Abstract
Background
Conditioned pain modulation (CPM) is a task that involves measuring pain in response to a test stimulus before and during a painful conditioning stimulus (CS). The CS pain typically inhibits pain elicited by the test stimulus; thus, this task is used to assess endogenous pain inhibition. Moreover, less efficient CPM-related inhibition is associated with chronic pain risk. Pain catastrophizing is a cognitive-emotional process associated with negative pain sequelae, and some studies have found that catastrophizing reduces CPM efficiency.
Purpose
The current study examined the relationship between catastrophizing (dispositional and situation specific) and CPM-related inhibition of pain and the nociceptive flexion reflex (NFR; a marker of spinal nociception) to determine whether the catastrophizing–CPM relationship might contribute to the higher risk of chronic pain in Native Americans (NAs).
Methods
CPM of pain and NFR was assessed in 124 NAs and 129 non-Hispanic Whites. Dispositional catastrophizing was assessed at the beginning of the test day, whereas situation-specific catastrophizing was assessed in response to the CS, as well as painful electric stimuli.
Results
Situation-specific, but not dispositional, catastrophizing led to less NFR inhibition but more pain inhibition. These effects were not moderated by race, but mediation analyses found that: (a) the NA race was associated with greater situation-specific catastrophizing, which led to less NFR inhibition and more pain inhibition, and (b) situation-specific catastrophizing was associated with greater CS pain, which led to more pain inhibition.
Conclusions
Catastrophizing may contribute to NA pain risk by disrupting descending inhibition.
Keywords: Pain modulation, Racial/ethnic differences, Coping, Catastrophizing, Chronic pain risk
Pain catastrophizing (rumination, magnification, and helplessness in the face of pain) may increase chronic pain risk in Native American by disrupting endogenous descending pain inhibitory processes
Introduction
Pain is a subjective experience that can be altered by motivational, emotional, and contextual variables [1]. Typically, this involves a descending circuit emanating from the brain that inhibits or facilitates nociceptive processing within the spinal cord dorsal horn [2, 3]. The supraspinal regions most notably involved with this descending circuit are the anterior cingulate cortex, amygdala, hippocampus, periaqueductal gray (PAG), and rostral ventromedial medulla (RVM) [4].
Conditioned Pain Modulation: A Tool to Study Pain Inhibition
Conditioned pain modulation (CPM) is an experimental task often used to assess the integrity of pain inhibitory circuitry. It involves assessing pain from a test stimulus that is inhibited by simultaneous application of a second painful conditioning stimulus (CS) at a distal, heterotopic site (i.e., pain inhibits pain) [5]. In animals, this effect is called diffuse noxious inhibitory controls (DNIC; 6), and studies with rodents have shown that: (a) the noxious CS inhibits nociceptive spinal neurons via a descending circuit from the brainstem and (b) the degree of inhibition is dependent on the intensity (and painfulness) of the CS. Human studies have similarly shown that the nociceptive flexion reflex (NFR; a spinally mediated withdrawal reflex used to assess spinal nociception) is inhibited by a painful CS, and the degree of inhibition is dependent on the intensity of the CS [6]. Moreover, NFR does not rely on self-report of pain, which can be biased by contextual factors [7, 8]. In this way, NFR is a useful physiological measure of the consequences of CPM on spinal nociceptive processes. However, few laboratories assess CPM of NFR.
Studies have shown that less efficient CPM-related pain inhibition is associated with a number of chronic pain states (e.g., osteoarthritis, irritable bowel syndrome, and fibromyalgia) [5, 9, 10]. It is also noteworthy that less efficient presurgical CPM-related pain inhibition is associated with the subsequent onset of postsurgical chronic pain [11, 12]. This not only points to the importance of CPM as a way to assess pain risk but also to the importance of identifying variables that influence CPM-related inhibition.
