Abstract
In the last few months, the number of cases of a new coronavirus-related disease (COVID-19) rose exponentially, reaching the status of a pandemic. Interestingly, early imaging studies documented that pulmonary vascular thickening was specifically associated with COVID-19 pneumonia, implying a potential tropism of the virus for the pulmonary vasculature. Moreover, SARS-CoV-2 infection is associated with inflammation, hypoxia, oxidative stress, mitochondrial dysfunction, DNA damage, and lung coagulopathy promoting endothelial dysfunction and microthrombosis. These features are strikingly similar to what is seen in pulmonary vascular diseases. Although the consequences of COVID-19 on the pulmonary circulation remain to be explored, several viruses have been previously thought to be involved in the development of pulmonary vascular diseases. Patients with preexisting pulmonary vascular diseases also appear at increased risk of morbidity and mortality. The present article reviews the molecular factors shared by coronavirus infection and pulmonary vasculature defects, and the clinical relevance of pulmonary vascular alterations in the context of COVID-19.
Keywords: coronavirus, COVID-19, pulmonary vascular diseases, SARS-CoV-1, SARS-CoV-2, vascular remodeling
INTRODUCTION
In December 2019, the first cases of pneumonia of unknown etiology were described in the city of Wuhan and linked to a seafood and wild-animal wholesale market, suggesting at first animal-to-person transmission. Rapidly, human-to-human transmission was confirmed (136), and the virus was isolated and identified as a novel β-coronavirus: severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (136). Compared with other members of the Coronaviridae family, which generally cause mild respiratory disease (111), the SARS-CoV-2-related disease (COVID-19) can lead to severe respiratory disease and death. However, reported mortality rates of 3–4% for SARS-CoV-2 infection appear lower than with SARS-CoV-1 (2002) and the Middle East Respiratory Syndrome (MERS-CoV; 2012), which were associated with case fatality rates of ≈9% (26) and ≈34% (137), respectively (111). Since its first description, the number of COVID-19 cases rose exponentially, rapidly reaching the status of a pandemic causing officially more than 507,000 deaths worldwide as of July 2020.
Interestingly, several viruses have been suspected of leading to the development of pulmonary vascular diseases over the years. The association between human immunodeficiency virus (HIV) infection and severe pulmonary hypertension (PH), with features identical to those seen in patients with idiopathic pulmonary arterial hypertension (PAH), was recognized early on in the AIDS epidemic (110). Although its specific role in PAH pathogenesis remains controversial, the human herpesvirus 8 (HHV-8) genome and HHV-8-encoded latency-associated nuclear antigen 1 were documented in plexiform lesions of patients with PAH (17, 22). However, other studies have since then failed to demonstrate this association (15, 62, 86, 119). Research on SARS-CoV-1, MERS-CoV, and more recently on SARS-CoV-2 suggests these viruses promote endothelial dysfunction, vascular leak, and pulmonary microthrombi (19, 37, 46, 55, 76, 100, 103, 112, 116, 120, 135) through mechanisms such as inflammation, hypoxia, oxidative stress, mitochondrial dysfunction, and DNA damage. Although their consequences on the pulmonary circulation remain unknown, these features are strikingly similar to what is seen in the development of pulmonary vascular disease (56). The present article highlights the molecular features shared by coronaviruses infection and pulmonary vasculature defects, examines the clinical relevance of pulmonary vascular diseases in the context of the COVID-19 pandemic. We also discuss the potential long-term effects of COVID-19 on the pulmonary circulation, as well as their management in light of current evidence.
SEARCH STRATEGY AND SELECTION CRITERIA
References for this review were identified through searches of PubMed for articles published from January 2000, to June 2020, by use of the terms “ARDS”, “acute respiratory distress syndrome”, “COVID-19”, “heparin”, MERS-Cov”, “pulmonary embolism”, “pulmonary arterial hypertension”, “pulmonary hypertension”, “SARS-CoV-1”, “SARS-CoV-2”, “severe acute respiratory syndrome”, and “thrombosis”. Articles published in English, French, and Chinese resulting from these searches and relevant references cited in those articles were reviewed.
CLINICAL PRESENTATION AND DISEASE SEVERITY
With the number of identified COVID-19 cases increasing worldwide, it has become clear that infected patients may present in a number of ways. The incubation period is 5 days on average, with initial symptoms being observed within 11.5 days in 97.5% of patients (68). However, asymptomatic carriers of the virus may represent up to 18–33% of cases and constitute a challenge because of their potential contribution to the silent spreading of the disease (85). The most common symptoms are fever, cough, dyspnea, myalgia, and fatigue (55, 98), but rhinorrhea, gastrointestinal symptoms, anosmia, and ageusia are also reported. A substantial proportion of patients have abnormal laboratory findings, such as lymphopenia, abnormal liver function tests, as well as elevated inflammatory markers, D-dimers, and prothrombin levels (134). Early observational studies also suggested that virtually all patients had parenchymal abnormalities on computed chest tomography (3). Typical features include multilobar ground-glass opacities and consolidations predominantly involving the posterior and lower lung zones. Interestingly, pulmonary vascular abnormalities were also observed on chest CT (129), where vascular thickening has been significantly associated with COVID-19 compared with non-COVID-19 pneumonia (10).
Despite a majority of patients presenting only mild-to-moderate symptoms, it is estimated that 10–20% will require hospitalization, and 10–40% of them will require intensive care unit (ICU) admission (42). Ultimately, up to 0.3–8% of infected individuals will succumb, most commonly of respiratory failure. The case fatality rate of COVID-19 has differed significantly around the world, being as high as 50% in critically ill patients (128). Increased patient age has repeatedly been shown to be associated with an enhanced risk of mortality (42). However, this association was at least partly explained by a higher prevalence of comorbidities in older individuals, as the age-adjusted relative risk of mortality or ICU admission was 1.6–3.5 for patients with malignancy, chronic obstructive pulmonary disease (COPD), diabetes, or hypertension (42). Of note, the clinical presentation of patients with comorbid conditions was strikingly similar, apart from a higher prevalence of dyspnea on admission (42). Surprisingly, less than 2% of hospitalized patients had self-reported COPD in large-scale cohorts from China. These findings, likely reflecting the lack of awareness or appropriate diagnosis of chronic lung disease in community settings and suggest that respiratory diseases were underdiagnosed, thus limiting the external validity of these early observational studies (42).
SIMILARITIES IN MOLECULAR AND CELLULAR DYSFUNCTIONS OBSERVED IN COVID-19 AND PULMONARY VASCULAR DISEASES
COVID-19 Structure, Function, and Interaction with the ACE2 Receptor
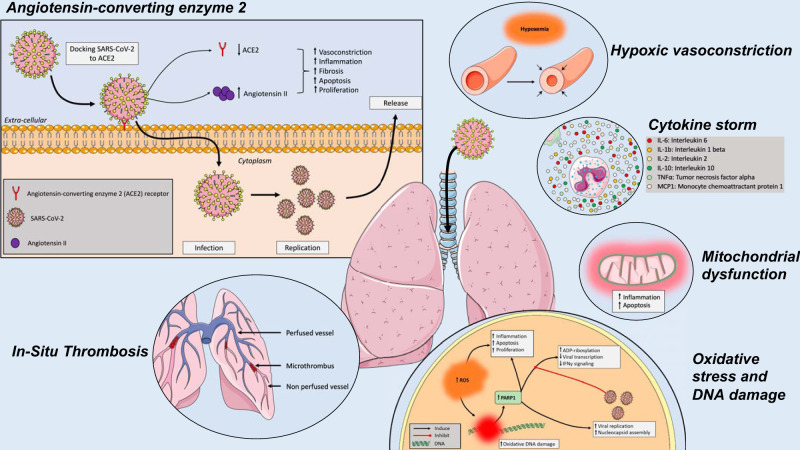
Coronaviruses are positive-stranded RNA viruses named after their crown-shaped appearance when viewed under an electron microscope. Like other coronaviruses, SARS-CoV-2 has four structural proteins, known as the S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins. SARS-CoV-2 cycle starts with the interaction between the viral S protein and its functional receptor from the host cells, the angiotensin-converting enzyme 2 (ACE2) receptor (127). This receptor is present on pneumocytes and macrophages, as well as on the surface of arterial endothelial and smooth muscle cells of virtually all organs, especially the heart, lungs, and kidneys (47). Importantly, the SARS-CoV-2 has a higher affinity for human ACE2 compared with SARS-CoV-1 (124). By binding the ACE2 receptor, SARS-CoV-2 enables the action of “transmembrane protease, serine 2” from the host cells to cut open the viral S protein, allowing for the fusion of viral and cellular membranes (52). Upon entry into the host cells, the viral RNA genome is then translated into two polyproteins and structural proteins in the cytoplasm (92) after which, proteins and RNA are packaged into progeny virions and released to infect more cells.
