Abstract
Background
Social support and social integration have been linked to lower rates of morbidity and mortality. However, the biological mechanisms responsible for such links need greater attention. Vaccine paradigms provide an integrative window into immune system involvement in the protective influence of social support/integration.
Purpose
The main aim of this article was to conduct a meta-analytic review of the association between social support/social integration and antibody responses to vaccines. Exploratory analyses also examined effect sizes and confidence intervals as a function of several factors to inform future research.
Method
A literature search was conducted using the ancestry approach and with PsycInfo, Medline, and the Psychology and Behavioral Science Collection by crossing the exact keywords of social support or social integration with vaccine or antibodies. The review identified nine studies with a total of 672 participants.
Results
The omnibus meta-analysis showed that social support/social integration was related to higher antibody levels following vaccination, but the average effect size was small and the lower bound of the confidence interval included zero (Zr = 0.06 [−.04, .15]). These results did not appear to differ much as a function of the operationalization of social relationships, participant age, or follow-up period, although effect sizes appeared larger for studies using a primary antigen.
Conclusions
These data provide some evidence that social support may be linked to antibody responses to vaccines. However, effect sizes are mostly small and zero overall effect cannot be ruled out. Future studies would benefit from larger sample sizes and greater consideration of methodological issues associated with secondary immune responses to antigen.
Keywords: Antibody titers, Social support, Relationships, Vaccination
This review shows that social support may beneficially influence antibody responses to vaccines, although effects appear small and null effects cannot be ruled out.
The quality and extent of one’s social relationships have been related to lower disease morbidity and mortality [1–3]. More specifically, both structural (e.g., social integration) and functional (e.g., perceived support) measures of social support have been linked to better health outcomes [1, 4, 5]. Thus, the link between social support and health is on strong ground, which sets the stage for theoretical questions on mechanisms.
One area of strong interest is the physiological mechanisms linking social support to disease outcomes. Prior research has examined a number of health-relevant biological pathways including neural, cardiovascular, neuroendocrine, and immune processes [6–8]. Such biological modeling is important because it can highlight important pathways that could be monitored or targeted for psychological and/or pharmacological interventions [9, 10].
Much research linking social support to biological outcomes has focused on the immune system, which is the bodies’ defense against infectious and malignant disease [11, 12]. These studies mostly focused on isolated measures of immune function starting with early work examining immune cell counts and their functional response to challenges in vitro [13, 14]. More recent work in the area has examined inflammatory cytokines given their link to numerous health outcomes such as cardiovascular disease and cancer [15–17]. Consistent with the epidemiological evidence linking social support to diverse health outcomes, a recent meta-analysis found that social support was associated with lower levels of inflammation [18]. In general, immune system involvement is consistent with the groundbreaking common cold studies by Cohen et al. who have showed that social network diversity is a predictor of lower infection rates following inoculation [19].
Given that most prior work has focused on isolated immune measures, a complementary approach would be to examine an integrative immune response to challenges. Vaccine paradigms (e.g., flu vaccine) provide a strong test of such processes as the immune response they trigger is a clinically significant outcome and there are numerous (and complex) steps that ultimately result in protective antibody titers to an antigen. In a typical immune response to infectious viruses, antigen-presenting cells (e.g., macrophages, dendritic cells) capture and process the antigen [11]. The antigen-presenting cells then present the antigen to the T-cell receptor of helper T cells in the context of major histocompatibility complex class II molecules (a genetic region in all mammals that signals between lymphocytes and cells that have major histocompatibility complex molecules). In response to antigen recognition, helper T cells undergo clonal expansion and secrete a variety of cytokines such as IL-2 and IL-4 that aide in the proliferation of B cells into plasma cells. These plasma cells then secrete antibody specific for the antigen. Upon initial exposure to antigen, the primary antibody response is IgM, which is effective as an immediate response given it has five active binding sites. However, there is an isotype switching from IgM to IgG later in the antibody response to promote longer-term immunity. A hallmark of the immune system is memory, and a subset of memory T and B cells are maintained that promote a more vigorous immune response (e.g., IgG is produced earlier and with greater affinity) upon subsequent exposure [11]. Given prior work linking social support to aspects of immune function, one might expect that this would translate to a stronger, more effective integrative immune response to vaccination.