Pain Catastrophizing and Pain Modulation
Pain catastrophizing is a cognitive-emotional process associated with rumination, magnification, and helplessness in the face of pain [13], and higher pain catastrophizing is associated with many negative pain-related sequelae (e.g., higher pain and greater pain-related disability) [14]. Recent studies have noted that both dispositional/trait-like pain catastrophizing (i.e., how a person catastrophizes during pain in general) [15] and situation-specific/state-like pain catastrophizing (i.e., how a person catastrophizes during a specific painful event) [16] can decrease CPM efficiency. However, the catastrophizing–CPM relationship is not always found [16–18] and, under some circumstances, greater catastrophizing is surprisingly associated with better CPM-related pain inhibition [19].
Native American Pain Risk
Research suggests that Native Americans (NAs) are at greater risk for chronic pain conditions as evidenced by higher pain prevalence rates than other U.S. groups [20]. Recently, we found that, compared to non-Hispanic Whites (NHWs), NAs experienced more situation-specific catastrophizing during painful events, even though their level of dispositional catastrophizing did not differ [21]. We speculated that this could promote chronic pain in NAs by contributing to a vicious cycle (pain→catastrophizing→pain), but it could also contribute to pain risk by decreasing the efficiency of pain inhibitory processes. To date, this issue has not been examined in NAs. Nonetheless, we have shown that NAs do not differ from NHWs in their ability to inhibit pain or NFR during CPM [22], but that study did not examine whether the relationship between pain catastrophizing and CPM was moderated by race nor did it examine whether increased pain catastrophizing could mediate the relationship between race and CPM-related inhibition. Indeed, Preacher and Hayes [23] have shown that a significant relationship between a predictor and a dependent variable is not necessary for there to be a significant indirect effect through a mediator. For this reason, it is important to test the indirect effect (i.e., NA race→catastrophizing→CPM efficacy) given that there is theoretical justification, even though we did not previously find a relationship between NA race and CPM [22].
The Present Study
This study examined the relationship between pain catastrophizing (dispositional and situation specific) and CPM of pain and CPM of NFR in healthy, pain-free participants (n = 124 NAs, n = 129 NHWs). Pain-free individuals were recruited so that we could identify potential chronic pain risk factors that are present before the onset of chronic pain because group differences found after chronic pain onset could be due to differences in disease status, access to health care, or other confounds.
There were two primary aims. First, we used moderation analysis to examine whether the relationship between catastrophizing and CPM of pain/NFR differed between NAs and NHWs. This allows us to determine whether catastrophizing might play a greater (or lesser) role in NA endogenous pain modulation. We predicted that pain catastrophizing would be associated with decreased efficiency of CPM-related inhibition and that this effect would be stronger in NAs (thus moderated by race). Second, we used bootstrapped mediation analyses to test whether the higher rate of situation-specific catastrophizing in NAs could provide an indirect pathway by which NAs could experience reduced CPM efficiency (i.e., NA race→situation-specific catastrophizing→reduced CPM). If supported, this would suggest a mechanistic linkage between NAs and reduced efficiency of endogenous pain modulation.
And finally, we assessed a novel, exploratory issue. As noted above, evidence suggests that the painfulness of the CS is associated with the degree of CPM-related inhibition (i.e., more CS pain = more CPM inhibition). Given that, we assessed situation-specific catastrophizing that occurred during the painful CS. This allowed us to use mediation to test whether situation-specific pain catastrophizing would influence the perceived intensity of the CS and, thus, influence the degree of CPM (i.e., situation-specific catastrophizing→CS pain→CPM inhibition).
Methods
Participants
Participants were healthy, pain-free individuals recruited as part of the Oklahoma Study of Native American Pain Risk (OK-SNAP). OK-SNAP is a 2 day study that investigated risk factors of chronic pain for NA and NHW populations. Participant recruitment involved fliers, email announcements, in-person meetings with community stakeholders, and newspaper and online ads (i.e., Facebook and Craigslist). Participants were excluded for the following criteria: (a) <18 years old, (b) history of cardiovascular, neuroendocrine, musculoskeletal, or neurological disorders, (c) current self-reported acute or chronic pain, (d) body mass index (BMI) ≥35 (due to difficulties obtaining the NFR), (e) use of antidepressant, anxiolytic, analgesic, stimulant, or antihypertensive medication, (f) current psychotic symptoms (assessed by Psychosis Screening Questionnaire) [24] or self-reported substance use problems, and/or (g) an inability to read/speak English. Participants who endorsed NA ancestry presented the verification of their heritage (e.g., Certificate of Degree of Indian Blood and tribal affiliation card). NA participants from this study represent tribal nations that predominately reside within the southern plains and eastern Oklahoma regions. All procedures for this study were approved by the institutional review boards of the Cherokee Nation, University of Tulsa, and the Indian Health Service Oklahoma City Area Office.