Maintenance of normal ACE2 levels within the host’s lung appears to be beneficial to combat inflammatory lung disease (60). Indeed, ACE2 hydrolyzes angiotensin II to generate angiotensin 1–7. Angiotensin II contributes to the lung damage observed in acute lung injury or acute respiratory distress syndrome (ARDS) by inducing vasoconstriction, proinflammatory, profibrotic, proapoptotic, and proproliferative phenotype (60). Increased angiotensin II circulating levels potentially resulting from COVID-19-mediated ACE2 downregulation correlate with the viral load and lung injury (74). Interestingly, angiotensin II is also upregulated in plexiform lesions observed in PAH patients, and circulating levels of angiotensin II correlates with disease severity (65). Experimentally, either inhibiting angiotensin-converting enzyme (ACE), blocking angiotensin receptor, or overexpressing ACE2 reverses adverse pulmonary vascular lesions in PH (102). Despite these experimental results, the use of angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARBs) is not recommended for treatment of PH due to the lack of convincing clinical studies on the efficacy and safety of such drugs (59, 81). However, a recent study documented reduced ACE2 activity in a small cohort of PAH patients (49). The same study reported improvements in pulmonary hemodynamics, oxidative and inflammatory markers in patients treated with recombinant human ACE2 (49). Altogether, these observations suggest similarities in the molecular signature of COVID-19 and pulmonary vascular diseases such as PAH.
Proinflammatory Storm and Hypercytokinemia
COVID-19 is also usually associated with lymphopenia and cytokine outburst, including IL-6, IL-1b, IL-2, IL-10, TNF-α, and monocyte chemoattractant protein-1, which correlate with COVID-19 severity (82). A recent meta-analysis reported that the mean concentrations of IL-6 were 2.9-fold higher in patients with complicated COVID-19 compared with those who had an uncomplicated disease course (23). Moreover, a single-arm nonrandomized trial showed that tocilizumab treatment in severe COVID-19 reduced oxygen requirements, improved radiographic abnormalities, and resulted in clinical improvement without serious adverse events or death in most patients (23).
Wilk and colleagues (126) investigated the production of inflammatory cytokines in circulating immune cells from COVID-19 patients. They applied a single-cell RNA sequencing to peripheral blood mononuclear cells of seven hospitalized COVID-19 patients and six healthy controls (126). They reported a profound reconfiguration of circulating immune cells in SARS-CoV-2-infected patients, including a novel B cell-derived granulocyte population in patients with severe acute respiratory failure. Intriguingly, they showed that circulating monocytes and lymphocytes do not express substantial amounts of proinflammatory cytokines, suggesting that circulating leukocytes do not hold a predominant role in the COVID-19-related cytokine storm.
In PAH, increased expression of proinflammatory cytokines also contributes to adverse pulmonary vascular remodeling (39) and predicts survival (109). Mechanistically, cytokines have pleiotropic effects, including promoting an antiapoptotic and proproliferative environment by activating several pathways like the JAK/STAT3 signaling (110a). Targeting the proinflammatory cytokines or their downstream effectors may thus minimize both lung damage and pulmonary vascular complications in COVID-19 patients, as suggested in PH preclinical models (91, 123). However, inflammatory cytokines and other components of the inflammatory cascade contribute to the host defense against infection. Thus, targeting cytokine expression in COVID-19 patients should be considered with caution. Consequently, the Centers for Disease Control and Prevention (CDC) advises avoiding the use of JAK inhibitors or interferon in COVID-19 treatments because of the lack of convincing studies on the efficacy and safety of such drugs.
Increased Oxidative Stress and DNA Damage
Reactive oxygen species (ROS) are key signaling molecules that play an important role in the progression of inflammatory disorders (84). In vitro, SARS-CoV-1 is associated with increased ROS production (133). In vivo, severe lung injury and proinflammatory host response are dependent on activation of the oxidative stress machinery in monkeys infected by SARS-CoV-1 (106). Increased intracellular ROS results in oxidative DNA damage (96). Single-strand DNA breaks are normally repaired by base excision repair involving the poly-ADP ribose polymerase (PARP). PARP also works as an antiviral agent through the ADP-ribosylation of the viral genome and inhibition of viral transcripts translation (11). Besides, ADP-ribosylation of the transcription factor NF-κB induces interferon-γ (IFNγ) transcription initiating the IFNγ signaling cascade, which contributes to the immune response against viral infection (30). Coronaviridae encodes for a macrodomain protein with poly (ADP-ribose) glycohydrolase activity, which binds and removes ADP-ribose, annealing the antiviral effects of PARP (40). Moreover, PARP expression is increased in cells infected by SARS-CoV-1 (133) and appears to be critical for viral replication, nucleocapsid assembly, and dissemination by promoting cell death (73). Interestingly, PARP inhibition reduced SARS-CoV-2 replication without an obvious cytopathic effect (36). It is thus reasonable to speculate that clinically available PARP-1 inhibitors might have therapeutic values in COVID-19 patients. Extensive studies have shown that DNA damage signaling and PARP activation similarly promote inflammation and mitochondrial alterations leading to severe pulmonary vascular lesions, and the development of pulmonary vascular diseases, such as PAH (83). These observations led to the first clinical trial investigating PARP inhibitors for PAH (NCT03782818).
Mitochondrial Dysfunction
Proteomic analysis of in vitro models of SARS CoV-1 infected cells showed that 36% of the upregulated proteins were located in the mitochondria (including apoptosis-inducing factor, ATP synthase β-chain and cytochrome-c oxidase) (66). A distinct report showed that cells infected by SARS CoV-1 displayed mitochondrial dysfunction, demonstrated by loss of mitochondrial potential (ΔΨm) (133). The authors observed that SARS-CoV-1 nucleocapsid protein induces mitochondrial-dependent cell apoptosis (increased apoptosis-inducing factor, ATP synthase β-chain, and cytochrome-c oxidase). Interestingly, it appears that SARS-CoV-1 can manipulate the host cell mitochondria and alter mitochondrial function to help evade the host’s innate immunity (103). For example, in vitro infection of cells by SARS-CoV-1 caused mitochondrial elongation by triggering ubiquitination and proteasomal degradation of dynamin-like protein 1, leading to the increase in mitochondrial fission and strong induction of autophagy (103). In parallel, the contribution of mitochondrial dysfunction is widely described as a mechanism involved in adverse pulmonary vascular remodeling observed in human and preclinical models of PH (24).
Despite being associated with coronavirus infections, including SARS-CoV-2, inflammation, oxidative stress, and mitochondrial dysfunction are unspecific manifestations also shared by other infections and hypoxemic processes (6, 7, 12, 13, 63, 107). Further investigations are warranted to better dissect and understand the specific molecular defects associated with SARS-CoV-2 infection.
PULMONARY VASCULAR DISEASE: A COMPLICATION AND A RISK FACTOR FOR COVID-19
In addition to common molecular pathways (Fig. 1), several lines of evidence suggest that COVID-19 specifically impacts the pulmonary circulation.
Fig. 1.