There have been a number of studies that have examined the link between social support and vaccine responses. Most of these have examined if social support predicts antibody titers to the influenza vaccine [20–23]. In general, results of these studies are mixed. For instance, Phillips et al. [21] found no link between perceived social support and antibody titers to the flu vaccine, whereas Pressman et al. [23] found that social integration was associated with higher antibody titers to the flu vaccine indicating a beneficial response. However, the study by Pressman et al. [23] only found a link to one strain of the trivalent flu vaccine (i.e., New Caledonia) and not the other two strains. Thus, a meta-analysis that looks at composite results across studies is needed in order to draw stronger inferences. This is especially important given the logistic challenges and invasive nature of such studies, which typically examine antibody responses from blood over several time points. The main aim of this meta-analytic review was thus to examine whether social support was related to a stronger antibody response to vaccination as might be expected given the epidemiological evidence to date.
An exploratory aim was to characterize the effect sizes and confidence intervals (CI) associated with several factors that might influence the link between social support and antibody titers to vaccines. This would help inform future research as an inferential moderation test (i.e., meta-regression) involving significance tests would likely be underpowered given the small number of studies identified in this review. One important conceptual question that can be explored relates to the different ways of measuring relationships, which have distinct theoretical implications [24, 25]. The classic review by Cohen and Wills [26] made the distinction between structural and functional measures of support. Social integration reflects the extent of one’s social connections and access to support (e.g., marriage, friendships, volunteer organizations [24]). Importantly, structural and functional measures of support are empirically and conceptually separable [1, 24, 27]. Functional measures of support can further be distinguished as perceived or received support. Perceived support reflects the perception that support would be available if needed whereas received support is the reported receipt of support during a particular time frame [28]. This distinction is also significant because perceived support is more consistently related to better health outcomes compared with received support [25, 28]. In fact, received support often has variable links to health as it might not be a good match to the needs or motivations of the recipient and can threaten a person’s sense of independence [29, 30]. For these reasons, it is possible that the effects of perceived support on antibody responses to vaccines might be stronger than the effects of received support.
There are several methodological aspects of studies that are important to consider as well. One is the age of participants as aging is associated with decreases in the number of naive T and B cells, the function of memory T cells, and changes in inflammation that negatively affect vaccine responses [31, 32]. For instance, the efficacy of the annual flu vaccine is only 30%–50% for adults over 65 compared with up to 90% in children and younger adults [31]. A second methodological factor is whether a study included multiple follow-up periods. This is important as associations might become stronger over time given the potential positive cumulative impact of social support on antibody production. Finally, an important methodological factor is whether the vaccine evokes a primary or secondary immune response. A primary immune response involves antigens that an individual has no prior exposure to, in contrast to a secondary immune response that is characterized by immunologic memory [11]. As a result, the secondary immune response is more vigorous and efficient as it can rely on memory T and B cells, which are “primed” to respond to the antigen. Secondary immune responses might be more difficult to find an association with social support given potential ceiling effects in antibody titers. Thus, an exploratory aim of this review was to examine these factors to inform future work in the area.
Method
Identification and Inclusion/Exclusion of Studies
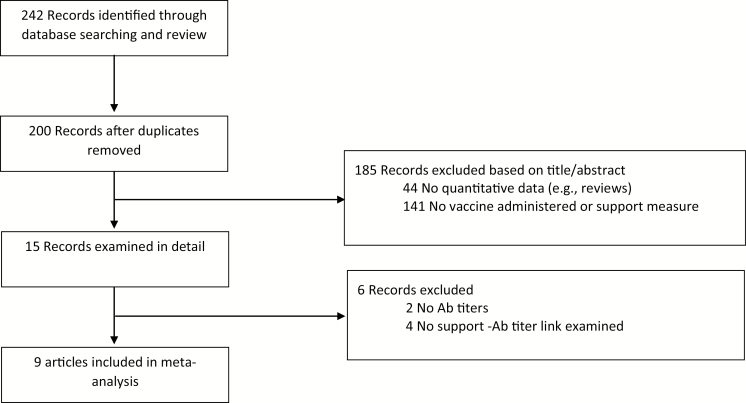
The review protocol used in this meta-analysis is detailed below. A literature search was first conducted using the major databases of PsycInfo, Medline, and the Psychology and Behavioral Science Collection by crossing the exact keywords of social support or social integration with vaccine or antibodies. This search was run up to March of 2019 and identified 242 records, with 200 remaining after duplicates were removed (see Fig. 1). One hundred and eighty-five articles were rejected based on a review of titles/abstracts as they did not contain any quantitative data or did not include measures of social support or antibody responses to vaccines. Of the remaining 14 records, 5 were excluded for various reasons, the most common being that no direct link between social support and antibody titers was examined given it was not a primary aim of the study. The ancestry approach was also used which searched the reference list of all eligible articles and review papers on the topic (e.g., Whittaker [33]). This resulted in a final count of nine articles. A search of EMBASE resulted in no additional articles being flagged.