Prior to study participation, participants were given an overview of all procedures and provided verbal and written consent. Participants were told that they could withdraw consent at any time and received $100 honorarium for the completion of each testing day (or $10 per hour for every hour completed). Of the 305 participants (165 women and 155 NAs) enrolled in the larger study, 253 attended the testing day that included CPM and completed some CPM testing (see Results for sample characteristics).
Power/Sensitivity Analysis
The present study is an ancillary analysis of data collected from OK-SNAP. Given this, a priori power analysis was not conducted for this particular study. However, sensitivity analysis using G*Power 3.1.9.2 (Kiel, Germany) suggests that, with 253 participants and α = .05, this would provide power = .80 to detect the significance of any individual predictor in our models as long as the predictor explained at least 3% of the variance (i.e., effect size f = .177) in the dependent variable. Given this, we believe that the current sample provides us adequate power to conduct these analyses.
General Testing Day Procedures
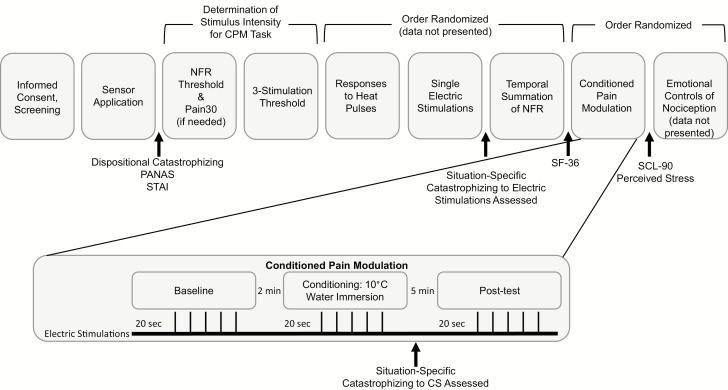
Testing was conducted over a 2 day period with each testing day approximately 4–6 hr long. Figure 1 depicts procedures performed on the CPM testing day (the testing day order was randomized across participants). Following participant consent, the experimenter administered a health screen to ensure eligibility. Following this procedure, the experimenter applied electrodes and sensors.
Fig. 1.
Study procedures that took place on the conditioned pain modulation (CPM) testing day. NFR = nociceptive flexion reflex.
Pain tests were pseudorandomized with the testing day, with the limitation that NFR threshold, Pain30, and three-stimulation threshold assessments were always administered first, second and third, respectively. These determined the electric test stimulus intensity that was used during the CPM procedure (see description below). The CPM task was administered near the end of the testing day. Breaks were provided in between tasks to minimize carryover effects.
Apparatus, Electrode Application, and Signal Acquisition
Procedures were controlled by a computer with dual monitors, analog-to-digital board (USB-6212 BNC; National Instruments, Austin, TX), and LabVIEW software (National Instruments). Participants completed study procedures in an experimental room and used one monitor to complete electronic questionnaires and provide pain ratings, while a researcher located in an adjacent room monitored participant physiology via the second monitor. In addition to monitoring physiology, the researcher monitored the participant via a video camera for study procedure compliance. Testing was conducted in a sound-attenuated and electrically shielded room. Throughout testing, participants wore sound-attenuating headphones to listen to prerecorded instructions and communicate with the experimenter.
Test stimuli
Electric test stimuli were delivered to the left ankle over the retromalleolar pathway of the sural nerve by a constant current stimulator (Digitimer DS7A; Hertfordshire, UK) and a bipolar electrode (Nicolet, Model#019-40400, Madison, WI). Each electric stimulation was delivered as a train of five 1 ms rectangular wave pulses at 250 Hz and was perceived as a single stimulus. The timing of the delivery of electric stimuli was computer controlled. For safety purposes, the maximum intensity of each electric stimulation intensity was set to 50 mA.