Effects of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on the lungs and the pulmonary vasculature. SARS-CoV-2 cycle starts with the interaction with the angiotensin-converting enzyme 2 (ACE2) receptor from the host cells. Upon entry into the host cells, the viral RNA genome is then translated and proteins and RNA are packaged into progeny virions being released to infect more cells. The SARS-CoV-2-related disease (COVID-19) results in cytokine outburst, including IL-6, IL-1b, IL-2, IL-10, TNF-α, and monocyte chemoattractant protein-1 (MCP-1). Reactive oxygen species (ROS) are key signaling molecules that play an important role in the progression of inflammatory disorders, resulting in oxidative stress, mitochondrial dysfunction, and DNA damage. In addition to parenchymal abnormalities, disseminated intravascular coagulation, endothelial dysfunction, and impaired hypoxic pulmonary vasoconstriction ultimately generate pulmonary microthrombi, ventilation-perfusion mismatch, and hypoxemia.
Hypoxemia and Impaired Hypoxic Pulmonary Vasoconstriction
In healthy adults, hypoxic pulmonary vasoconstriction (HPV) occurs in response to alveolar hypoxia. HPV diverts blood flow from poorly ventilated to well-ventilated alveoli, therefore optimizing ventilation-perfusion matching (108). Importantly, when HPV is compromised, intrapulmonary shunts cause a decrease in oxygenation of the pulmonary venous blood flow, ultimately contributing to poor oxygen delivery, organ dysfunction, and failure. Intriguingly, preliminary reports suggest that severe COVID-19 can present as an atypical form of ARDS with significant dissociation between relatively well-preserved lung mechanics and severe hypoxemia. For example, Guan et al. (41) reported dyspnea in only 19% of 1,099 hospitalized COVID-19 pneumonia patients, despite low : ratios, abnormal CT scans, and common requirement for supplemental oxygen. These observations could be explained by impaired HPV but also by the loss of lung perfusion regulation, and pulmonary microthrombi (8, 35, 67). Whether patients with severe COVID-19 have impaired hypoxic pulmonary vasoconstriction remains, however, controversial.
Endotheliitis, Vasculitis, and Angiogenesis
Perivascular lymphocytic inflammation induced by SARS-CoV-2 has been observed in post-mortem studies (1, 120). This endotheliitis and vasculitis provoke severe endothelial injury and, consequently, increase the risk of thrombosis. Ackermann and colleagues (1) compared autopsy findings from seven COVID-19-infected patients, seven influenza A (H1N1)-infected patients with ARDS to 10 uninfected control patients. COVID-19-infected lungs exhibited severe endothelial injury, vascular thrombosis associated with microangiopathy, occlusion of alveolar capillaries, and intussusceptive angiogenesis. Interestingly, SARS-CoV-2 infection is also associated with systemic vasculitis, such as cutaneous vasculitis, which could be related to either the viral infection or to the immune response triggered by SARS-Cov-2 in affected patients (18, 21, 29, 61). Interestingly, Endotheliitis and vasculitis have also been observed in some cases of PAH associated with connectivity diseases and describe in the advance stage of the diseases (e.g., grade 6 of the Heath and Edwards classification) (27, 43, 48, 71, 93).
Pulmonary In Situ Thrombosis and Embolism
In COVID-19 cohorts, disseminated intravascular coagulation, a condition characterized by the generation of microthrombi in different organs, including the pulmonary circulation (77), was diagnosed in 11% of the patients overall, including 71% of the nonsurvivors (114). The same group noted pulmonary microthrombi at lung dissection from a critically ill COVID-19 patient. Also, a series of 12 consecutive autopsies showed the presence of venous thromboembolism in 58%, contributing to death in four of them (125). Consistently, high levels of D-dimers were repeatedly shown to be associated with the need for ICU admission and mortality among COVID-19 patients (72). This is not surprising since lung coagulopathy is relevant in the pathogenesis of ARDS (89). The cytokine storm and pulmonary microthrombi observed with COVID-19 are thus consistent with the immunothrombosis model, which highlights a bidirectional relationship between the immune system and thrombin generation during severe infection (33). Tang and colleagues (113) suggested that the use of anticoagulant therapy with heparin was associated with decreased mortality, especially so in patients with significant sepsis-induced coagulopathy or markedly elevated D-dimer levels. Paranjpe et al. (90) also showed a positive association between systemic anticoagulation and survival in hospitalized patients with COVID-19, requiring mechanical ventilation. On the other hand, Tremblay and colleagues (117) showed no difference in mortality in hospitalized patients already on anticoagulation compared with patients without such treatment. As recognized by the authors, these observational studies are subjected to bias and confounding and thus require further confirmation. Moreover, these beneficial effects could be related to the nonanticoagulant properties of heparin, including its anti-inflammatory (131), antiviral (105), and protective effects on the pulmonary endothelium (57), as well as the overall prevention of venous thromboembolism. Indeed, hospitalized COVID-19 patients are also at higher risk of venous thromboembolism due to their intense inflammatory state (41), elevated hypoxia-inducible transcription factors, increased blood viscosity (45), and immobilization. While initial anecdotal reports described cases of pulmonary embolism diagnosed concomitantly with COVID-19 (25, 129), more recent observational studies suggest that venous thromboembolic events are common among COVID-19 patients hospitalized in the ICU despite systematic thrombosis prophylaxis (64). Poissy and colleagues (94) reported that among 107 consecutive confirmed COVID-19 patients admitted to the ICU from Feb 27 to March 31, 2020, 20.6% experienced pulmonary embolism (PE) within a median of 6 days from ICU admission. Interestingly, in a cohort of 196 patients during the same period in 2019, only 6.1% had PE despite a similar severity score upon admission to the ICU. Between January 1 and December 31, 2019, nearly 8% of patients admitted in the ICU (n = 40) due to influenza suffered from PE (94). The high incidence of PE related to COVID-19 was supported by another study performed in 184 ICU patients, where 31% developed thrombotic complications, which comprised 81% of total thrombotic events (64). In that context, it is recommended that COVID-19 patients receive appropriate thrombosis prophylaxis. However, drug-drug interactions between antiplatelet agents or anticoagulants with investigational COVID-19 therapies should be considered (16). Physicians should also be vigilant for signs of venous thromboembolic events. The efficacy, dosage, and characteristics of patients suitable for high-prophylactic doses or systemic anticoagulation remain to be demonstrated.