Fig. 1.
Meta-analytic flow chart describing selection of studies.
Analysis Plan and Data Extraction
Major details regarding studies (e.g., sample, type of support measure, outcomes) were first characterized and examined in tabular form. The subsequent meta-analysis was performed using a commercially available software package (MetaWin, Rosenberg et al. [34]) that provided results regarding effect sizes, CI, tests of variability regarding effect sizes, and a fail-safe number for any significant associations. These analyses were based on a random effects model, so that inferences can be made to studies on the topic more generally [35]. Correlation coefficients (r) were used as the common metric for data entry. When correlations were not presented, measures of effect size were converted to r values. Standardized regression weights were converted using the formula: r = β + .05 λ where λ = 1 when β is not negative and λ = 0 when β is negative [36]. When p-values were the only source of data, they were transformed using the equation based on the one-tailed z-score. Results reported as nonsignificant utilized a conservative significance level of .50 [37]. To reduce the problem of nonindependence in omnibus analyses, when multiple effects were reported (e.g., different vaccine strains, assessment points), they were first transformed to a common metric (i.e., z-scores), averaged into a single effect, then entered into the meta-analysis. Publication bias for the omnibus meta-analysis was done at the outcome level by calculating Kendall’s Tau and the fail-safe number. Kendall’s Tau examines the association between effect sizes and sample sizes, whereas the fail-safe number estimates the number of nonsignificant studies needed to make the results nonsignificant [37]. Finally, exploratory analyses were done to characterize the effect sizes and CI for the different types of support (i.e., structural, perceived support, received support), participant age (i.e., young, older), follow-up period (first, second), and type of immune response (i.e., primary, secondary).
Results
Overview of Studies
The main characteristics of these studies are shown in Table 1. At least two authors verified the accuracy of the details listed in the table that was subsequently also used in the exploratory analyses. In total, 672 participants were included in the meta-analysis. Most of these studies used relatively young, healthy samples under the age of 25 (67%). Several studies had multiple support assessments (33%), and the most common measures examined were perceived support (77%), followed by social integration (57%), and received support (22%).
Table 1.
Main study characteristics
| Study | n | Age | Vaccine type | Periods | Support measure | Main outcome | Effect size Zr | Var(Zr) |
|---|---|---|---|---|---|---|---|---|
| Snyder et al. (1990) [38] | 89 | 21 | KLH | Baseline, 3 weeks, 8 weeks | RSS | IgG Ab titers | .000 | .011 |
| Glaser et al. (1992) [39] | 35 | 23.3 | Hep B | Baseline, 1 month, 6 m months | PSS | Composite of HBsAg Ab titers and T-cell response from 2nd to 3rd periods | .485 | .031 |
| Moynihan et al. (2004) [20] | 37 | 84 | Flu: NC, HK, Pan | Baseline, 3 weeks | PSS | HAI Ab titers | -.192 | .029 |
| Phillips et al. (2005) [21] | 57 | 19.8 | Flu: NC, Pan, Shan; Mening A, C | Baseline, 5 weeks, 5 months | PSS/SI | HAI/IgG Ab titers | .023 | .018 |
| Pressman et al. (2005) [23] | 83 | 18–25 | Flu: NC, Pan, Yam/Vict | Baseline, 1 month, 4 months | SI | HAI Ab titers | .090 | .013 |
| Phillips et al. (2006) [23] | 104 | 74.6 | Flu: NC, Pan, Shan | Baseline, 1 month, 12 months | SI, PSS | HAI Ab titers | .000 | .010 |
| Li et al. (2007) [41] | 119 | 57.1 | Tetanus | Baseline, 4 weeks | RSS | IgG Ab titers | .131 | .009 |
| Gallagher et al. (2008) [42] | 74 | 23 | Pneum | Baseline, 5 days | PSS | IgM Ab titers | .070 | .014 |
| Gallagher et al. (2008) [43] | 74 | 22.9 | Primary (Pneum), Hep A | Baseline, 4 weeks, 18 weeks | PSS, SI | IgG Ab titers | .030 | .014 |
Cumulative Zr = .06, 95% CI [−0.04, 0.16]. PSS perceived social support; RSS received social support; SI social integration.
