Keywords: COVID-19, gastrointestinal symptoms, SARS-CoV-2
Abstract
In addition to the typical respiratory response, new coronavirus disease 2019 (COVID-19) is also associated with very common gastrointestinal symptoms. Cases with gastrointestinal symptoms are more likely to be complicated by liver injury and acute respiratory distress syndrome (ARDS). If not treated in time, coma and circulatory failure may ensue. As severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infects the human body through the combination of angiotensin-converting enzyme 2 (ACE2) in the gastrointestinal tract, the mechanism underlying the gastrointestinal symptoms may involve damage to the intestinal mucosal barrier and promotion of the production of inflammatory factors. Indeed, after cells in the lungs become infected by SARS-CoV-2, effector CD4+ T cells reach the small intestine through the gut-lung axis, causing intestinal immune damage and diarrhea; early extensive use of antibacterial and antiviral drugs can also lead to diarrhea in patients. Thus, treatment options for COVID-19 patients should be promptly adjusted when they have gastrointestinal symptoms. As SARS-CoV-2 has been detected in the feces of COVID-19 patients, future prevention and control efforts must consider the possibility of fecal-oral transmission of the virus.
BACKGROUND
At the end of December 2019, a highly contagious viral pneumonia occurred in Wuhan, Hubei, China. Researchers then isolated and identified a new coronavirus by collecting bronchial lavage fluid from patients. The World Health Organization (WHO) named it the 2019 new coronavirus (2019-novel coronavirus, 2019-nCoV), and the disease was called COVID-19.
To date, 2019-nCoV is the seventh coronavirus known to infect humans. Among them, 229E, NL63, OC43, and HKU1 only cause symptoms of the common cold and upper respiratory tract infection (56). Conversely, SARS-CoV and middle east respiratory syndrome coronavirus (MERS-CoV) can cause large-scale atypical pneumonia (12, 73, 74), leading to serious lower respiratory tract infections accompanied by acute respiratory distress syndrome (ARDS) and extrapulmonary manifestations (48, 68). According to viral sequencing analysis, 2019-nCoV belongs to the genus β-coronavirus, and its genome sequence shows up to 82% similarity to SARS-CoV (4). On February 13, 2020, the International Virus Taxonomy Committee officially renamed “2019-nCoV” as “SARS-CoV-2.” SARS-CoV-2 is extremely contagious. It is currently believed that the virus is mainly spread through respiratory droplets and close contact (72, 76). Moreover, it has been proposed that SARS-CoV-2 likely can be transmitted through the fecal-oral route (67). This article reviews the incidence of gastrointestinal symptoms caused by SARS-CoV-2 infection, related mechanisms, treatment, and protection, providing a reference for clinical prevention and treatment.
EPIDEMIOLOGICAL EVIDENCE OF GASTROINTESTINAL INVOLVEMENT AND CLINICAL SYMPTOMS
Both SARS-CoV and MERS-CoV can cause respiratory and gastrointestinal symptoms, with an incidence of gastrointestinal symptoms higher than 20% (25). In general, all people are susceptible to COVID-19, but research shows that the median age of patients is ~47 yr (25). The most common underlying diseases among patients are hypertension (14.9–35.2%), diabetes (7.4–16.7%), and cardiovascular disease (2.5–14.5%) (25, 53, 59, 72). Analysis of the clinical characteristics of COVID-19 patients found that the main symptoms were fever (72.0–98.6%), cough (39.3–83.0%), and fatigue (38.1–69.6%) accompanied by gastrointestinal symptoms (25, 53, 59, 72). Dan Fang et al. (14) reported that among the 305 COVID-19 patients in Wuhan, the incidence of gastrointestinal symptoms was as high as 79.1%, and the incidence of diarrhea was 49.5%. Among them, the positive rate of fecal microscopic leukocytes was 5.2% and no red blood cells were found, which was consistent with viral diarrhea characteristics. The incidence of nausea and vomiting were 29.4% and 15.9%, respectively, though the incidence of abdominal pain was relatively low at 6.0%. Consistent with the results of the above studies diarrhea was also the main gastrointestinal symptom in two other studies on Wuhan COVID-19 patients (53, 59), with an incidence of 10.1% and 22.4%; however, the incidence of abdominal pain was lower at 2.2% and 3.7%. Among 106 COVID-19 patients in Henan (72), the incidence of diarrhea was 6.6%, whereas that of nausea and vomiting was only 0.9%, with no symptoms of abdominal pain. In addition, the team of academician Zhong Nanshan reported 1,099 cases of COVID-19 patients all over China, with an incidence of diarrhea of 3.7% and nausea and vomiting of 5%. Moreover, using the stool of 62 patients, 4 cases were positive for viral nucleic acids. In another study, SARS-CoV-2 nucleic acid was detected in the stool of up to 53.4% of patients (22). The results of these studies indicate that the gastrointestinal tract is a site where SARS-CoV-2 invades and is released. Diarrhea is the most common gastrointestinal symptom, and the incidence of gastrointestinal symptoms in patients with COVID-19 in Wuhan was significantly higher than the national level. This may be because Wuhan is the origin of the SARS-CoV-2 outbreak and because priority was given to the treatment of critically ill patients due to a shortage of medical resources (35).