Conditioning stimulus
The CS was a circulating cold-water bath (Thermo Fisher Scientific, Pittsburgh, PA) maintained at a constant temperature of 10 ± 0.1°C. This temperature was chosen because pilot testing determined that it elicited strong pain that could be tolerated for 2 mins by the majority of participants. During conditioning, participants were instructed to submerge their right hand up to their forearm in the water (~6” deep) and to keep their hand palm down with fingers spread for 2 mins.
NFR recording
To assess the NFR electromyography (EMG), two Ag–AgCl electrodes were applied over the left biceps femoris muscle (located approximately 10 cm superior to the popliteal fossa). The EMG signal was filtered (10–300 Hz) and amplified (×10,000) using a Grass Technologies (West Warwick, RI) Model 15LT amplifier (with AC Module 15A54). An electrode was placed over the lateral epicondyle of the femur to serve as a common ground. The participant’s skin was cleaned with alcohol and exfoliated (NuPrep gel; Weaver and Company, Aurora, CO) to achieve impedances ≤5 kΩ for EMG and stimulating electrode. Electrodes were filled with conductive gel (EC60, Grass Technologies), and EMG signals were sampled at 1,000 Hz.
Questionnaires
The Pain Catastrophizing Scale was administered to assess dispositional and situation-specific pain catastrophizing, whereas several other questionnaires were collected to ensure that groups did not differ on important background characteristics that might confound group comparisons on primary pain outcomes.
Demographics and health exclusion
To assess background information (e.g., age, biological sex, socioeconomic status, and health status), a custom-made demographics form was administered. This information was used to describe the sample and evaluate eligibility criteria. BMI was calculated using a medical scale.
Current affect
Positive and negative affect was assessed at the beginning of the study from the Positive and Negative Affect Schedule (PANAS; [25]). Each subscale for this measure consists of 10 items that measure positive and negative emotions, with subscales ranging from 0 to 40. Higher scores on each scale indicate greater positive and negative affects.
State anxiety
State anxiety at the beginning of the study was assessed with the 20-item state anxiety subscale of the State Trait Anxiety Inventory [26]. This subscale ranges from 20 to 80, with higher scores indicating greater current anxiety.
Psychological symptoms/problems
Psychological symptoms were assessed with the Global Severity Index (GSI) of the Symptom Checklist-90-Revised (SCL-90-R) [27]. Scores range from 0 to 4, with higher scores indicating more problems.
Perceived stress
The Perceived Stress Scale (PSS) is a 10-item measure that assesses psychological stress within the past month. Scores range from 0 to 40, with higher scores indicating more perceived stress [28].
Physical and mental health perceptions
Perceptions of health were assessed from two subscales of the Medical Outcomes Study 36-item Short Form Health Survey (SF-36) [29]: Bodily Pain Scale (assessing pain that occurred in the past 4 weeks) and General Health. Both subscales were standardized to range from 0 to 100, with higher scores indicating better functioning.
Pain catastrophizing
Dispositional (trait-like) pain catastrophizing was assessed using the 13-item Pain Catastrophizing Scale (PCS) [30]. Scores ranged from 0 to 52, with higher scores indicating greater tendencies for catastrophic thoughts during pain. Situation-specific pain catastrophizing represents catastrophic thoughts that happened during a specific painful event. To assess this, a modified version of PCS was used [31]. Rather than have participants report on catastrophic thoughts about pain in general, participants were asked to think back on their thoughts during a painful task and indicate the degree to which they catastrophized [14]. Thus, the situation-specific version was administered immediately after painful tasks. Scores for the situation-specific PCS also ranged from 0 to 52, with higher scores indicating greater catastrophic thoughts. Situation-specific catastrophizing was measured in response to the cold-water CS used in the CPM task. Although situation-specific catastrophizing to the electric test stimuli used during CPM was not measured, it was measured in reaction to electric stimulations delivered during another task (see Fig. 1). Situation-specific catastrophizing to the CS was used in all primary analyses, but we also reran all analyses using situation-specific catastrophizing in response to the electric stimuli to determine whether results were specific to catastrophizing during the CS. In the present study, the correlation between dispositional catastrophizing and situation-specific catastrophizing was r = .37 (during CS pain) and r = .47 (during electric pain), whereas the correlation between the two situational catastrophizing variables was r = .61. This provides evidence that dispositional and situational catastrophizing are not perfectly overlapping constructs. In the current study, means, standard deviations (SDs), and min/max values for pain catastrophizing variables were: trait catastrophizing (M = 9.81, SD = 7.87, min = 0, max = 34), situation-specific catastrophizing to cold-water CS (M = 17.54, SD = 13.87, min = 0, max = 52), situation-specific catastrophizing to electric pain (M = 8.95, SD = 10.73, min=0, max=52).