What Is the Overall Link Between Social Support, Social Integration, and Antibody Titers?
As shown in Table 1, 67% of the studies showed a positive association between social support/integration and antibody titers. Across nine studies, the weighted random effect size was in the expected direction (i.e., social support being linked to higher antibody titers to vaccination), but the CI included 0 (Zr = .06, 95% CI [−0.04, 0.16]). The test of study-level heterogeneity was not significant (Q [8] = 8.52, p = .38), but given the likelihood of true study heterogeneity in biomedical research [40], a random effects analysis was used. A direct test of the association between effect sizes and sample sizes revealed a nonsignificant link suggesting no sample size bias (Kendall’s Tau = .03, p = .92).
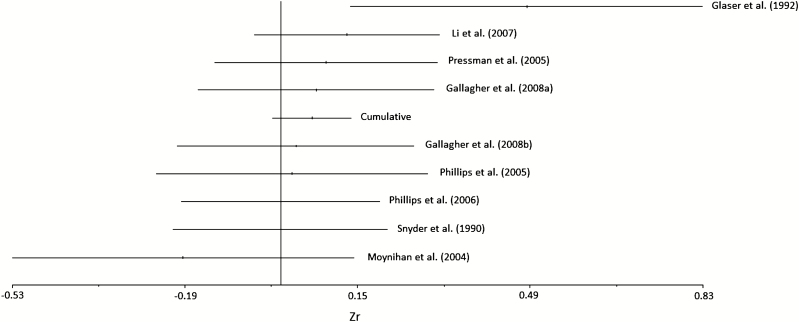
Effect sizes for the studies ranged from Zr = −.19 to Zr = .49 (see Fig. 2). Of these, only one study showed a negative correlation (i.e., social support being related to lower antibody titers, Moynihan et al. [20]). In addition, this was one of the only studies that did not control or take into account baseline antibody titers which could influence post-vaccine analyses. As a result, this study was deleted to examine whether the links were stronger across the remaining studies. However, the effect size only increased slightly and was still not significant although it pointed in the expected direction (Zr = .08, 95% CI [−0.02, 0.17]).
Fig. 2.
Forest plot of effect sizes and confidence intervals.
Exploratory analyses were conducted to descriptively characterize the effect sizes based on several conceptual and methodological variables. First, the type of support was examined. The effect size appeared only slightly larger for received support (Zr = .07, 95% CI [−0.88, 1.02]) compared with perceived support (Zr = .04, 95% CI [−0.10, 0.18]) and social integration (Zr = .04, 95% CI [−0.15, 0.23]). Second, older individuals tend to have lower seroconversion rates to vaccines given age-related declines in immune functioning [31]. The effect size for younger individuals (i.e., less than 30 years) appeared only slightly larger compared with older individuals (Zr = .08, 95% CI [−0.06, 0.23] vs. Zr = .03, 95% CI [−0.29, 0.35]). Variations based on the follow-up assessment periods were also examined. In total, five studies included an early and later follow-up of antibody titers and related them to social support. The effect sizes appeared comparable for the first (Zr = .04, 95% CI [−0.10, 0.18]) and second (Zr = .02, 95% CI [−0.12, 0.16]) assessment periods in these studies. Finally, a distinction was also made between primary and secondary vaccine responses. This classification produced the largest absolute difference as associations were larger for primary versus secondary responses (Zr = .15, 95% CI [−1.16, 1.45] vs. Zr = .04, 95% CI [−0.08, 0.16], respectively). Of course, the relatively large CI for primary responses suggests some caution in making conclusions.
Discussion
The main aim of this meta-analytic review was to examine whether social support predicted higher antibody titers to vaccination. Such an association would be consistent with epidemiological research that has linked support to lower overall mortality rates (Holt-Lunstad [3]). Indeed, respiratory infections are one of the leading cause of death globally, especially in low-income countries [44]. Across all studies to date, the effect size linking social support to antibody titers following vaccination was small (Zr = .06) and its CI included zero [−0.04, 0.16]. Although the bulk of the plausible values are positive, more evidence is needed on the topic [45].