As studies have shown that SARS-CoV-2 can be transmitted through feces (31), the clinical characteristics and prognosis analysis of COVID-19 patients with gastrointestinal symptoms are of great significance to prevent the spread of disease, and statistics on the epidemiology and clinical characteristics of COVID-19 patients with gastrointestinal symptoms have been reported. When compared with patients without gastrointestinal symptoms, those with gastrointestinal symptoms are more prone to fatigue (35, 72), cough (35), and headache (35). Routine blood tests show increased levels of neutrophils in patients with gastrointestinal symptoms, and the inflammation index C-reactive protein is also significantly increased, indicating that inflammation occurs (35, 72). Based on biochemical analyses, alanine aminotransferase, aspartate aminotransferase, and bilirubin, which are related to liver function, are higher in those with gastrointestinal symptoms (35, 72). Liver damage among COVID-19 patients with gastrointestinal symptoms is as high as 17.57% compared with the 8.84% among those without gastrointestinal symptoms. The incidence of ARDS in patients with COVID-19 with gastrointestinal symptoms was 6.76%, significantly higher than that in patients without gastrointestinal symptoms (2.08%) (35, 72). The proportion of severe cases (25%) among patients with gastrointestinal symptoms is higher than the overall proportion of severe cases (10.4%) (72). Furthermore, in patients with gastrointestinal symptoms, diarrhea may lead to a decrease in serum sodium levels and electrolyte disturbances (35), causing nausea, vomiting, fatigue, and headache if not treated in time and even leading to coma and circulatory failure. As the severity of the disease increases, also digestive system symptoms become more obvious. This may be caused by the replication of the virus in the gastrointestinal tract, or it may be related to delayed treatment because the patient initially did not have typical respiratory symptoms. Studies have found that patients in the intensive care unit have a higher frequency of abdominal pain than do ordinary patients, and 10% only experience diarrhea and nausea as the first symptoms (59). Patients with gastrointestinal symptoms have a longer detoxification time, and the possibility of a positive test for SARS-CoV-2 virus in feces is greater (27). These data indicate that the clinical symptoms of COVID-19 patients with gastrointestinal symptoms are more significant, that inflammation is more serious, that complications are more common, and that they are more likely to develop severe disease. Therefore, during the diagnosis and treatment of COVID-19, close attention should be paid to the gastrointestinal symptoms of patients.
Studies have shown that similar to SARS-CoV, SARS-CoV-2 uses ACE2 as a receptor to enter cells; as ACE2 is expressed in the gastrointestinal tract, it may be a target organ for SARS-CoV-2 (65). Therefore, clinicians should pay attention to gastrointestinal symptoms and other atypical symptoms of COVID-19 patients to prevent and cure their diseases.
MECHANISMS THAT CAUSE GASTROINTESTINAL DAMAGE
Direct Infection of Gastrointestinal Cells
Virus entry into cells is an important part of cross-species transmission. All coronaviruses encode a surface glycoprotein and spike protein, which binds to host cell receptors and mediates virus entry (43). It is well known that the receptors of β-CoVs mainly include ACE2, for SARS-CoV (38), and dipeptidyl peptidase 4 (DPP4), for MERS-CoV (49). Some studies have found that the spike (S) protein of SARS-CoV-2 has a high affinity for human angiotensin-converting enzyme 2 (ACE2) (58), and SARS-CoV-2 mainly enters cells through the ACE2 receptor (74). Three-dimensional reconstruction and computer simulation experiments found that the structure of the receptor binding domain and external region of SARS-CoV-2 is very similar to that of SARS-CoV (64). Michael, et al. (45) infected young hamster kidney cells (BHK) specifically expressing human ACE2 with SARS-CoV and SARS-CoV-2 and observed that SARS-CoV-2 can only enter cells expressing ACE2, which fully indicates that SARS-CoV-2 enters cells through ACE2. Therefore, the expression and distribution of ACE2 in humans is a potential infection pathway for SARS-CoV-2.
ACE2 is widely found in human small intestinal epithelial cells. ACE2 is more strongly expressed in type II epithelial cells (26, 77). Other studies provided additional evidence that coronavirus may infect the gastrointestinal tract, since high expression of ACE2 has been detected in intestinal epithelial cells, esophagus, and lungs (23). Many studies (25, 53, 59, 72) have proven that COVID-19 patients have a certain proportion of gastrointestinal symptoms, indicating that SARS-CoV-2 may invade target organs of the digestive tract through ACE2 receptors and cause primary damage. Compared with SARS-CoV, SARS-CoV-2 is more transmissible. Through surface plasmonic resonance, Daniel, et al. (63) found that the binding affinity of ACE2 to the outer domain of SARS-CoV-2 is ~10–20 times higher than that of SARS-CoV. It has been reported that the accumulation of angiotensin II (ANG II) level in COVID-19 patients is significantly increased and that the degree of elevation is related to the severity of the disease. This may be because SARS-CoV-2 can bind to the receptor ACE2, resulting in its downregulation. However, the correlation between SARS-CoV-2 virus and ANG II needs further study (44).