Determination of Stimulus Intensity for Electric Test Stimuli Used During CPM
To determine the electric stimulus intensity (in mA) to use during CPM, three procedures were conducted: NFR threshold, Pain30 (if necessary; see below), and three-stimulation threshold. These procedures were employed prior to CPM and were used to ensure that stimuli reliably evoked NFRs and were perceived as painful.
Following each electric stimulus during these procedures, participants rated their pain intensity on a computer-presented visual analog scale (VAS) that ranged from “no pain sensation” to “the most intense pain sensation imaginable.” The computer converted the rating to a score that ranged from 0 to 100.
NFR threshold
NFR threshold was assessed using an ascending–descending staircase technique [32]. An NFR was said to have occurred if the mean rectified biceps femoris EMG in the 90 to 150 ms poststimulus interval exceeded the mean rectified biceps femoris EMG activity during the 60 ms prestimulus baseline interval by at least 1.4 SD of the −60 to 0 ms prestimulation baseline EMG activity [32]. The first ascending staircase began at 0 mA and increased in 2 mA increments until a reflex was observed (peak). Following the first reflex, the stimulus intensity decreased in 1 mA steps until the reflex was no longer present (trough). The subsequent two ascending–descending staircases used 1 mA step increments until all three peaks and troughs were obtained. The interval between electric stimulations varied randomly (8–12 s) to minimize reflex habituation and predictability. NFR threshold was defined as the average stimulus intensity (mA) of the last two peaks and troughs.
Pain30
In the event that stimuli at NFR threshold did not elicit at least mild pain (determined by a rating ≥30 on the VAS), the Pain30 task was implemented. If assessed, the computer started the stimulus intensity at NFR threshold and increased the intensity in 2 mA increments (8–12 s interstimulus interval) until a VAS rating ≥30 was obtained.
Three-stimulation threshold
Three-stimulation threshold was defined as the stimulus intensity required to evoke an NFR on the last stimulus of a series of three electric stimulations. To assess this, a series of three electric stimulations were delivered with an interstimulus interval of 0.5 s (2.0 Hz). The series started at 0 mA and increased in 2 mA steps until an NFR was evoked by the third stimulus in the series (8–12 s interseries interval). The stimulus intensity at which the NFR was evoked was used as three-stimulation threshold.
Conditioned Pain Modulation
CPM is a validated paradigm used to assess pain inhibition circuitry [10] (a human analog of DNIC used with animals) [33]. The CPM task involves the assessment of pain in response to a test stimulus before, during, and after a tonic CS delivered at a distal body site from the test stimulus. In healthy humans, the CS should inhibit pain evoked by the test stimuli [33]. In the present study, the test stimuli were electrical stimulations delivered to the left ankle using an 8-12 s random interstimulus interval and the CS was a circulating cold-water bath in which participants placed their right hand/forearm.
The CPM task consisted of three 2 min phases: baseline (five electric test stimuli delivered prior to cold water), conditioning (five electric test stimuli delivered while hand/arm is submerged in cold water), and posttest (five electric test stimuli delivered after removing the hand from cold water). A 2 min rest occurred between baseline and conditioning and a 5 min rest occurred between conditioning and posttest. During each 2 min phase, electric test stimuli were delivered after a 20 s wait period to allow the onset of inhibition during the CS. For the current study, only data during the baseline and conditioning phases were used in order to allow the use of multilevel growth model analyses.