It is important to note that the number of studies included in this meta-analysis was small. However, small meta-analyses are frequently conducted, especially when they ask a specific question with similar approaches [45]. It is also important to conduct such an analysis given the logistics and costs of running such studies. Vaccine protocols are time intensive and relatively invasive compared with other common biological assessments. They involve longitudinal designs with multiple intravenous blood draws and assays to quantify antibody titers. The expense and difficulty of doing such studies is reflected by the fact that these nine studies were published over an 18-year period (1990–2008), with the last one published over 10 years ago. It is thus unlikely that this literature will grow considerably in the foreseeable future and this review can inform current theory and future work in the area.
A second, exploratory aim was to examine effect sizes and CI to guide future research. Effect sizes appeared to vary little as a function of support type, participant age, and follow-up period. Effect sizes appeared to vary more considerably for primary challenges (Zr = .15) versus secondary challenges (Zr = .04). Primary antigens are new to the body and hence prior exposure is limited as a complicating factor. This is an important issue as social support is associated with greater social contact [46], which is a primary mode of infectious disease transmission. This might make it more difficult to detect links between social support and Ab titers due to ceiling effects and/or reduced variability. The use of a primary antigen reduces such concerns and hence may be a stronger test of links between social support and vaccine responses. Of course, there were only two studies that examined a primary immune response and the CI around this estimate was relatively large [39, 47]. If this trend is correct, future studies examining secondary immune responses might need larger samples sizes to detect smaller associations.
The exploratory analyses also suggested that the effect size for perceived support was small, which appears inconsistent with prior epidemiological work. One possible area for future work is to examine the stress-buffering model of support [26]. Although several studies assessed both stress and support, only one study directly examined the stress-buffering hypothesis. This study found preliminary evidence that social support buffered the influence of anxiety on antibody responses to the vaccine [47]. Future research examining the stress-buffering model will not only aide in a conceptual understanding of how social support operates in vaccine models but also potentially explain additional variance in outcomes as the above study did not find a significant main effect of social support.
More generally, this research also suggests the importance of examining other indicators of immune responses to vaccination in order to examine which aspects of the integrative immune system may be compromised by low social support. For instance, the first study in this area by Snyder et al. [47] did not find that social support was significantly related to antibody titers to keyhole limpet hemocyanin (a primary antigen). However, a subsequent paper did show social support to predict a greater proliferative response of immune cells to stimulation by keyhole limpet hemocyanin with effect sizes ranging from r = .13 to .23 [38]. This is consistent with early studies showing that social support predicts proliferative lymphocyte responses to mitogens [48–50]. This is important because although antibody titers do confer protection, the immune system has other mechanisms of protection depending on the challenge at hand. For instance, the T-cell response is also critical for the resolution of intracellular pathogens such as viruses and some bacteria [11].
Research in this domain should also examine potential neuroendocrine mediators. Prior work has linked social support to lower levels of catecholamines, cortisol, and higher oxytocin levels [7, 51]. Importantly, many immune cells (e.g., lymphocytes) have functional receptors for neuroendocrine hormones, which provide a direct route for neuroimmune modulation [52, 53]. Recent research is also highlighting the role of the parasympathetic nervous system (as reflected by respiratory sinus arrhythmia) on social/regulatory functioning [54, 55]. Future work will be needed to directly model these neuroendocrine pathways as mediators of the link between social support and vaccine responses.
Given the correlational nature of the links between social support and vaccine responses in this review, future research should consider alternative designs that could produce larger effect sizes and stronger inferences. For instance, support interventions that include the involvement of family, peers, and individuals with experiential similarity could be conducted over time following vaccination and compared with relevant control conditions [3, 56]. In addition, although largely untested in the health domain, compassion-based practices (e.g., loving-kindness meditation) could be used as they appear to positively influence health-relevant psychological outcomes such as depression and positive affect [57, 58]. Finally, daily life protocols might also be possible using text-based short message services to enhance support although such research is in its infancy [59, 60]. Relatedly, results suggested a positive effect of received support, although the CI around this estimate was wide. Nevertheless, given mixed evidence regarding links between received support and health, future work may benefit from examining this association in greater detail.
It should be emphasized that the conclusion of this meta-analysis is not that researchers should abandon using vaccine protocols for social support studies given the nonsignificant link. The CI associated with the omnibus test highlights the importance of more research on this topic given the larger range for a protective link [45]. Indeed, meta-analytic evidence exists linking other psychosocial processes such as chronic and perceived stress to lower antibody titers to the flu vaccine [61]. It is likely that the increased social contact associated with social support complicates many such studies that often rely on secondary immune responses. Future research will need to consider this issue and carefully consider how to take them into account in future studies (e.g., assessments of social contact, prior vaccine history, etc.). In addition, the overall effect size is relatively small, so larger sample sizes will be needed to potentially detect associations (n’s for the current studies ranged from 35 to 154), with potentially even larger sample sizes for detecting antibody responses to secondary antigen. Modeling stress-buffering influences or implementing social support manipulations over time as part of the vaccination protocol may also be useful to increase effect sizes as noted earlier.