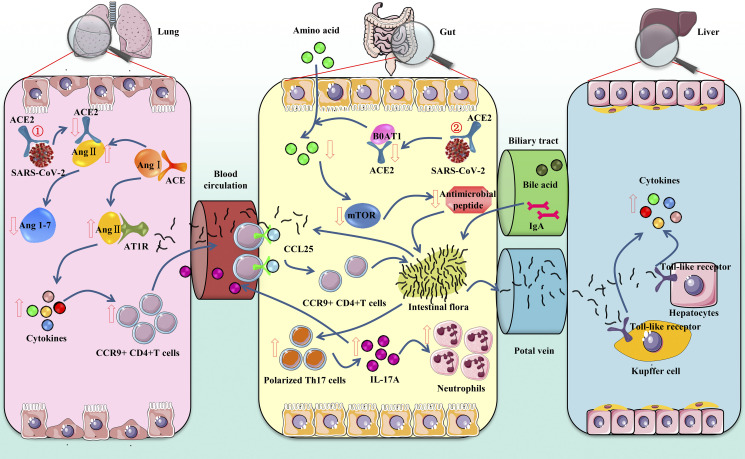
ACE2 is a key enzyme in the renin-angiotensin system (RAS) (10, 37) and plays an important role in regulating intestinal inflammation and diarrhea (28). Loss of ACE2 leads to the ANG II accumulation (34). Although ACE2 knockdown results in elevated levels of ANG II in the mouse colon, ACE2 does not perform its function primarily through the RAS system in the intestine but rather directly regulates the homeostasis of intestinal amino acids, expression of antimicrobial peptides, and ecology of the gut microbiome (28). For instance, mice with ACE2 knockdown have significantly reduced serum tryptophan levels. Tryptophan is essential for niacin synthesis in the body (15), and insufficient intake of niacin or tryptophan is the main cause of pellagra (55). Tryptophan is mainly absorbed through the B0AT1/ACE2 transport pathway on the lumen surface of intestinal epithelial cells; mammalian target of rapamycin (mTOR) becomes activated, which in turn regulates the expression of antimicrobial peptides, thereby affecting the composition of the intestinal flora (28). Accordingly, more than 90% of patients with pellagra will eventually develop colitis (52). Therefore, we speculate that binding of SARS-CoV-2 with ACE2 in the gastrointestinal tract reduces the level available receptors, affects the absorption of tryptophan, and ultimately destroys the steady state of the intestinal flora, which is one of the causes of gastrointestinal symptoms such as diarrhea.
He et al. (29) performed autopsy on patients who died of SARS-CoV. Pathological results showed that in ACE2-expressing cells, proinflammatory cytokines (PICs), including monocyte chemokine-1 (MCP-1), tumor growth factor-β1 (TGF-β1), tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-6, were highly expressed but that no PIC expression occurred in cells that do not express ACE2. A large number of PICs causes a cytokine storm, which further leads to multiple organ damage. Huang et al. (33) found that the plasma cytokine and chemokine levels of COVID-19 patients were higher than those of healthy people; the plasma IL-2, IL-7, IL-10, granulocyte colony stimulating factor (G-SCF), and recombinant human interferon-induced protein-10 (IP-10), MCP-1, macrophage inflammatory protein-1A (MIP-1A), and TNF-α levels of patients with severe disease were all higher than those of patients without severe disease. In the early stage of SARS-COV infection, dendritic cells and macrophages show delayed release of cytokines and chemokines, followed by low levels of antiviral interferon and high levels of pro-inflammatory cytokines and chemokines (8, 39, 40). After that, rapidly elevated cytokines and chemokines attract a large number of inflammatory cells such as neutrophils and monocytes, causing tissue damage. Therefore, we speculate that SARS-CoV-2 might be similar to SARS-CoV and can also cause cells expressing ACE2 to secrete a large number of cytokines, which eventually involved in causing a cytokine storm and damage to multiple organs throughout the body. Direct tissue damage caused by viral infection of target tissues such as kidney, intestine, and brain has been reported (24). In addition, cytokine storms may directly cause immune cell death. Lymphocytosis is a common feature in patients with severe COVID-19 infection, with significant reductions in the number of CD4+ T cells, CD8+ T cells, B cells, and natural killer cells, resulting in spleen atrophy and necrosis, lymph node necrosis, renal hemorrhage, hepatomegity, and degeneration of central nervous system neurons (3).