NFR magnitudes
NFR magnitudes in response to electric test stimuli were used to assess within-subject changes in spinal nociception [34]. NFR magnitudes were calculated as a d-score (NFR d = [mean rectified EMG of 90 to 150 ms poststimulation interval minus mean of rectified EMG from −60 to 0 ms prestimulation interval] divided by the average SD of the rectified EMG from the two intervals) [35].
Pain ratings of test stimuli
During each CPM phase, a numerical rating scale (NRS) was displayed on a computer screen in front of the participants. Anchors on the scale were: 0, “no pain”; 20, “mild pain”; 40, “moderate pain”; 60, “severe pain”; 80, “very severe pain”; and 100, “worst possible pain.” Participants provided pain ratings verbally and the experimenter, in an adjacent room, recorded the ratings.
Pain ratings of CS
Following the conditioning phase, participants used the same NRS to rate the pain evoked by the cold water.
Data Analysis
Prior to analyses, variables were examined for nonnormality. Skewed distributions were transformed (e.g., log10 for right skew). Also, outliers were identified according to Wilcox’s recommended MAD-Median procedure with the criteria set to 2.24 [36]. Identified outliers were winsorized to the nearest nonoutlier value. Alpha level was set to p < .05 (two tailed) for all analyses.
Between-group differences in participant characteristics were analyzed using independent t-tests (continuous variables) or chi-square tests (categorical variables). Analyses of CPM of pain ratings and NFR magnitudes were conducted with multilevel growth models. Data for these models were kept in “long form,” meaning that each participant had multiple rows of data that corresponded to each stimulation they received. CPM testing involved three phases (baseline, conditioning, and posttest). For the current study, only data during the baseline and conditioning phases were used; therefore, each participant had 10 rows of data corresponding to the 10 stimulations delivered during these phases. Participants served as Level 2 units in the multilevel growth models and reactions to electric stimulations served as Level 1 units. The within-subject variance–covariance structure was modeled as a first-order autoregressive matrix (AR1) to account for the autocorrelation across the repeated measures. A variable called Stimulus Number (Stim 1 through Stim 5, coded 0–4) was entered into these models as a continuous predictor to model the linear trend of any habituation/sensitization effects that occurred across the five stimulations within each CPM phase. The primary predictor of interest was a Level 1 predictor called CPM phase that was coded 0 for baseline and 1 for conditioning (this codes for the slope of CPM-related modulation). This was entered as a continuous predictor and the intercept and slope were allowed to randomly vary across Level 2 units (participants). Situation-specific catastrophizing and dispositional catastrophizing were added as grand mean-centered continuous predictors, as well as their interactions with the CPM phase variable. These interactions tested whether CPM was influenced by catastrophizing. The random intercepts and slopes of CPM phase were allowed to covary to control for the law of initial values [37] (i.e., pain/NFR that is higher during baseline is more likely to decrease during conditioning and vice versa). The race/ethnicity variable was coded −1 for NHWs and +1 for NAs and was also entered in the models. This variable was also placed in a three-way interaction with catastrophizing and CPM phase to assess whether any relationship between catastrophizing and CPM modulation was moderated by race/ethnicity. Control variables that were entered included: age, sex (−1 = male, +1 = female), BMI, psychological symptoms (SCL-90-R GSI subscale), perceived stress (PSS), SF-36 General Health subscale, pain in response to the cold water CS (CS pain), and the electric stimulation intensity. All continuous variables were grand mean centered to reduce multicollinearity and/or aid interpretation. Chi-square difference tests were used to determine whether the multilevel growth curve models were significantly different from a no-predictor model (akin to the omnibus F-test used in ordinary least squares [OLS] linear regression).
