor predictor of plant species richness. Science 333, 1750–1753 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Hempson G. P., Archibald S., Bond W. J., The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 7, 17196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratajczak Z., Nippert J. B., Collins S. L., Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 93, 697–703 (2012). [DOI] [PubMed] [Google Scholar]
- 37.Hautier Y. et al., Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 508, 521–525 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Hillebrand H. et al., Biodiversity change is uncoupled from species richness trends: Consequences for conservation and monitoring. J. Appl. Ecol. 55, 169–184 (2018). [Google Scholar]
- 39.Rozendaal D. M. et al., Biodiversity recovery of Neotropical secondary forests. Sci. Adv. 5, eaau3114 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruprecht E., Successfully recovered grassland: A promising example from Romanian old‐fields. Restor. Ecol. 14, 473–480 (2006). [Google Scholar]
- 41.Komatsu K. J. et al., Global change effects on plant communities are magnified by time and the number of global change factors imposed. Proc. Natl. Acad. Sci. U.S.A. 116, 17867–17873 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirkman L. K., Coffey K. L., Mitchell R. J., Moser E. B., Ground cover recovery patterns and life‐history traits: Implications for restoration obstacles and opportunities in a species‐rich savanna. J. Ecol. 92, 409–421 (2004). [Google Scholar]
- 43.Zaloumis N. P., Bond W. J., Grassland restoration after afforestation: No direction home? Austral Ecol. 36, 357–366 (2011). [Google Scholar]
- 44.Brudvig L. A. et al., Interpreting variation to advance predictive restoration science. J. Appl. Ecol. 54, 1018–1027 (2017). [Google Scholar]
- 45.Helsen K., Hermy M., Honnay O., Spatial isolation slows down directional plant functional group assembly in restored semi‐natural grasslands. J. Appl. Ecol. 50, 404–413 (2013). [Google Scholar]
- 46.Damschen E. I. et al., Ongoing accumulation of plant diversity through habitat connectivity in an 18-year experiment. Science 365, 1478–1480 (2019). [DOI] [PubMed] [Google Scholar]
- 47.Bradshaw A., Restoration of mined lands—using natural processes. Ecol. Eng. 8, 255–269 (1997). [Google Scholar]
- 48.Grman E., Suding K. N., Within‐year soil legacies contribute to strong priority effects of exotics on native California grassland communities. Restor. Ecol. 18, 664–670 (2010). [Google Scholar]
- 49.Martin L. M., Moloney K. A., Wilsey B. J., An assessment of grassland restoration success using species diversity components. J. Appl. Ecol. 42, 327–336 (2005). [Google Scholar]
- 50.Fuhlendorf S. D. et al., “The combined influence of grazing, fire, and herbaceous productivity on tree–grass interactions” in Western North American Juniperus Communities, (Springer, New York, NY, 2008), pp. 219–238. [Google Scholar]
- 51.Platt W. J., Pyrogenic fuels produced by savanna trees can engineer humid savannas. Ecol. Monogr. 86, 352–372 (2016). [Google Scholar]
- 52.Archibald S., Hempson G. P., Lehmann C., A unified framework for plant life-history strategies shaped by fire and herbivory. New Phytol. 224, 1490–1503 (2019). [DOI] [PubMed] [Google Scholar]
- 53.Hempson G. P., Archibald S., Donaldson J. E., Lehmann C. E. R., Alternate grassy ecosystem states are determined by palatability–flammability trade-offs. Trends Ecol. Evol. 34, 286–290 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Pullin A. S., Stewart G. B., Guidelines for systematic review in conservation and environmental management. Conserv. Biol. 20, 1647–1656 (2006). [DOI] [PubMed] [Google Scholar]
- 55.Moher D., Liberati A., Tetzlaff J., Altman D. G.; PRISMA Group , Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151, 264–269, W64 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Abràmoff M. D., Magalhães P. J., Ram S. J., Image processing with ImageJ. Biophoton. Int. 11, 36–42 (2004). [Google Scholar]
- 57.Weir C. J. et al., Dealing with missing standard deviation and mean values in meta-analysis of continuous outcomes: A systematic review. BMC Med. Res. Methodol. 18, 25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Magurran A. E., Measuring Biological Diversity, (Blackwell, Oxford, 2004). [Google Scholar]
- 59.QGIS Development Team , QGIS Geographic Information System-version 2.10.1. Open Source Geospatial Foundation Project. https://qgis.org/en/site/. Accessed 1 June 2019 (2010).