Gastrointestinal Damage Caused by Lung Infection
Changes in the composition and function of the gastrointestinal flora affect the respiratory tract through the common mucosal immune system. Disorders of the respiratory tract flora also impact the digestive tract through immune regulation. This effect is called the “gut-lung axis” (1, 17). One study found that SARS-CoV-2 cannot always be detected in the stool of COVID-19 patients with gastrointestinal symptoms, so we speculate that the gastrointestinal symptoms of some patients may not be caused by direct damage due to the viral infection. Effector CD4+ T cells entering the intestinal mucosa are key for mucosal immunity and chronic enteritis. C-C chemokine receptor type 9 (CCR9) is a necessary chemokine receptor for CD4+ T cells to enter the small intestine (60). Jian Wang et al. (60) found that lung-derived CCR9+CD4+ T cells were increased after viral infection. The small intestinal epithelium can express CCL25 (47), which promotes the recruitment of CCR9+CD4+T cells into the small intestine (54), leading to intestinal immune damage and destroying the homeostasis of the intestinal flora. Disturbance of the intestinal flora will promote the polarization of Th17 cells in the small intestine, and the production of too much IL-17A will lead to the recruitment of neutrophils (11), causing intestinal immune damage, diarrhea, and other gastrointestinal symptoms. When inflammation occurs in the intestine, cytokines and bacteria also can enter the lung through the bloodstream, further affecting the lung immune response and inflammation (70). Intestinal mucosal damage and bacterial imbalance can also influence the gut-liver axis (which refers to the bidirectional relationship of the portal vein between the intestine, microorganisms and liver through the portal vein). In the intestine, host and microbial metabolites are transferred to the liver through the portal vein and affect liver function. The liver releases bile acids and bioactive media into the biliary and systemic circulation and transports them to the intestine (57). This leads to liver function damage in patients, which may also explain the abnormality of liver function indicators in COVID-19 patients in some studies (32).
Gastrointestinal Symptoms Caused by Drug Side Effects
Antibiotic-associated diarrhea is the most common adverse reaction to antibacterial drugs, especially macrolides, cephalosporins, and β-lactam antibiotics. A retrospective analysis from Guangzhou, China, of the treatment process for 260 SARS-CoV patients found that macrolides, fluoroquinolones, or cephalosporin antibiotics were used and that the proportion of patients with diarrhea was 24.2% (71). Another study that included 138 SARS-CoV patients found that 38% of patients had diarrhea during treatment, with a median duration of 3.7 days (41). The above studies suggested that some diarrhea symptoms may be related to the early use of large amounts of antibacterial drugs. Antiviral drugs are also widely used in the treatment of patients with COVID-19. Some serious intractable diarrhea may be related to the use of oseltamivir and arbidol; the incidence of diarrhea in patients using these drugs is ~55.2% (18). Other antiviral drugs that can cause diarrhea include chloroquine phosphate, lopinavir, and remdesivir, as well as Chinese patent medicines such as lianhuaqingwen capsules (8a). Additionally, exposure to broad-spectrum antibiotics is the main risk factor for primary Clostridioides difficile infection (CDI), the leading cause of nosocomial diarrhea (36). The stated research above suggests that drug-induced diarrhea caused by the large-scale use of antibacterial drugs and antiviral drugs is also one of the causes of gastrointestinal symptoms in some patients (Fig. 1).
Fig. 1.
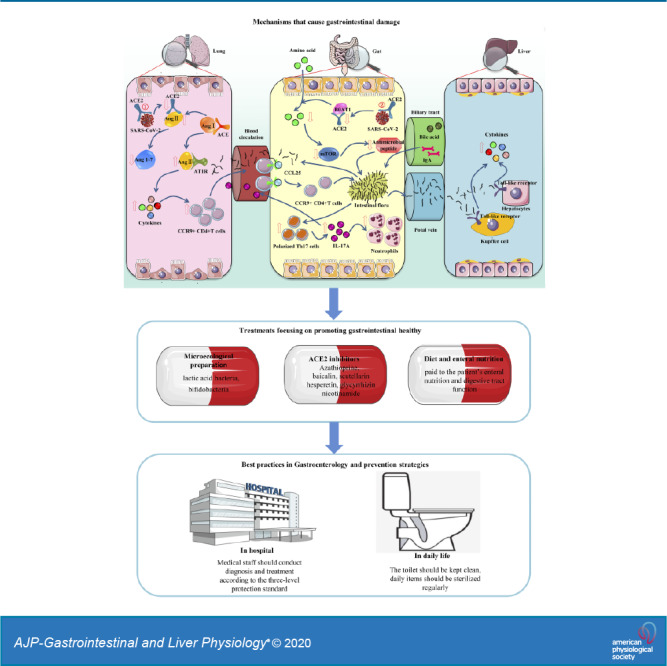
Mechanism of gastrointestinal symptoms in patients with coronavirus disease 2019 (COVID-19). ①Gut-lung axis: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds with angiotensin-converting enzyme 2 (ACE2) to enter the lung, resulting in the accumulation of angiotensin II (ANG II) and the decrease of angiotensin (1-7) (Ang1–7). ANG II combined with AT1R promotes cytokine release and increases CCR9+CD4+T cells. CCL25 promotes the recruitment of C-C chemokine receptor type 9 (CCR9)+CD4+T cells into the small intestine. The changed flora then promotes the polarization of Th17 cells, and finally IL-17A causes the recruitment of neutrophils. Cytokines and bacteria also enter the lung through the bloodstream, further affecting the lung inflammation. ②Gut-liver axis: SARS-CoV-2 binds with ACE2 to enter the intestine, inhibits the absorption of the B0AT1/ACE2 transport pathway, and then affects the activation of mammalian target of rapamycin (mTOR) to reduce the expression of antimicrobial peptides. The intestinal flora is transferred to the liver through the portal vein, where it binds to toll-like receptors, causing hepatitis. The liver also can transport metabolites to the intestine through the biliary tract.