To test primary hypotheses, the main effects of catastrophizing and race examined whether these variables were associated with overall levels (i.e., the intercept) of pain/NFR. The interactions of Catastrophizing × CPM Phase and Race × CPM Phase tested whether catastrophizing or race (respectively) moderated the CPM of pain or NFR. The Catastrophizing × Race × CPM Phase interactions tested whether the relationship between catastrophizing and CPM modulation differed between NAs and NHWs. The following are examples of Level 1 and Level 2 equations in the prediction of pain during CPM:
Level 1
Paini,j=β0,j+β1,jStimNum + β2,jCPMphase + εi,j
Level 2
β0,j= γ0,0 + γ0,1 Race + γ0,2 SScatas + γ0,3 Dcatas + γ0,4Race × SScatas + γ0,5 Race × Dcatas +u0,j
β1,j= γ1,0
β2,j= γ2,0 + γ2,1 Race + γ2,2 SScatas + γ2,3 Dcatas + γ2,4Race × SScatas + γ2,5 Race × Dcatas +u2,j
Control variables were omitted from the above equations to reduce complexity; however, they would be entered as predictors in the Level 2 equation for the random intercept (β0,j). SScatas = ld be entered as predictors in tesssituation-specific catastrophizing; Dcatas = dispositional catastrophizing.
Bootstrapped mediation analyses were also conducted to examine whether situation-specific catastrophizing provided an indirect pathway between race and CPM outcomes. These analyses were conducted with the PROCESS macro (PROCESS 2.16.3) for SPSS [38] using 10,000 bootstrap iterations. To accomplish this, CPM data that were in long form had to be aggregated to create a change score that coded for the modulation of pain during CPM (i.e., test stimulus pain during the CS minus test stimulus pain during the baseline phase) and NFR during CPM (i.e., NFR in response to test stimuli during CS minus NFR in response to test stimuli during baseline phase). For both of these change scores, negative values indicate CPM-related inhibition, whereas positive scores indicate CPM-related facilitation. These were used as dependent variables in the mediation analyses. Situation-specific catastrophizing was entered as the mediator and race (0 = NHW, 1 = NA) was entered as the independent variable.
Exploratory mediation analyses were also conducted in PROCESS to investigate whether pain from the CS mediated the relationship between situation-specific catastrophizing and CPM-related modulation. The same change scores described above were used as dependent variables. In separate analyses, we also examined whether these mediated models (i.e., the a path and the b path of the models) were moderated by race.
Results
Final Sample Characteristics
A total of 329 participants were enrolled in the overall OK-SNAP study, but 22 were not considered for the current analysis because they were non-NA minorities and two persons’ data were lost due to a computer malfunction. This left 305 potential participants. Twenty-nine completed some or all of the testing day that did not involve CPM but did not return for the CPM testing day. Another 23 attended the CPM testing day but quit before CPM testing. Thus, 253 participants were available for the current study (129 NHWs, 124 NAs). Table 1 compares those 253 participants with the 52 who did not complete CPM (the 2 with lost data were not included in this analysis). As shown, there were mostly no significant differences between completers and noncompleters on background characteristics, except that noncompleters were slightly older, had fewer psychological problems (GSI), and reported less bodily pain. Importantly, there were no differences in dispositional pain catastrophizing.
Table 1.
Characteristics of persons with and without conditioned pain modulation data
| CPM (n = 253) | No CPM (n = 52) | |||||||
|---|---|---|---|---|---|---|---|---|
| Continuous variable | N | M | SD | N | M | SD | t | P |
| Age (years) | 253 | 28.921 | 12.771 | 52 | 33.365 | 14.554 | 2.046 | .045 |
| Body mass index (kg/m2) | 248 | 24.939 | 4.230 | 52 | 25.656 | 4.283 | 1.109 | .268 |
| Negative affect (PANAS; 0–40) | 252 | 2.929 | 2.617 | 49 | 2.939 | 2.520 | 0.025 | .980 |
| Positive affect (PANAS; 0–40) | 252 | 18.512 | 7.282 | 17 | 19.235 | 8.326 | 0.393 | .695 |
| State anxiety (STAI; 20–80) | 252 | 32.770 | 7.089 | 49 | 31.694 | 7.335 | −0.967 | .334 |
| SCL-90—Global Severity Index (0–4) | 251 | 0.389 | 0.379 | 44 | 0.266 | 0.257 | −2.127 | .034 |
| Perceived stress (PSS; 0–40) | 251 | 13.845 | 5.994 | 44 | 13.091 | 6.160 | −0.766 | .444 |
| SF-36 Bodily Pain Scale (0–100) | 252 | 42.929 | 7.300 | 18 | 46.667 | 4.947 | 2.136 | .034 |
| SF-36 General Health (0–100) | 252 | 64.010 | 10.499 | 44 | 64.786 | 10.701 | 0.451 | .652 |
| Dispositional pain catastrophizing (PCS; 0–52) | 252 | 9.679 | 7.569 | 49 | 9.143 | 7.853 | −0.451 | .653 |
| Categorical variable | n | % | n | % | χ 2 | p | ||
| Sex (female) | 133 | 52.6% | 32 | 61.5% | 1.397 | .237 | ||
| Race (Native American) | 124 | 49.0% | 31 | 59.6% | 1.940 | .164 | ||
Items in bold are statistically significant.