Effects of SARS-CoV-2 on Pulmonary Hemodynamics, Right Ventricular Function, and Myocardial Injury
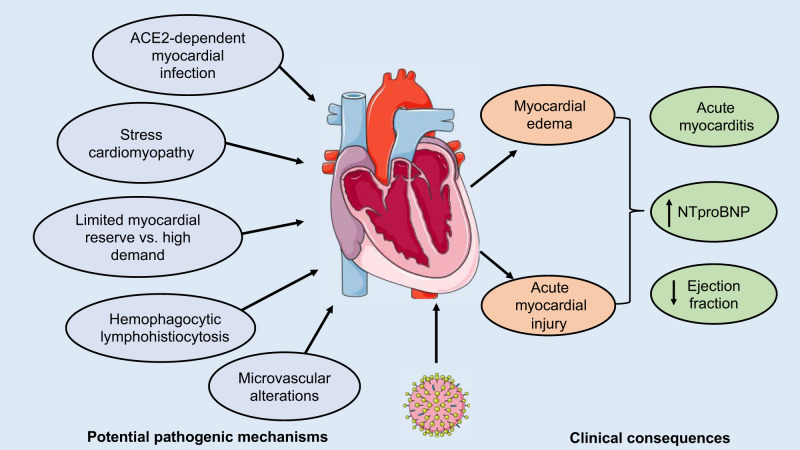
Similar to ARDS, patients with severe COVID-19 are expected to exhibit a high prevalence of pulmonary vascular defects, which can lead to PH and subsequent right ventricular (RV) dysfunction (122). In a cohort of 120 consecutive patients with COVID-19, Yuan and colleagues (70) showed that low RV longitudinal strain is a predictor of higher mortality. In a cohort of 110 consecutive patients, Argulian et al. (9) also reported that RV dilation (31% of COVID-19 patients) is associated with increased mortality risk. Consistent with this observation, Fox and colleagues (31) reported a massive RV dilation in 10 patients who died from COVID-19. In addition to the COVID-19-specific pulmonary vascular abnormalities described above, the process of pulmonary hemodynamic alterations in ARDS is multifactorial and involves hypoxia, hypercapnia, vascular compression by pulmonary edema and fibrosis, and ventilator-induced increases in alveolar and intrathoracic pressures (122). Together with this increased afterload, ARDS is associated with increased sympathetic stimulation and hypoxia, resulting in myocardial oxygen imbalance, myocardial depression, as well as an increased risk of infarction, acute RV decompensation, and arrhythmia (79). Importantly, in most influenza pandemics, cardiovascular events surpassed all other causes of mortality, including superimposed pneumonia (78). There is also evidence that MERS-CoV caused acute myocarditis (5). Similarly, acute cardiac injury, manifesting as reduced ejection fraction, and increased troponin and natriuretic peptide levels are observed in 7–22% of admitted patients with COVID-19, and independently associated with a higher case fatality rate (104). Myocardium from patients who died from COVID-19 exhibited scattered individual cell myocyte necrosis (31). Supporting this finding, Sharma and colleagues (101) demonstrated that SARS-CoV-2 can enter and replicate in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), resulting in hiPSC-CM apoptosis and cessation of beating. Consistent with this observation, autopsies of patients who died from SARS-CoV-1 revealed that 35% of heart samples displayed the presence of viral RNA, which, in turn, was associated with reduced ACE2 protein expression (88). Similarly, in a murine model, infection with SARS-CoV-1 also precipitated an ACE2‐dependent myocardial infection (88). Thus, as observed for SARS-CoV-1, the expression of ACE2 in the human heart could be a potential mechanism of heart injury among patients infected with SARS-CoV-2 (19). A prospective study of SARS-CoV-1 patients without preexisting cardiac disease also confirmed subclinical diastolic dysfunction in the acute stage of the disease (69). However, whether the cardiac injury in COVID-19 results from direct ACE2-dependent myocardial infection, stress cardiomyopathy, high demand in patients with limited myocardial reserve, or indirect myocardial injury due to secondary cytokine storm and microvascular alterations requires further evaluation (Fig. 2) (44). Similarly, the mechanism of RV dilation and dysfunction is likely multifactorial and may involve an interplay between thrombotic events, hypoxemic vasoconstriction, cytokine storm, and direct viral damages on cardiomyocytes or endothelial cells.
Fig. 2.
Effects of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on pulmonary hemodynamics and the heart. Acute cardiac injury, manifesting as an ejection fraction decline, and troponin and natriuretic peptide elevation are commonly observed in patients admitted with SARS-CoV-2-related disease (COVID-19). Diastolic dysfunction has also been described in the acute stage of SARS-CoV-1 in patients without preexisting cardiac disease. Whether cardiac injury in COVID-19 results from direct myocardial infection, stress cardiomyopathy, high demand in patients with significantly increased afterload due to secondary pulmonary hypertension in the context of hypoxemia and limited myocardial reserve, or indirect myocardial injury due to secondary hemophagocytic lymphohistiocytosis and microvascular alterations requires further evaluation.
Pulmonary Vascular Disease as a Risk Factor for COVID-19
The presence of severe PH is associated with poorer outcomes among patients with ARDS (122), secondary RV hemodynamic instability being the leading cause of mortality over hypoxemia (97). In patients with preexisting PH, the RV chronically faces increased afterload, initially resulting in adaptive RV hypertrophy. In many cases, however, the RV progresses inexorably toward a maladaptive phenotype culminating in RV failure and death. Importantly, infection remains a common cause of superimposed acute RV decompensation among patients with chronic PH (51). As observed for PH patients, increased circulating lactate dehydrogenase (LDH) (54), and high-sensitivity C-reactive protein (hsCRP) (95) can accurately predict 10-day mortality of COVID-19 patients (130). Taken together, it makes little doubt that the present pandemic represents a serious threat for patients with preexisting pulmonary vascular diseases, such as PAH and chronic thromboembolic PH, as well as in patients with significant PH related to comorbid disease, such as left heart and respiratory diseases. Although no comprehensive study to date has evaluated whether COVID-19 occurs more frequently or is more severe in these patients compared with the general population, we surprisingly found no evidence for such association based on a PubMed search on June 1, 2020 (64). This most likely reflects the lack of awareness of preexisting PH in early observational studies. On the basis of the apparent key role of the pulmonary vasculature in COVID-19, indirect evidence suggests that current therapies in PAH targeting the nitric oxide (NO), the endothelin, and the prostaglandin pathways may partially influence the COVID-19 prognosis. Indeed, NO has frequently been considered a protective mediator in viral infection due to its microbicide function, although it could also potentiate inflammation or promote virus latency (118). While inhaled NO (iNO) treatments showed promising results in a patient with concomitant idiopathic pulmonary arterial hypertension (iPAH) and COVID-19 (132), the utility of iNO in treating respiratory manifestations of COVID-19 needs to be proven and supported by clinical trials. Interestingly, over 20 clinical trials investigating the effect of inhaled NO in COVID-19 are currently ongoing. Similarly, endothelin-1 contributes toward increasing the expression of leukocyte adhesion molecules and promotes the synthesis of inflammatory mediators leading to vascular dysfunction during viral pneumonia (32), whereas prostacyclin regulates both the innate and adaptive immune systems (28). Whether current PAH vasoactive therapies have any protective effects clinically remains however unknown.
PH patients are also at higher risk of poorer outcomes owing to indirect consequences of the current pandemic. Indeed, actions to manage the COVID-19 crisis include the cancellation of all nonemergency services, as well as drastic restriction of interhospital patient transfers and hospital-based outpatient clinics. Moreover, access to laboratories and imaging studies is altered by infection control procedures. Thus, reduced delivery of health care to PH patients is a potential adverse consequence of the global response to control the SARS-CoV-2 pandemic. During other pandemics, such as the influenza outbreaks, increased cardiovascular and other non-influenza-related deaths have been observed due to limited access to care (53). Prolonged and unprecedented containment procedures are expected to be associated with suboptimal patient management.
PREVENTION AND TREATMENT OF COVID-19 INFECTION IN PATIENTS WITH PULMONARY VASCULAR DISEASES
In the absence of a vaccine or approved therapies against SARS-CoV-2, preventive measures currently remain the best strategy for COVID-19 in patients with pulmonary vascular diseases, such as PH. It is also recommended that PH guideline-oriented therapies should be provided and continued (34). In addition to heparin, several investigational therapies, reviewed elsewhere (2), are also in development. These include colchicine, monoclonal antibodies against SARS-CoV-2, recombinant human ACE2, serine protease inhibitor, broad-spectrum antiviral, remdesivir, lopinavir/ritonavir, favipiravir, human IL1-b receptor antagonist, as well as antibodies against the interleukin-6 or its receptor. Remdesivir, a prodrug of an adenosine analog with antiviral activity, has initially shown promising results with a 68% clinical improvement in patients, with severe COVID-19 receiving the drug under compassionate use (38). Recently, a randomized controlled trial comparing remdesivir with placebo in hospitalized COVID-19 patients reported a significant reduction of 4 days in the time to recovery with this antiviral therapy (14). Chloroquine and hydroxychloroquine, currently used for the treatment of malaria and rheumatoid arthritis, respectively, are also under the spotlight for COVID-19 treatment. An earlier open-label and nonrandomized study in France (n = 36) reported improved virologic clearance in COVID-19 patients receiving hydroxychloroquine. On the other hand, a prospective cohort study conducted in China (n = 30) found no difference in virologic clearance after hydroxychloroquine treatment (99). Two recent studies confirmed that the use of hydroxychloroquine did not increase the likelihood of virus elimination in a cohort of 150 Chinese patients with mild-to-moderate COVID-19 (115), nor did it have any effect on reducing admissions to intensive care or mortality in a cohort of 181 French patients with more severe illness (80). Both studies also found a higher rate of adverse events in patients treated with the drug. Of interest, previous studies demonstrated that both chloroquine and hydroxychloroquine prevent adverse pulmonary vascular remodeling and progression of PH through inhibition of autophagy in preclinical models of the disease (75). Thus, the preliminary outcomes described with these therapies could be in part by preserving pulmonary vasculature functions in COVID-19 patients. However, the use of hydroxychloroquine or chloroquine against COVID-19 is not supported by large clinical trials and the potential for cardiac adverse effects, namely, arrhythmias have been cautioned by the US Food and Drug Administration (4).