TREATMENTS FOCUSING ON PROMOTING GASTROINTESTINAL HEALTHY
There is currently no effective treatment for COVID-19 patients. High viral titers and subsequent intense inflammatory cytokine and chemokine responses are associated with high morbidity and mortality during pathogenic HCoV infection (46). Therefore, antiviral and immunomodulatory drugs might be the main routine treatment regimens. Antiviral drugs mainly include lopinavir-ritonavir, chloroquine and hydroxychloroquine, remdesivir, etc. However, studies found that lopinavir-ritonavir treatment has no significant effect on clinical improvement, mortality reduction, or laryngeal viral RNA detection in patients with severe COVID-19 (2). Notably, the window between the treatment dose and toxic dose of chloroquine is narrow and has been linked to cardiovascular symptoms, which can be life-threatening (16). Remdesivir is an experimental drug being developed to treat Ebola virus-infected patients (61). The most promising treatment is remdesivir, which has a strong activity against SARS-COV-2 in vitro but has not been approved by the United States Food and Drug Administration and is currently in randomized trials (51). High inflammatory status can lead to deterioration in COVID-19 patients, so it is also critical to take immunomodulatory therapies-including glucocorticoids, convalescent plasma, and anticytokine therapy (19). For COVID-19 patients with gastrointestinal symptoms, it is important to identify the cause of the disease, then improve diarrhea symptoms and maintain intestinal microflora homeostasis (13).
Microecological Preparation
The intestinal flora produces various vitamins, fatty acids, bile acids, and immune factors through the fermentation and decomposition of food and participate in immune function regulation (42). If the intestinal flora is dysfunctional and the intestinal mucosa is damaged, a virus may further induce infection through this pathway. Studies have confirmed that probiotics can treat diarrhea caused by rotavirus (62). Taking lactic acid bacteria and bifidobacteria can also promote the body to produce antiviral antibodies, thereby hastening the removal of viruses. In addition, probiotic treatment may improve the symptoms of diarrhea caused directly by SARS-CoV-2 or by the use of antiviral drugs/antibacterial drugs. In China's COVID-19 diagnosis and treatment protocols, the use of microecological regulators is recommended to maintain the intestinal microecological balance and prevent secondary bacterial infections (45a).
ACE2 Inhibitors
SARS-CoV-2 enters the cell by binding to ACE2. Therefore, preventing interaction between the SARS-CoV-2 receptor-binding domain (RBD) and ACE2 may be an effective strategy to prevent viral infections. Studies have found that ACE2 inhibitors can regulate intestinal amino acid metabolism, antibacterial peptide secretion, intestinal microbial homeostasis, and innate immunity (42). The ACE2 transport pathway on the surface of the lumen of small intestinal epithelial cells activate mTOR through nutrient induction and/or the tryptophan-nicotinamide pathway, thereby affecting the composition of intestinal flora, reducing gastrointestinal symptoms in mice (28). Similar to chloroquine, azathioprine is an immunosuppressive agent; recent studies have screened azathioprine through database analysis, and it may be an ACE2 inhibitor (7). In addition, in vitro experiments proved that azathioprine can inhibit vaccinia virus (14). In terms of molecular immunity, it can competitively bind to ACE2 through the soluble viral receptor-binding domain (RBD) using a single-chain antibody fragment (scFv) that binds to the ACE2 protein or SARS-CoV-2. The human recombinant ACE2 antibody Fc segment directly bound by the S protein inhibits binding of the virus to ACE2 (74). Chen et al. (5) also selected medicines that may be combined with ACE2 from the Chinese medicine library, including baicalin in the Chinese medicine Scutellaria baicalensis, scutellarin in Erigeron breviscapus, hesperetin in lime and orange peel, glycyrrhizin in liquorice and nicotinamide in soybean.
Diet and Enteral Nutrition
Many COVID-19 patients have a marked decrease in appetite, and those with severe disease are taking many types of drugs. On the basis of ensuring effective treatment, attention should be paid to the patient's enteral nutrition and digestive tract function (8a). In addition to providing the necessary energy, enteral nutrition can help restore the digestion, absorption, and physiological peristalsis of the intestine and maintain the normal function of the gastrointestinal tract microecology and mucosal immunity. For severe COVID-19 patients with gastrointestinal symptoms, nutritional risk assessment can be conducted (30). If the patient has gastrointestinal lesions and cannot tolerate enteral nutrition, parenteral nutrition may be appropriately supplemented to maintain a normal energy supply. Once the risk of affecting enteral nutrition is removed, enteral nutrition should be restored as soon as possible, and oral consumption should be encouraged. Patients in poor condition can take digestive enzymes.
For, those who are unable to consume food orally (such as receiving mechanical ventilation), a nasogastric tube can be placed for enteral nutrition. If the patient has a high risk of reflux aspiration or cannot tolerate nasogastric tube feeding, a nasal jejunal tube can be placed. In general, the patient's energy needs and gastrointestinal tolerance should be evaluated in a timely manner and the enteral nutrition program adjusted accordingly.