- 60.Fick S. E., Hijmans R. J., WorldClim 2: New 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 61.Öster M., Ask K., Cousins S. A., Eriksson O., Dispersal and establishment limitation reduces the potential for successful restoration of semi‐natural grassland communities on former arable fields. J. Appl. Ecol. 46, 1266–1274 (2009). [Google Scholar]
- 62.Brudvig L. A. et al., Land-use history and contemporary management inform an ecological reference model for longleaf pine woodland understory plant communities. PLoS One 9, e86604 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hedges L. V., Gurevitch J., Curtis P. S., The meta‐analysis of response ratios in experimental ecology. Ecology 80, 1150–1156 (1999). [Google Scholar]
- 64.Koricheva J., Gurevitch J., Uses and misuses of meta‐analysis in plant ecology. J. Ecol. 102, 828–844 (2014). [Google Scholar]
- 65.Borenstein M., Hedges L. V., Higgins J. P. T., Rothstein H. R., Introduction to Meta-Analysis, (John Wiley & Sons Ltd, New York, 2009). [Google Scholar]
- 66.Hedges L. V., Olkin I., Statistical Methods for Meta-Analysis, (Academic Press, San Diego, 1985). [Google Scholar]
- 67.Spake R., Doncaster C. P., Use of meta-analysis in forest biodiversity research: Key challenges and considerations. For. Ecol. Manage. 400, 429–437 (2017). [Google Scholar]
- 68.Davies G. M., Gray A., Don’t let spurious accusations of pseudoreplication limit our ability to learn from natural experiments (and other messy kinds of ecological monitoring). Ecol. Evol. 5, 5295–5304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wallace B. C. et al., OpenMEE: Intuitive, open-source software for meta-analysis in ecology and evolutionary biology. Methods Ecol. Evol. 8, 941–947 (2017). [Google Scholar]
- 70.Viechtbauer W., Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 36, 1–48 (2010). [Google Scholar]
- 71.Mayerhofer M. S., Kernaghan G., Harper K. A., The effects of fungal root endophytes on plant growth: A meta-analysis. Mycorrhiza 23, 119–128 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Doncaster C. P., Spake R., Correction for bias in meta-analysis of little-replicated studies. Methods Ecol. Evol. 9, 634–644 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia L. C., Eubanks M. D., Overcompensation for insect herbivory: A review and meta-analysis of the evidence. Ecology 100, e02585 (2019). [DOI] [PubMed] [Google Scholar]
- 74.R Development Core Team , R: A Language and Environment for Statistical Computing. Version 3.6.3, (R Foundation for Statistical Computing, Vienna, Austria, 2020). [Google Scholar]
- 75.Wickham H., ggplot2: Elegant Graphics for Data Analysis, (Springer-Verlag, New York, 2016). [Google Scholar]
- 76.Pinheiro J., Bates D., DebRoy S., Sarkar D., R Core Team; nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-140, https://cran.r-project.org/web/packages/nlme/index.html (2019).
- 77.Brustolin M. C., Nagelkerken I., Fonseca G., Large-scale distribution patterns of mangrove nematodes: A global meta-analysis. Ecol. Evol. 8, 4734–4742 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Venables W. N., Ripley B. D., Modern Applied Statistics with S, (Springer, New York, ed. 4, 2002). [Google Scholar]
- 79.Lefcheck J. S., piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579 (2016). [Google Scholar]
- 80.Leimu R., Koricheva J., Cumulative meta-analysis: A new tool for detection of temporal trends and publication bias in ecology. Proc. Biol. Sci. 271, 1961–1966 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg M. S., The file-drawer problem revisited: A general weighted method for calculating fail-safe numbers in meta-analysis. Evolution 59, 464–468 (2005). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used for the analyses are provided in Dataset S1.