COVID-19 AS A RISK FACTOR FOR SUBSEQUENT PULMONARY VASCULAR DYSFUNCTION?
As the world progressively recovers from the acute stages of the pandemic, we will be facing new challenges regarding the long-term consequences of COVID-19. As can be extrapolated from data on ARDS (50) and SARS-CoV-1 (87), survivors of severe COVID-19 are expected to experience persistent impairment in lung-diffusing capacity and gas exchanges. While generally attributed to mild restrictive patterns commonly observed in these patients, we could speculate that impaired diffusing capacity may also partly be of vascular origin. Indeed, SARS-CoV-1 and SARS-CoV-2 share several deleterious mechanisms involved in pulmonary vascular dysfunction, as described above. These coronaviruses could thus be risk factors for persistent pulmonary vascular defects and subsequent PH development, especially in patients with persistent lung impairment post-COVID-19, and susceptible patients with genetic predisposition or chronic lung and heart diseases. Although this remains speculative, this could become a major health problem in the future given the large number of patients infected by SARS-CoV-2 worldwide. Systematic post-mortem evaluations, long-term studies assessing the risk of developing pulmonary vascular lesions or clinical PH following COVID-19, and basic research will thus be of interest to inform on the putative ongoing vascular remodeling effects of COVID-19 and best long-term management of survivors.
CONCLUSIONS
COVID-19 is a global pandemic evolving in real time that is associated with significant morbidity and mortality. Lungs remain the organ mostly affected by SARS-CoV-2 infection, leading to severe respiratory disease in many individuals. Patients with preexisting comorbid conditions, including patients with pulmonary vascular diseases, are at particularly high risk of hospitalization and death. While the effects of COVID-19 for the pulmonary circulation are being defined, several lines of evidence suggest that the molecular features of SARS-CoV-2 infection are strikingly similar to what is seen in pulmonary vascular disease development, promoting endothelial dysfunction, lung coagulopathy and microthrombi, and hemodynamic impairments (Table 1). The involvement of the pulmonary vasculature is also supported by imaging studies. While there are a number of treatments under investigation, pathologic assessments and long-term studies assessing the risk of developing chronic pulmonary vascular lesions following COVID-19 infection will be of great interest for both basic and clinical research.
Table 1.
Similarities between COVID-19 and pulmonary hypertension
| COVID-19 | PH | |
|---|---|---|
| Symptom | ||
| Dyspnea | +++ | +++ |
| Fatigue | +++ | + |
| Inflammation | ||
| Endotheliitis | +++ | +* |
| Vasculitis | +++ | +* |
| Myocarditis | + | − |
| Proinflammatory cytokines | ↑↑↑ | ↑↑† |
| Thrombosis, microthrombi | ||
| D-dimers | ↑↑↑ | ↑ |
| Prothrombin | ↑ | ↑ |
| DNA damage | ||
| PARP | ↑ | ↑↑ |
| RAA activation | + | +++ |
| ACE 2 | ↓↓ | ↓ |
| Angiotensin 2 | ↑↑↑ | ↑↑ |
| Cardiac injury | ||
| Ejection fraction | ↓* | ↓‡ |
| Troponin | ↑↑ | ↑ |
| Natriuretic peptide | ↑ | ↑↑↑ |
| RV dilatation | ↑ | ↑↑↑ |
| Pulmonary vascular thickness | ↑ | ↑↑↑ |
| Mitochondrial dysfunction | ↑ | ↑↑ |
| ROS | ↑ | ↑↑ |
| Endothelial dysfunction | ↑ | ↑↑ |
| HPV | ↑ | ↑↑↑§ |
HPV, hypoxic pulmonary vasoconstriction; PARP, poly-ADP ribose polymerase; PH, pulmonary hypertension; RAA, renin-angiotensin-aldosterone system; ROS, reactive oxygen species; RV, right ventricle.
Occasionally;
group 1 pulmonary hypertension;
group 2 pulmonary hypertension;
group 3 pulmonary hypertension.
↑ increased, ↑↑ generally increased, ↑↑↑ frequently increased, ↓ decreased, + observed, ++ generally observed, +++, frequently observed.
NOTE ADDED IN PROOF
On June 15, 2020, after this article was accepted, the FDA revoked the emergency use authorization (EUA) that allowed for chloroquine or hydroxychloroquine to be used to treat hospitalized patients with COVID-19 when a clinical trial was unavailable or participation in a clinical trial was not feasible (https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and). This decision reflects the lack of beneficial effects of these medications for decreasing the likelihood of death or speeding recovery in large clinical trials and the serious cardiac adverse events associated with chloroquine or hydroxychloroquine treatment.
DISCLOSURES
S.P. reports grants from Actelion, grants from AstraZeneca, grants from Resverlogix, outside of the submitted work. M.L. reports personal fees from Air Liquide Health Care, outside the submitted work. S.P. received speaker fees from Actelion Pharmaceuticals and unrestricted grant from Actelion Pharmaceuticals, AstraZeneca (in-kind), Glaxo-Smith-Kline and Resverlogix outside the context of the submitted work. M.L. received speaker fees, and nonfinancial support from Air Liquide Healthcare and SEFAM outside the context of the submitted work. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
F.P. prepared figures; F.P., V.M., M.L., S.M., E.B.-G., A.C.L., O.B., S.B., and S.P. drafted manuscript; F.P., V.M., M.L., S.M., E.B.-G., A.C.L., O.B., S.B., and S.P. edited and revised manuscript; F.P., V.M., M.L., S.M., A.C.L., O.B., S.B., and S.P. approved final version of manuscript.