BEST PRACTICES IN GASTROENTEROLOGY AND PREVENTION STRATEGIES
SARS-CoV-2 transmission from person to person occurs mainly through direct contact or through droplets from an infected person coughing and/or sneezing (50). However, SARS-CoV-2 nucleic acid has been detected in the stool of COVID-19 patients in multiple studies (25, 69). Further research also found that some patients remain positive for fecal virus nucleic acids for a long time after viral nucleic acid testing using throat swabs becomes negative (66). Moreover, some patients will only be positive for SARS-CoV-2 virus nucleic acid in stool (6). SARS-CoV-2 in feces may be spread mainly through contact with hands or other parts after excretion. In addition, feces may dry to form small particles suspended in the air, which may be inhaled by susceptible persons. Thus, attention should be paid to preventing contact or airborne transmission of the digestive tract secretions of COVID-19 patients (8a). When handling the feces of COVID-19 patients, strict precautions must be followed, emphasizing the importance of hand hygiene, and hospital sewage should also be properly disinfected (21).
Digestive tract secretion contact is one of the main transmission routes. Digestive endoscopy can easily stimulate a patient's mouth and pharynx, with secretion and discharge mucus, causing choking, vomiting, or diarrhea. Therefore, there is a great risk of infection during digestive endoscopy, biopsy, and treatment (75). When performing necessary digestive endoscopy, medical staff should conduct diagnosis and treatment in a special endoscopic operation room or negative pressure operating room and in accordance with the three-level protection standard. After diagnosis and treatment, the inspection equipment and operation room should be disinfected according to the standard (8a).
In daily life, the toilet lid should be covered and flushed after use, and the toilet paper should not be placed in the trash. The toilet should be kept clean, paying attention to sewers to check for leakage. Overall, people should reduce contact with others, ventilation in living and working places should be strengthened, and daily items should be sterilized regularly.
CONCLUSION
The main symptoms of COVID-19 are respiratory system responses, and gastrointestinal symptoms are also very common. COVID-19 cases with gastrointestinal symptoms are more likely to be complicated by acute respiratory distress (ARDS) and liver damage, and the prognosis is poor. In the process of diagnosis and treatment, attention should be paid to the gastrointestinal symptoms of the patient, and virus transmission caused by the fecal-oral route should be prevented.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.Y. edited and revised manuscript; B.W. drafted manuscript; J.X. and S.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Qiang Shu for assistance in editing, formatting, and in submitting the manuscript for publication.
REFERENCES
- 1.Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15: 55–63, 2017. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 2.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N Engl J Med 382: 1787–1799, 2020. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20: 269–270, 2020. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 9: 221–236, 2020. [Erratum in Emerg Microbes Infect 9: 540, 2020] doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H-S, Du Q-H. Potential Natural Compounds for Preventing SARS-CoV-2 (2019-nCoV) Infection. www.preprints.org, 30 January 2020.
- 6.Chen L, Lou J, Bai Y, Wang M. COVID-19 disease with positive fecal and negative pharyngeal and sputum viral tests. Am J Gastroenterol 115: 790, 2020. doi: 10.14309/ajg.0000000000000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun 525: 7819–7826, 2020. doi: 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, Chan KH, Yuen KY, Gordon S, Guan Y, Peiris JS. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol 79: 7819–7826, 2005. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Chinese Society of Gastroenterology . Expert consensus on diagnosis and treatment of covid-19 digestive system. National Medical J China 100, 2020. doi: 10.3760/cma.j.cn112137-20200308-00645. [DOI] [Google Scholar]
- 10.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveira-dos-Santos AJ, da Costa J, Zhang L, Pei Y, Scholey J, Ferrario CM, Manoukian AS, Chappell MC, Backx PH, Yagil Y, Penninger JM. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417: 822–828, 2002. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 11.Crowe CR, Chen K, Pociask DA, Alcorn JF, Krivich C, Enelow RI, Ross TM, Witztum JL, Kolls JK. Critical role of IL-17RA in immunopathology of influenza infection. J Immunol 183: 5301–5310, 2009. doi: 10.4049/jimmunol.0900995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol 17: 181–192, 2019. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention, and management. Clin Gastroenterol Hepatol 18: 1663–1672, 2020. doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damaso CR, Oliveira MF, Massarani SM, Moussatché N. Azathioprine inhibits vaccinia virus replication in both BSC-40 and RAG cell lines acting on different stages of virus cycle. Virology 300: 79–91, 2002. doi: 10.1006/viro.2002.1534. [DOI] [PubMed] [Google Scholar]
- 15.Darby WJ, McNutt KW, Todhunter EN. Niacin. Nutr Rev 33: 289–297, 1975. doi: 10.1111/j.1753-4887.1975.tb05075.x. [DOI] [PubMed] [Google Scholar]