In summary, the link between social support and antibody responses during vaccination appears to be small and positive, but is statistically inconclusive and hence is in need of more research. The relatively small effect size appears inconsistent with the epidemiological evidence showing an association between social support and all-cause mortality given that infectious diseases are among the leading causes of death worldwide. This review also raised several important issues that will need to be considered in future work including sample size, type of immune response, and the incorporation of other indicators of immune system function. Nevertheless, the vaccination protocol remains an important paradigm for this area (and others in psychoneuroimmunology) given its ability to test an integrative and clinically significant immune response to potential pathogens.
Acknowledgment
The authors have full control of all primary data and that they agree to allow the journal to review their data if requested.
Funding
This research was generously supported by the National Heart, Lung, and Blood Institute (grant number R01HL137606; PI: Bert N. Uchino).
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors declare that they have no conflict of interest.
Authors’ Contributions B.N.U. produced a draft of the manuscript, conducted the literature review, and performed the primary meta-analysis. J.L. provided comments on earlier and the final drafts of the paper. He also served as a reliability check for the main study characteristics. K.Z. provided comments on earlier and final drafts of the paper. She also served as a reliability check for the main study characteristics. N.B. provided comments on both earlier and final drafts of the paper. He also served as a consultant on the meta-analytic procedures.
References
- 1. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: A meta-analytic review. PLoS Med. 2010;7:e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. [DOI] [PubMed] [Google Scholar]
- 3. Holt-Lunstad J. Why social relationships are important for physical health: A systems approach to understanding and modifying risk and protection. Annu Rev Psychol. 2019;69:437–458. [DOI] [PubMed] [Google Scholar]
- 4. Cohen S, Lemay EP. Why would social networks be linked to affect and health practices? Health Psychol. 2007;26:410–417. [DOI] [PubMed] [Google Scholar]
- 5. Uchino BN, Bowen K, Kent R, Mikal J, Fisher EB. Social support and physical health: Models, mechanisms, and opportunities. In: Fisher EB, Cameron L, Christensen A, Ehlert U, Oldenburg B, Snoek F, eds. Principles and Concepts of Behavioral Medicine: A Global Handbook. New York: Springer; 2018:341–372. [Google Scholar]
- 6. Coan JA, Beckes L, Gonzalez MZ, Maresh EL, Brown CL, Hasselmo K. Relationship status and perceived support in the social regulation of neural responses to threat. Soc Cogn Affect Neurosci. 2017;12:1574–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uchino BN, Way BM. Integrative pathways linking close family ties to health: A neurochemical perspective. Am Psychol. 2017;72:590–600. [DOI] [PubMed] [Google Scholar]
- 8. Stadler G, Snyder KA, Horn AB, Shrout PE, Bolger NP. Close relationships and health in daily life: A review and empirical data on intimacy and somatic symptoms. Psychosom Med. 2012;74:398–409. [DOI] [PubMed] [Google Scholar]
- 9. Bakermans-Kranenburg MJ, van I Jzendoorn MH. Sniffing around oxytocin: Review and meta-analyses of trials in healthy and clinical groups with implications for pharmacotherapy. Transl Psychiatry. 2013;3:e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: Epidemiologic evidence and implications for treatment. Psychosom Med. 2005;67:869–878. [DOI] [PubMed] [Google Scholar]
- 11. Abbas AK, Lichtman AH, Pillai S.. Basic Immunology: Functions and Disorders of the Immune System. 5th ed. St. Louis, MO: Elsevier; 2016. [Google Scholar]
- 12. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. [DOI] [PubMed] [Google Scholar]
- 13. Levy SM, Herberman RB, Whiteside T, Sanzo K, Lee J, Kirkwood J. Perceived social support and tumor estrogen/progesterone receptor status as predictors of natural killer cell activity in breast cancer patients. Psychosom Med. 1990;52:73–85. [DOI] [PubMed] [Google Scholar]
- 14. McNaughton ME, Smith LW, Patterson TL, Grant I. Stress, social support, coping resources, and immune status in elderly women. J Nerv Ment Dis. 1990;178:460–461. [DOI] [PubMed] [Google Scholar]
- 15. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 16. Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: An expanded “cardiovascular continuum”. J Am Coll Cardiol. 2016;67:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. [DOI] [PubMed] [Google Scholar]
- 18. Uchino BN, Trettevik R, Kent de Grey RG, Cronan S, Hogan J, Baucom BRW. Social support, social integration, and inflammatory cytokines: A meta-analysis. Health Psychol. 2018;37:462–471. [DOI] [PubMed] [Google Scholar]