REFERENCES
- 1.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. In press. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, Kim BT, Kim SJ. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19). J Microbiol Biotechnol 30: 313–324, 2020. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1,014 cases. Radiology. In press. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander PE, Debono VB, Mammen MJ, Iorio A, Aryal K, Deng D, Brocard E, Alhazzani W. COVID-19 coronavirus research has overall low methodological quality thus far: case in point for chloroquine/hydroxychloroquine. J Clin Epidemiol 123: 120–126, 2020. doi: 10.1016/j.jclinepi.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saudi Med 36: 78–80, 2016. doi: 10.5144/0256-4947.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anand SK, Tikoo SK. Viruses as modulators of mitochondrial functions. Adv Virol 2013: 738794, 2013. doi: 10.1155/2013/738794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Araneda OF, Tuesta M. Lung oxidative damage by hypoxia. Oxid Med Cell Longev 2012: 856918, 2012. doi: 10.1155/2012/856918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Archer SL, Sharp WW, Weir EK. Differentiating COVID-19 pneumonia from acute respiratory distress syndrome (ARDS) and high altitude pulmonary edema (HAPE): therapeutic implications. Circulation. In press. doi: 10.1161/CIRCULATIONAHA.120.047915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, Lerakis S, Narula J. Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. In press. doi: 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi LB, Wang DC, Mei J, Jiang XL, Zeng QH, Egglin TK, Hu PF, Agarwal S, Xie F, Li S, Healey T, Atalay MK, Liao WH. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. In press. doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai P. Biology of poly(ADP-ribose) polymerases: the factotums of cell maintenance. Mol Cell 58: 947–958, 2015. doi: 10.1016/j.molcel.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 12.Bartels K, Grenz A, Eltzschig HK. Hypoxia and inflammation are two sides of the same coin. Proc Natl Acad Sci USA 110: 18351–18352, 2013. doi: 10.1073/pnas.1318345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck MA, Handy J, Levander OA. The role of oxidative stress in viral infections. Ann NY Acad Sci 917: 906–912, 2000. doi: 10.1111/j.1749-6632.2000.tb05456.x. [DOI] [PubMed] [Google Scholar]
- 14.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC; ACTT-1 Study Group Members . Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. In press. doi: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 15.Bendayan D, Sarid R, Cohen A, Shitrit D, Shechtman I, Kramer MR. Absence of human herpesvirus 8 DNA sequences in lung biopsies from Israeli patients with pulmonary arterial hypertension. Respiration 75: 155–157, 2008. doi: 10.1159/000097495. [DOI] [PubMed] [Google Scholar]
- 16.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, Ageno W, Madjid M, Guo Y, Tang LV, Hu Y, Giri J, Cushman M, Quéré I, Dimakakos EP, Gibson CM, Lippi G, Favaloro EJ, Fareed J, Caprini JA, Tafur AJ, Burton JR, Francese DP, Wang EY, Falanga A, McLintock C, Hunt BJ, Spyropoulos AC, Barnes GD, Eikelboom JW, Weinberg I, Schulman S, Carrier M, Piazza G, Beckman JA, Steg PG, Stone GW, Rosenkranz S, Goldhaber SZ, Parikh SA, Monreal M, Krumholz HM, Konstantinides SV, Weitz JI, Lip GYH; Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol 75: 2950–2973, 2020. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bull TM, Cool CD, Serls AE, Rai PR, Parr J, Neid JM, Geraci MW, Campbell TB, Voelkel NF, Badesch DB. Primary pulmonary hypertension, Castleman’s disease and human herpesvirus-8. Eur Respir J 22: 403–407, 2003. doi: 10.1183/09031936.03.00006903. [DOI] [PubMed] [Google Scholar]
- 18.Castelnovo L, Capelli F, Tamburello A, Faggioli PM, Mazzone A. Symmetric cutaneous vasculitis in COVID-19 pneumonia. J Eur Acad Dermatol Venereol. In press. doi: 10.1111/jdv.16589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res 116: 1097–1100, 2020. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Pradhan S, Xue S. What are we doing in the dermatology outpatient department amidst the raging of the 2019 novel coronavirus? J Am Acad Dermatol 82: 1034, 2020. doi: 10.1016/j.jaad.2020.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cool CD, Rai PR, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, Voelkel NF. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med 349: 1113–1122, 2003. doi: 10.1056/NEJMoa035115. [DOI] [PubMed] [Google Scholar]
- 23.Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis (Preprint). medRxiv 2020. doi: 10.1101/2020.03.30.20048058. [DOI] [PMC free article] [PubMed]
- 24.Culley MK, Chan SY. Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J Clin Invest 128: 3704–3715, 2018. doi: 10.1172/JCI120847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 41: 1858, 2020. doi: 10.1093/eurheartj/ehaa254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol 14: 523–534, 2016. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dion J, Terrier B, Jaïs X, Mehdaoui A, Sattler C, Amar D, Cohen P, Guillevin L, Mouthon L. Atypical vasculitis mimicking chronic thromboembolic pulmonary hypertension. Am J Med 128: e47–e49, 2015. doi: 10.1016/j.amjmed.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Dorris SL, Peebles RS Jr. PGI2 as a regulator of inflammatory diseases. Mediators Inflamm 2012: 926968, 2012. doi: 10.1155/2012/926968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estébanez A, Pérez-Santiago L, Silva E, Guillen-Climent S, García-Vázquez A, Ramón MD. Cutaneous manifestations in COVID-19: a new contribution. J Eur Acad Dermatol Venereol 34: e250–e251, 2020. doi: 10.1111/jdv.16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fehr AR, Singh SA, Kerr CM, Mukai S, Higashi H, Aikawa M. The impact of PARPs and ADP-ribosylation on inflammation and host-pathogen interactions. Genes Dev 34: 341–359, 2020. doi: 10.1101/gad.334425.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 27: 30243-5, 2020. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman BD, Machado FS, Tanowitz HB, Desruisseaux MS. Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci 118: 110–119, 2014. doi: 10.1016/j.lfs.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaertner F, Massberg S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin Immunol 28: 561–569, 2016. doi: 10.1016/j.smim.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Galiè N, Channick RN, Frantz RP, Grünig E, Jing ZC, Moiseeva O, Preston IR, Pulido T, Safdar Z, Tamura Y, McLaughlin VV. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 53: 1801889, 2019. doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am J Respir Crit Care Med 201: 1299–1300, 2020. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ge Y, Tian T, Huang S, Wan S, Li J, Li S, Yang H, Hong L, Wu N, Yuan E, Cheng L, Lei Y, Shu H, Feng X., Jiang Z, Chi Y, Guo X, Cui L, Xiao L, Li Z, Yang C, Miao Z, Tang H, Chen L, Zeng H, Zhao D, Zhu F, Shen X, and Zeng J. A data-driven drug repositioning framework discovered a potential therapeutic agent targeting COVID-19 (Preprint). bioRxiv 2020. doi: 10.1101/2020.03.11.986836. [DOI] [PMC free article] [PubMed]
- 37.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol 127: 104362, 2020. doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, Green G, Green ML, Lescure F-X, Nicastri E, Oda R, Yo K, Quiros-Roldan E, Studemeister A, Redinski J, Ahmed S, Bernett J, Chelliah D, Chen D, Chihara S, Cohen SH, Cunningham J, D’Arminio Monforte A, Ismail S, Kato H, Lapadula G, L’Her E, Maeno T, Majumder S, Massari M, Mora-Rillo M, Mutoh Y, Nguyen D, Verweij E, Zoufaly A, Osinusi AO, DeZure A, Zhao Y, Zhong L, Chokkalingam A, Elboudwarej E, Telep L, Timbs L, Henne I, Sellers S, Cao H, Tan SK, Winterbourne L, Desai P, Mera R, Gaggar A, Myers RP, Brainard DM, Childs R, Flanigan T. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 382: 2327–2336, 2020. doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groth A, Vrugt B, Brock M, Speich R, Ulrich S, Huber LC. Inflammatory cytokines in pulmonary hypertension. Respir Res 15: 47, 2014. doi: 10.1186/1465-9921-15-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grunewald ME, Chen Y, Kuny C, Maejima T, Lease R, Ferraris D, Aikawa M, Sullivan CS, Perlman S, Fehr AR. The coronavirus macrodomain is required to prevent PARP-mediated inhibition of virus replication and enhancement of IFN expression. PLoS Pathog 15: e1007756, 2019. doi: 10.1371/journal.ppat.1007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen K-Y, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX; China Medical Treatment Expert Group for COVID-19 . Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 55: 2000547, 2020. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guillevin L. Vasculopathy and pulmonary arterial hypertension. Rheumatology 48, Suppl 3: 54–57, 2009. doi: 10.1093/rheumatology/ken484. [DOI] [PubMed] [Google Scholar]