- 16.Delang L, Neyts J. Medical treatment options for COVID-19. Eur Heart J Acute Cardiovasc Care 9: 209–214, 2020. doi: 10.1177/2048872620922790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Domínguez-Díaz C, García-Orozco A, Riera-Leal A, Padilla-Arellano JR, Fafutis-Morris M. Microbiota and its role on viral evasion: is it with us or against us? Front Cell Infect Microbiol 9: 256, 2019. doi: 10.3389/fcimb.2019.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang D, Ma J-D, Guan J-L, Wang M-R, Song Y, Tian D-A, Li P-Y. A single-center, descriptive study on the digestive system of COVID-19 inpatients in wuhan. Chinese J Digest 40: E005–E005, 2020. [Google Scholar]
- 19.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med NEJMcp2009249, 2020. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 21.Geller C, Varbanov M, Duval RE. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses 4: 3044–3068, 2012. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonato G, Dioscoridi L, Mutignani M. Faecal-oral transmission of SARS-COV-2: practical implications. Gastroenterology In press, 2020. doi: 10.1053/j.gastro.2020.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 158: 1518–1519, 2020. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol 170: 1136–1147, 2007. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 . Clinical Characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han C-Q, Duan C-H, Zhang S-Y, Spiegel B, Shi H-Y, Wang W-J, Zhang L, Lin R, Liu J, Ding Z, Hou X-H. Digestive symptoms in COVID-19 patients with mild disease severity: clinical presentation, stool viral RNA Testing, and outcomes. Am J Gastroenterol 115: 916–923, 2020. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487: 477–481, 2012. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, Wang H, Li Z, Zhao L, Geng J, Deng Y, Yang L, Li J, Cai J, Qiu L, Wen K, Xu X, Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 210: 288–297, 2006. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hersberger L, Bargetzi L, Bargetzi A, Tribolet P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Kägi-Braun N, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brändle M, Benz C, Henzen C, Nigg M, Thomann R, Brand C, Rutishauser J, Aujesky D, Rodondi N, Donzé J, Stanga Z, Mueller B, Schuetz P. Nutritional risk screening (NRS 2002) is a strong and modifiable predictor risk score for short-term and long-term clinical outcomes: secondary analysis of a prospective randomised trial. Clin Nutr 14: S0261-5614(19)33171-1, 2019. doi: 10.1016/j.clnu.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 31.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK; Washington State 2019-nCoV Case Investigation Team . First case of 2019 novel coronavirus in the United States. N Engl J Med 382: 929–936, 2020. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y, Lu G-Z, Xu J. Discussion on symptoms of digestive tract caused by novel coronavirus infection. Chinese J Infect Dis 38: 2020. doi: 10.3760/cma.j.cn311365-20200220-00094. [DOI] [Google Scholar]
- 33.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 436: 112–116, 2005. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin X, Lian JS, Hu JH, Gao J, Zheng L, Zhang YM, Hao SR, Jia HY, Cai H, Zhang XL, Yu GD, Xu KJ, Wang XY, Gu JQ, Zhang SY, Ye CY, Jin CL, Lu YF, Yu X, Yu XP, Huang JR, Xu KL, Ni Q, Yu CB, Zhu B, Li YT, Liu J, Zhao H, Zhang X, Yu L, Guo YZ, Su JW, Tao JJ, Lang GJ, Wu XX, Wu WR, Qv TT, Xiang DR, Yi P, Shi D, Chen Y, Ren Y, Qiu YQ, Li LJ, Sheng J, Yang Y. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut 69: 1002–1009, 2020. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kociolek LK, Gerding DN. Breakthroughs in the treatment and prevention of Clostridium difficile infection. Nat Rev Gastroenterol Hepatol 13: 150–160, 2016. doi: 10.1038/nrgastro.2015.220. [DOI] [PubMed] [Google Scholar]
- 37.Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 11: 875–879, 2005. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn JH, Li W, Choe H, Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell Mol Life Sci 61: 2738–2743, 2004. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau SKP, Lau CCY, Chan KH, Li CPY, Chen H, Jin DY, Chan JFW, Woo PCY, Yuen KY. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: implications for pathogenesis and treatment. J Gen Virol 94: 2679–2690, 2013. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 40.Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 106: 2366–2374, 2005. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leung WK, To KF, Chan PK, Chan HL, Wu AK, Lee N, Yuen KY, Sung JJ. Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125: 1011–1017, 2003. doi: 10.1016/j.gastro.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li C, Liu P, Guo S-S, Zhao Z-G. Study on the mechanism and treatment of COVID-19, SARS and MERS with gastrointestinal symptoms. Chinese J Digest 40: 2020. doi: 10.3760/cma.j.issn.0254-1432.2020.0009. [DOI] [Google Scholar]