- 19. Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM. Social ties and susceptibility to the common cold. JAMA. 1997;278:1231. [PubMed] [Google Scholar]
- 20. Moynihan JA, Larson MR, Treanor J, et al. Psychosocial factors and the response to influenza vaccination in older adults. Psychosom Med. 2004;66:950–953. [DOI] [PubMed] [Google Scholar]
- 21. Phillips AC, Burns VE, Carroll D, Ring C, Drayson M. The association between life events, social support, and antibody status following thymus-dependent and thymus-independent vaccinations in healthy young adults. Brain Behav Immun. 2005;19:325–333. [DOI] [PubMed] [Google Scholar]
- 22. Phillips AC, Carroll D, Burns VE, Ring C, Macleod J, Drayson M. Bereavement and marriage are associated with antibody response to influenza vaccination in the elderly. Brain Behav Immun. 2006;20:279–289. [DOI] [PubMed] [Google Scholar]
- 23. Pressman SD, Cohen S, Miller GE, Barkin A, Rabin BS, Treanor JJ. Loneliness, social network size, and immune response to influenza vaccination in college freshmen. Health Psychol. 2005;24:297–306. [DOI] [PubMed] [Google Scholar]
- 24. Cohen S. Social relationships and health. Am Psychol. 2004;59:676–684. [DOI] [PubMed] [Google Scholar]
- 25. Uchino BN. Understanding the links between social support and physical health: A life-span perspective with emphasis on the separability of perceived and received support. Perspect Psychol Sci. 2009;4:236–255. [DOI] [PubMed] [Google Scholar]
- 26. Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 27. Uchino BN. Social Support and Physical Health: Understanding the Health Consequences of Relationships. Yale University Press; 2004. [Google Scholar]
- 28. Wills TA, Shinar O. Measuring perceived and received social support. In: Cohen S, Gordon L, Gottlieb B, eds. Social Support Measurement and Intervention: A Guide for Health and Social Scientists. New York: Oxford University Press; 2000:86–135. [Google Scholar]
- 29. Bolger N, Amarel D. Effects of social support visibility on adjustment to stress: Experimental evidence. J Pers Soc Psychol. 2007;92:458–475. [DOI] [PubMed] [Google Scholar]
- 30. Zee KS, Cavallo JV, Flores AJ, Bolger N, Higgins ET. Motivation moderates the effects of social support visibility. J Pers Soc Psychol. 2018;114:735–765. [DOI] [PubMed] [Google Scholar]
- 31. Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: The challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. [DOI] [PubMed] [Google Scholar]
- 32. Akha AAS. Aging and the immune system: An overview. J Immunol Methods. 2018;463:21–26. [DOI] [PubMed] [Google Scholar]
- 33. Whittaker AC. The vaccination model in psychoneuroimmunology research: A review. Methods Mol Biol. 2018;1781:309–326. [DOI] [PubMed] [Google Scholar]
- 34. Rosenberg MS, Adams DC, Gurevitch J.. MetaWin: Statistial Software for Meta-Analysis. Sunderland, MA: Sinauer Associates; 2000. [Google Scholar]
- 35. Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 36. Peterson RA, Brown SP. On the use of beta coefficients in meta-analysis. J Appl Psychol. 2005;90:175–181. [DOI] [PubMed] [Google Scholar]
- 37. Rosenthal R. Meta-Analytic Procedures for Social Research. Newbury Park, CA: Sage; 1984. [Google Scholar]
- 38. Snyder BK, Roghmann KJ, Sigal LH. Stress and psychosocial factors: Effects on primary cellular immune response. J Behav Med. 1993;16:143–161. [DOI] [PubMed] [Google Scholar]
- 39. Glaser R, Kiecolt-Glaser JK, Bonneau RH, Malarkey W, Kennedy S, Hughes J. Stress-induced modulation of the immune response to recombinant hepatitis B vaccine. Psychosom Med. 1992;54:22–29. [DOI] [PubMed] [Google Scholar]
- 40. Kenny DA, Judd CM. The unappreciated heterogeneity of effect sizes: Implications for power, precision, planning of research, and replication. Psychol Methods. 2019;1:1–12. [DOI] [PubMed] [Google Scholar]
- 41. Li J, Cowden LG, King JD, Briles DA, et al. Effects of chronic stress and interleukin-10 gene polymorphisms on antibody response to tetanus vaccine in family caregivers of patients with Alzheimer’s disease. Psychosom Med. 2007;69(6):551–559. [DOI] [PubMed] [Google Scholar]
- 42. Gallagher S, Phillips AC, Ferraro AJ, Drayson MT, Carroll D. Social support is positively associated with the immunoglobulin M response to vaccination with pneumococcal polysaccharides. Biol Psychol. 2008;78(2):211–215. [DOI] [PubMed] [Google Scholar]
- 43. Gallagher S, Phillips AC, Ferraro AJ, Drayson MT, Carroll D. Psychosocial factors are associated with the antibody response to both thymus-dependent and thymus-independent vaccines. Brain Behav Immun. 2008;22(4):456–460. [DOI] [PubMed] [Google Scholar]
- 44. WHO. The Top 10 Causes of Death 2018. https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death. Accessibility verified March 20, 2019.