- 44.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. In press. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res 181: 77–83, 2019. doi: 10.1016/j.thromres.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Guzzi PH, Mercatelli D, Ceraolo C, Giorgi FM. Master regulator analysis of the SARS-CoV-2/human interactome. J Clin Med 9: 982, 2020. doi: 10.3390/jcm9040982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203: 631–637, 2004. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 18: 533–547, 1958. doi: 10.1161/01.cir.18.4.533. [DOI] [PubMed] [Google Scholar]
- 49.Hemnes AR, Rathinasabapathy A, Austin EA, Brittain EL, Carrier EJ, Chen X, Fessel JP, Fike CD, Fong P, Fortune N, Gerszten RE, Johnson JA, Kaplowitz M, Newman JH, Piana R, Pugh ME, Rice TW, Robbins IM, Wheeler L, Yu C, Loyd JE, West J. A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur Respir J 51: 1702638, 2018. doi: 10.1183/13993003.02638-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, Cooper AB, Guest CB, Mazer CD, Mehta S, Stewart TE, Barr A, Cook D, Slutsky AS; Canadian Critical Care Trials Group . One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med 348: 683–693, 2003. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 51.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med 184: 1114–1124, 2011. doi: 10.1164/rccm.201104-0662CI. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181: 271–280.e8, 2020. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Housworth J, Langmuir AD. Excess mortality from epidemic influenza, 1957–1966. Am J Epidemiol 100: 40–48, 1974. doi: 10.1093/oxfordjournals.aje.a112007. [DOI] [PubMed] [Google Scholar]
- 54.Hu EC, He JG, Liu ZH, Ni XH, Zheng YG, Gu Q, Zhao ZH, Xiong CM. High levels of serum lactate dehydrogenase correlate with the severity and mortality of idiopathic pulmonary arterial hypertension. Exp Ther Med 9: 2109–2113, 2015. doi: 10.3892/etm.2015.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 53: 1801887, 2019. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iba T, Hashiguchi N, Nagaoka I, Tabe Y, Kadota K, Sato K. Heparins attenuated histone-mediated cytotoxicity in vitro and improved the survival in a rat model of histone-induced organ dysfunction. Intensive Care Med Exp 3: 36, 2015. doi: 10.1186/s40635-015-0072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther 92: 1– 20, 2001. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 60.Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock 46: 239–248, 2016. doi: 10.1097/SHK.0000000000000633. [DOI] [PubMed] [Google Scholar]
- 61.Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol 82: e177, 2020. doi: 10.1016/j.jaad.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Katano H, Ito K, Shibuya K, Saji T, Sato Y, Sata T. Lack of human herpesvirus 8 infection in lungs of Japanese patients with primary pulmonary hypertension. J Infect Dis 191: 743–745, 2005. doi: 10.1086/427824. [DOI] [PubMed] [Google Scholar]
- 63.Kleinerman ES, Daniels CA, Polisson RP, Snyderman R. Effect of virus infection on the inflammatory response. Depression of macrophage accumulation in influenza-infected mice. Am J Pathol 85: 373–382, 1976. [PMC free article] [PubMed] [Google Scholar]
- 64.Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 191: 145–147, 2020. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kümpers P, Nickel N, Lukasz A, Golpon H, Westerkamp V, Olsson KM, Jonigk D, Maegel L, Bockmeyer CL, David S, Hoeper MM. Circulating angiopoietins in idiopathic pulmonary arterial hypertension. Eur Heart J 31: 2291–2300, 2010. doi: 10.1093/eurheartj/ehq226. [DOI] [PubMed] [Google Scholar]
- 66.Lai CC, Jou MJ, Huang SY, Li SW, Wan L, Tsai FJ, Lin CW. Proteomic analysis of up-regulated proteins in human promonocyte cells expressing severe acute respiratory syndrome coronavirus 3C-like protease. Proteomics 7: 1446–1460, 2007. doi: 10.1002/pmic.200600459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lang M, Som A, Mendoza DP, Flores EJ, Reid N, Carey D, Li MD, Witkin A, Rodriguez-Lopez JM, Shepard JO, Little BP. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. In press. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med 172: 577–582, 2020. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li SS, Cheng CW, Fu CL, Chan YH, Lee MP, Chan JW, Yiu SF. Left ventricular performance in patients with severe acute respiratory syndrome: a 30-day echocardiographic follow-up study. Circulation 108: 1798–1803, 2003. doi: 10.1161/01.CIR.0000094737.21775.32. [DOI] [PubMed] [Google Scholar]
- 70.Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Zhang Y, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. In press. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li Y, Yi Q. Pulmonary arterial hypertension associated with rare cause of ANCA-associated vasculitis misdiagnosed as idiopathic one. Int J Clin Exp Med 8: 16,850–16,863, 2015. [PMC free article] [PubMed] [Google Scholar]
- 72.Lippi G, Favaloro EJ. D-dimer is associated with severity of coronavirus disease 2019: a pooled analysis. Thromb Haemost 120: 876–878, 2020. doi: 10.1055/s-0040-1709650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu L, Lear Z, Hughes DJ, Wu W, Zhou EM, Whitehouse A, Chen H, Hiscox JA. Resolution of the cellular proteome of the nucleocapsid protein from a highly pathogenic isolate of porcine reproductive and respiratory syndrome virus identifies PARP-1 as a cellular target whose interaction is critical for virus biology. Vet Microbiol 176: 109–119, 2015. doi: 10.1016/j.vetmic.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 63: 364–374, 2020. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Long L, Yang X, Southwood M, Lu J, Marciniak SJ, Dunmore BJ, Morrell NW. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ Res 112: 1159–1170, 2013. doi: 10.1161/CIRCRESAHA.111.300483. [DOI] [PubMed] [Google Scholar]
- 76.Luftig MA. Viruses and the DNA damage response: activation and antagonism. Annu Rev Virol 1: 605–625, 2014. doi: 10.1146/annurev-virology-031413-085548. [DOI] [PubMed] [Google Scholar]
- 77.Luo W, Yu H, Gou J, Li X, Sun Y, Li J, Liu L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19) (Preprint). Preprints 2020.
- 78.Madjid M, Casscells SW. Of birds and men: cardiologists’ role in influenza pandemics. Lancet 364: 1309, 2004. doi: 10.1016/S0140-6736(04)17176-6. [DOI] [PubMed] [Google Scholar]
- 79.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol In press. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 80.Mahévas MA-O, Tran VT, Roumier M, Chabrol A, Paule R, Guillaud C, Fois E, Lepeule R, Szwebel TA, Lescure FX, Schlemmer F, Matignon M, Khellaf M, Crickx E, Terrier B, Morbieu C, Legendre P, Dang J, Schoindre Y, Pawlotsky JM, Michel M, Perrodeau E, Carlier N, Roche N, de Lastours V, Ourghanlian C, Kerneis S, Ménager P, Mouthon L, Audureau E, Ravaud P, Godeau B, Gallien S, and Costedoat-Chalumeau N. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ 369: m1844, 2020. doi: 10.1136/bmj.m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mascitelli L, Pezzetta F. Inhibition of the renin-angiotensin system in patients with COPD and pulmonary hypertension. Chest 131: 938, 2007. doi: 10.1378/chest.06-2018. [DOI] [PubMed] [Google Scholar]
- 82.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033–1034, 2020. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meloche J, Pflieger A, Vaillancourt M, Paulin R, Potus F, Zervopoulos S, Graydon C, Courboulin A, Breuils-Bonnet S, Tremblay E, Couture C, Michelakis ED, Provencher S, Bonnet S. Role for DNA damage signaling in pulmonary arterial hypertension. Circulation 129: 786–797, 2014. doi: 10.1161/CIRCULATIONAHA.113.006167. [DOI] [PubMed] [Google Scholar]
- 84.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 25: 2000180, 2020. doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nicastri E, Vizza CD, Carletti F, Cicalini S, Badagliacca R, Poscia R, Ippolito G, Fedele F, Petrosillo N. Human herpesvirus 8 and pulmonary hypertension. Emerg Infect Dis 11: 1480–1482, 2005. doi: 10.3201/eid1109.0408801480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ong KC, Ng AW, Lee LS, Kaw G, Kwek SK, Leow MK, Earnest A. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J 24: 436–442, 2004. doi: 10.1183/09031936.04.00007104. [DOI] [PubMed] [Google Scholar]