- 43.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol 3: 237–261, 2016. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364–374, 2020. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letko M, Munster V. Functional assessment of cell entry and receptor usage for lineage B β-coronaviruses, including 2019-nCoV. bioRxiv 2020.01.22.915660, 2020. doi: 10.1101/2020.01.22.915660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.National Health Commission and National Administration of Traditional Chinese Medicine . Diagnosis and treatment of pneumonia caused by novel coronavirus (Trial version 7). Chinese Medical J 133: 1087–1095, 2020. doi: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, Almakhlafi GA, Albarrak MM, Memish ZA, Albarrak AM. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis 14: 1090–1095, 2014. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol 165: 5069–5076, 2000. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 48.Peiris JS, Lai ST, Poon LL, Guan Y, Yam LY, Lim W, Nicholls J, Yee WK, Yan WW, Cheung MT, Cheng VC, Chan KH, Tsang DN, Yung RW, Ng TK, Yuen KY; SARS study group . Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361: 1319–1325, 2003. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raj VS, Mou H, Smits SL, Dekkers DH, Müller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495: 251–254, 2013. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 109: 102433, 2020. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA In press. 2020. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 52.Segal I, Ou Tim L, Demetriou A, Paterson A, Hale M, Lerios M. Rectal manifestations of pellagra. Int J Colorectal Dis 1: 238–243, 1986. doi: 10.1007/BF01648345. [DOI] [PubMed] [Google Scholar]
- 53.Shi J-H, Wang Y-R, Li W-B, Gang R, Liu X, Xu L, Luo Q-F. A single-center descriptive study of COVID-19 inpatients in Wuhan, analysis of digestive system performance and disease severity in 54 cases. Chinese J Digest 40, 2020. doi: 10.3760/cma.j.issn.0254-1432.2020.0010. [DOI] [Google Scholar]
- 54.Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood 107: 3447–3454, 2006. doi: 10.1182/blood-2005-07-2860. [DOI] [PubMed] [Google Scholar]
- 55.Stratigos JD, Katsambas A. Pellagra: a still existing disease. Br J Dermatol 96: 99–106, 1977. doi: 10.1111/j.1365-2133.1977.tb05197.x. [DOI] [PubMed] [Google Scholar]
- 56.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24: 490–502, 2016. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, Knight R. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol 15: 397–411, 2018. [Erratum in Nat Rev Gastroenterol Hepatol 15: 785, 2018]. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94: e00127-20, 2020. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 Novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med 211: 2397–2410, 2014. [Erratum in J Exp Med 211: 2683, 2014]. doi: 10.1084/jem.20140625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508: 402–405, 2014. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkins T, Sequoia J. Probiotics for gastrointestinal conditions: a summary of the evidence. Am Fam Physician 96: 170–178, 2017. [PubMed] [Google Scholar]
- 63.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, Zhong W, Hao P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 63: 457–460, 2020. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan R-H, Zhang Y-Y, Guo Y-Y, Xia L, Zhou Q. Structural basis for the recognition of the 2019-nCoV by human ACE2 (Preprint). bioRxiv: 2020.02.19.956946, 2020. doi: 10.1101/2020.02.19.956946. [DOI] [Google Scholar]
- 66.Yang Z-W, Li G-W, Dai X-L, Liu G-R, Li G, Jie Y-S. Three cases of COVID-19 pharyngeal swab were still positive for fecal nucleic acid. Chinese J Digest 40, 2020. doi: 10.3760/cma.j.issn.0254-1432.2020.0002. [DOI] [Google Scholar]
- 67.Yeo C, Kaushal S, Yeo D. Enteric involvement of coronaviruses: is faecal-oral transmission of SARS-CoV-2 possible? Lancet Gastroenterol Hepatol 5: 335–337, 2020. doi: 10.1016/S2468-1253(20)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeung ML, Yao Y, Jia L, Chan JF, Chan KH, Cheung KF, Chen H, Poon VK, Tsang AK, To KK, Yiu MK, Teng JL, Chu H, Zhou J, Zhang Q, Deng W, Lau SK, Lau JY, Woo PC, Chan TM, Yung S, Zheng BJ, Jin DY, Mathieson PW, Qin C, Yuen KY. MERS coronavirus induces apoptosis in kidney and lung by upregulating Smad7 and FGF2. Nat Microbiol 1: 16004, 2016. doi: 10.1038/nmicrobiol.2016.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC; Singapore 2019 Novel Coronavirus Outbreak Research Team . Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323: 1488, 2020. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang D, Li S, Wang N, Tan HY, Zhang Z, Feng Y. The Cross-talk between gut microbiota and lungs in common lung diseases. Front Microbiol 11: 301, 2020. doi: 10.3389/fmicb.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang F-C, Yin Z-B, Tang X-P. Clinical analysis of 260 cases of SARS in Guangzhou. Chinese J Infectious Dis 21: 84–88, 2003. [Google Scholar]
- 72.Zhao Y, Zhong S-P, Li F, Liu G-Q, Wang X-F, Liu Z-J. Analysis of clinical characteristics and risk factors of gastrointestinal symptoms of COVID-19 patients in Xinyang, Henan province. Chinese J Digest 40: E011–E011, 2020. doi: 10.3760/cma.j.issn.0254-1432.2020.0011. [DOI] [Google Scholar]
- 73.Zhong NS, Zheng BJ, Li YM, Poon LLM, Xie ZH, Chan KH, Li PH, Tan SY, Chang Q, Xie JP, Liu XQ, Xu J, Li DX, Yuen KY, Peiris JSM, Guan Y. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People’s Republic of China, in February, 2003. Lancet 362: 1353–1358, 2003. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579: 270–273, 2020. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu F-S, Chen L, Yang C-Q. Protective measures in the gastroenterology department during the outbreak of COVID-19. Chinese J Digest Dis Imaging 10: 2020. doi: 10.3877/cma.j.issn.2095-2015.2020.02.001. [DOI] [Google Scholar]
- 76.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus I, Research T; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med 14: 185–192, 2020. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]