- 45. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? J Educ Behav Stat. 2010;35:215–247. [Google Scholar]
- 46. Peirce RS, Frone MR, Russell M, Cooper ML, Mudar P. A longitudinal model of social contact, social support, depression, and alcohol use. Health Psychol. 2000;19:28–38. [DOI] [PubMed] [Google Scholar]
- 47. Snyder BK, Roghmann KJ, Sigal LH. Effect of stress and other biopsychosocial factors on primary antibody response. J Adolesc Health Care. 1990;11:472–479. [DOI] [PubMed] [Google Scholar]
- 48. Kiecolt-Glaser JK, Dura JR, Speicher CE, Trask OJ, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. [DOI] [PubMed] [Google Scholar]
- 49. Thomas PD, Goodwin JM, Goodwin JS. Effect of social support on stress-related changes in cholesterol level, uric acid level, and immune function in an elderly sample. Am J Psychiatry. 1985;142:735–737. [DOI] [PubMed] [Google Scholar]
- 50. Baron RS, Cutrona CE, Hicklin D, Russell DW, Lubaroff DM. Social support and immune function among spouses of cancer patients. J Pers Soc Psychol. 1990;59:344–352. [DOI] [PubMed] [Google Scholar]
- 51. Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377–387. [DOI] [PubMed] [Google Scholar]
- 52. Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–669. [DOI] [PubMed] [Google Scholar]
- 53. Uchino B., Glaser R, Kiecolt-Glaser JK. Psychological modulation of cellular immunity. In: Cacioppo JT, Tassinary LG, Berntson GG, eds. Handbook of Psychophysiology. New York, NY: Cambridge University Press; 2000:397–424. [Google Scholar]
- 54. Kok BE, Coffey KA, Cohn MA, et al. How positive emotions build physical health: Perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol Sci. 2013;24:1123–1132. [DOI] [PubMed] [Google Scholar]
- 55. Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dam AE, de Vugt ME, Klinkenberg IP, Verhey FR, van Boxtel MP. A systematic review of social support interventions for caregivers of people with dementia: Are they doing what they promise? Maturitas. 2016;85:117–130. [DOI] [PubMed] [Google Scholar]
- 57. Kirby JN, Tellegen CL, Steindl SR. A meta-analysis of compassion-based interventions: Current state of knowledge and future directions. Behav Ther. 2017;48:778–792. [DOI] [PubMed] [Google Scholar]
- 58. Zeng X, Chiu CP, Wang R, Oei TP, Leung FY. The effect of loving-kindness meditation on positive emotions: A meta-analytic review. Front Psychol. 2015;6:1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Volpp KG, Troxel AB, Mehta SJ, et al. Effect of electronic reminders, financial incentives, and social support on outcomes after myocardial infarction: The HeartStrong randomized clinical trial. JAMA Intern Med. 2017;177:1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schwebel FJ, Larimer ME. Using text message reminders in health care services: A narrative literature review. Internet Interv. 2018;13:82–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pedersen AF, Zachariae R, Bovbjerg DH. Psychological stress and antibody response to influenza vaccination: A meta-analysis. Brain Behav Immun. 2009;23:427–433. [DOI] [PubMed] [Google Scholar]