- 88.Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 39: 618–625, 2009. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ozolina A, Sarkele M, Sabelnikovs O, Skesters A, Jaunalksne I, Serova J, Ievins T, Bjertnaes LJ, Vanags I. Activation of coagulation and fibrinolysis in acute respiratory distress syndrome: a prospective pilot study. Front Med (Lausanne) 3: 64, 2016. doi: 10.3389/fmed.2016.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paranjpe I, Fuster V, Lala A, Russak A, Glicksberg BS, Levin MA, Charney AW, Narula J, Fayad ZA, Bagiella E, Zhao S, Nadkarni GN. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. In press. doi: 10.1016/j.jacc.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paulin R, Courboulin A, Meloche J, Mainguy V, Dumas de la Roque E, Saksouk N, Côté J, Provencher S, Sussman MA, Bonnet S. Signal transducers and activators of transcription-3/pim1 axis plays a critical role in the pathogenesis of human pulmonary arterial hypertension. Circulation 123: 1205–1215, 2011. doi: 10.1161/CIRCULATIONAHA.110.963314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol 7: 439–450, 2009. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pilania RK, Dhawan SR, Mathew JL, Singh S, Sodhi KS, Singh M. ANCA-associated vasculitis presenting as severe pulmonary hypertension and right heart failure. Indian J Pediatr 84: 799–801, 2017. doi: 10.1007/s12098-017-2379-0. [DOI] [PubMed] [Google Scholar]
- 94.Poissy J, Goutay J, Caplan M, Parmentier E, Duburcq T, Lassalle F, Jeanpierre E, Rauch A, Labreuche J, Susen S; Lille ICU Haemostasis COVID-19 Group . Pulmonary embolism in COVID-19 patients: awareness of an increased prevalence. Circulation In press. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 95.Quarck R, Nawrot T Meyns B. Delcroix M. C-reactive protein: a new predictor of adverse outcome in pulmonary arterial hypertension. J Am Coll Cardiol 53: 1211–1218, 2009. doi: 10.1016/j.jacc.2008.12.038. [DOI] [PubMed] [Google Scholar]
- 96.Ranchoux B, Meloche J, Paulin R, Boucherat O, Provencher S, Bonnet S. DNA damage and pulmonary hypertension. Int J Mol Sci 17: 990, 2016. doi: 10.3390/ijms17060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Repessé X, Charron C, Vieillard-Baron A. Acute respiratory distress syndrome: the heart side of the moon. Curr Opin Crit Care 22: 38–44, 2016. doi: 10.1097/MCC.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, Alvarado-Arnez LE, Bonilla-Aldana DK, Franco-Paredes C, Henao-Martinez AF, Paniz-Mondolfi A, Lagos-Grisales GJ, Ramírez-Vallejo E, Suárez JA, Zambrano LI, Villamil-Gómez WE, Balbin-Ramon GJ, Rabaan AA, Harapan H, Dhama K, Nishiura H, Kataoka H, Ahmad T, Sah R; Latin American Network of Coronavirus Disease 2019-COVID-19 Research (LANCOVID-19) . Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 34: 101623, 2020. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 323: 1824–1836, 2020. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 100.Schwarz KB. Oxidative stress during viral infection: a review. Free Radic Biol Med 21: 641–649, 1996. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 101.Sharma A, Garcia G, Arumugaswami V, Svendsen CN. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection (Preprint). bioRxiv 2020. doi: 10.1101/2020.04.21.051912.32511402. [DOI] [PMC free article] [PubMed]
- 102.Shenoy V, Kwon KC, Rathinasabapathy A, Lin S, Jin G, Song C, Shil P, Nair A, Qi Y, Li Q, Francis J, Katovich MJ, Daniell H, Raizada MK. Oral delivery of angiotensin-converting enzyme 2 and angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64: 1248–1259, 2014. doi: 10.1161/HYPERTENSIONAHA.114.03871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Shi CS, Qi HY, Boularan C, Huang NN, Abu-Asab M, Shelhamer JH, Kehrl JH. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol 193: 3080–3089, 2014. doi: 10.4049/jimmunol.1303196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. In press. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shukla D, Spear PG. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J Clin Invest 108: 503–510, 2001. doi: 10.1172/JCI200113799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Smits SL, van den Brand JM, de Lang A, Leijten LM, van Ijcken WF, van Amerongen G, Osterhaus AD, Andeweg AC, Haagmans BL. Distinct severe acute respiratory syndrome coronavirus-induced acute lung injury pathways in two different nonhuman primate species. J Virol 85: 4234–4245, 2011. doi: 10.1128/JVI.02395-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Solaini G, Baracca A, Lenaz G, Sgarbi G. Hypoxia and mitochondrial oxidative metabolism. Biochim Biophys Acta 1797: 1171–1177, 2010. doi: 10.1016/j.bbabio.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 108.Sommer N, Strielkov I, Pak O, Weissmann N. Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction. Eur Respir J 47: 288–303, 2016. doi: 10.1183/13993003.00945-2015. [DOI] [PubMed] [Google Scholar]
- 109.Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 122: 920–927, 2010. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 110.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 100: 1268–1271, 1991. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 110a.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 104: 236–244, 2009. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, Liu W, Bi Y, Gao GF. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 24: 490–502, 2016. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tang D, Comish P, Kang R. The hallmarks of COVID-19 disease. PLoS Pathog 16: e1008536, 2020. doi: 10.1371/journal.ppat.1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 18: 1094–1099, 2020. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18: 844–847, 2020. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, and Xie QA-O. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 369: m1849, 2020. doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 20: 363–374, 2020. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tremblay D, van Gerwen M, Alsen M, Thibaud S, Kessler AJ, Venugopal S, Makki I, Qin Q, Dharmapuri S, Jun T, Bhalla S, Berwick S, Feld J, Mascarenhas J, Troy K, Cromwell C, Dunn A, Oh WK, Naymagon L. Impact of anticoagulation prior to COVID-19 infection: a propensity score-matched cohort study. Blood. In press. doi: 10.1182/blood.2020006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Uehara EU, Shida BS, de Brito CA. Role of nitric oxide in immune responses against viruses: beyond microbicidal activity. Inflamm Res 64: 845–852, 2015. doi: 10.1007/s00011-015-0857-2. [DOI] [PubMed] [Google Scholar]
- 119.Valmary S, Dorfmüller P, Montani D, Humbert M, Brousset P, Degano B. Human γ-herpesviruses Epstein-Barr virus and human herpesvirus-8 are not detected in the lungs of patients with severe pulmonary arterial hypertension. Chest 139: 1310–1316, 2011. doi: 10.1378/chest.10-1200. [DOI] [PubMed] [Google Scholar]
- 120.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Crit Care Med 29: 1551–1555, 2001. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 123.Voelkel NF, Tuder RM, Bridges J, Arend WP. Interleukin-1 receptor antagonist treatment reduces pulmonary hypertension generated in rats by monocrotaline. Am J Respir Cell Mol Biol 11: 664–675, 1994. doi: 10.1165/ajrcmb.11.6.7946395. [DOI] [PubMed] [Google Scholar]
- 124.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol 94: e00127-20, 2020. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. In press. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilk AJ, Rustagi A, Zhao NQ, Roque J, Martinez-Colon GJ, McKechnie JL, Ivison GT, Ranganath T, Vergara R, Hollis T, Simpson LJ, Grant P, Subramanian A, Rogers AJ, and Blish CA. A single-cell atlas of the peripheral immune response to severe COVID-19 (Preprint). medRxiv 20069930, 2020. [DOI] [PMC free article] [PubMed]
- 127.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367: 1260–1263, 2020. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323: 1239–1242, 2020. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 129.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. In press. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yan L, Zhang H-T, Goncalves J, Xiao Y, Wang M, Guo Y, Sun C, Tang X, Jing L, Zhang M, Huang X, Xiao Y, Cao H, Chen Y, Ren T, Wang F, Xiao Y, Huang S, Tan X, Huang N, Jiao B, Cheng C, Zhang Y, Luo A, Mombaerts L, Jin J, Cao Z, Li S, Xu H, Yuan Y. An interpretable mortality prediction model for COVID-19 patients. Nature Machine Intelligence 2: 283–288, 2020. doi: 10.1038/s42256-020-0180-7. [DOI] [Google Scholar]
- 131.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res 122: 743–752, 2008. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 132.Zamanian RT, Pollack CV Jr, Gentile MA, Rashid M, Fox JC, Mahaffey KW, de Jesus Perez V. Outpatient inhaled nitric oxide in a patient with vasoreactive IPAH and COVID-19 infection. Am J Respir Crit Care Med. In press. doi: 10.1164/rccm.202004-0937LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L, Wei L, Jiang D, Wang J, Cong X, Fei R. SARS-CoV nucleocapsid protein induced apoptosis of COS-1 mediated by the mitochondrial pathway. Artif Cells Blood Substit Immobil Biotechnol 35: 237–253, 2007. doi: 10.1080/10731190601188422. [DOI] [PubMed] [Google Scholar]
- 134.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov 6: 14, 2020. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet 386: 995–1007, 2015. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]