CPM conditioned pain modulation; PANAS Positive and Negative Affect Schedule; PCS Pain Catastrophizing Scale; PSS Perceived Stress Scale; SCL-90 Symptom Checklist 90; SD standard deviation; SF-36 Medical Outcomes Study 36-item Short Form Health Survey; STAI State Trait Anxiety Inventory.
Table 2 presents the differences between the 129 NHWs and 124 NAs on background characteristics. The only significant differences were for BMI (NA > NHW), SCL-90 GSI (NA > NHW), SF-36 General Health (NAs had poorer health), situation-specific catastrophizing to the cold-water CS pain (NA > NHW), situation-specific catastrophizing to electric pain (NA > NHW), education (NAs were more likely to have only a high school diploma), and employment (NAs were more likely to be unemployed). Notably, groups did not differ on the suprathreshold stimulus intensity used to elicit electric pain or on pain ratings of the CS cold water. Thus, any group differences for CPM cannot be attributed to these variables.
Table 2.
Characteristics of Native American (NA) and non-Hispanic White (NHW) participants
| NHW (n = 129) | NA (n = 124) | |||||
|---|---|---|---|---|---|---|
| Continuous variable | M | SD | M | SD | t | p |
| Age (years) | 28.519 | 13.569 | 29.339 | 11.925 | −0.509 | .611 |
| Body mass index (kg/m2) | 24.210 | 3.757 | 25.717 | 4.570 | −2.827 | .005 |
| Negative affect (PANAS; 0–40) | 2.806 | 2.613 | 3.057 | 2.625 | −0.760 | .448 |
| Positive affect (PANAS; 0–40) | 17.938 | 6.875 | 19.114 | 7.669 | −1.283 | .201 |
| State anxiety (STAI; 20–80) | 32.411 | 6.903 | 33.146 | 7.288 | −0.823 | .411 |
| SCL−90—Global Severity Index (0–4) | 0.336 | 0.332 | 0.445 | 0.416 | −3.040 | .003 |
| Perceived stress (PSS; 0–40) | 13.039 | 5.631 | 14.697 | 6.265 | −2.207 | .028 |
| SF-36 Bodily Pain Scale (0–100) | 43.434 | 6.786 | 42.398 | 7.796 | 1.126 | .261 |
| SF-36 General Health (0–100) | 65.504 | 9.482 | 62.444 | 11.298 | 2.333 | .020 |
| Suprathreshold stimulus intensity (0–50 mA) | 25.034 | 12.558 | 27.425 | 12.156 | −1.538 | .125 |
| Cold-water (10˚C) pain (0–100) | 51.820 | 24.266 | 55.910 | 24.281 | −1.334 | .184 |
| Dispositional pain catastrophizing (PCS; 0–52) | 9.806 | 7.562 | 9.545 | 7.604 | 0.274 | .785 |
| Cold (CS) situation-specific Catas (PCS; 0–52) | 15.155 | 11.962 | 20.066 | 15.283 | −2.824 | .005 |
| Electric situation-specific Catas (PCS; 0–52) | 7.364 | 9.606 | 10.610 | 11.595 | −2.854 | .005 |
| Categorical variables | n | % | n | % | χ 2 | p |
| Sex (female) | 64 | 49.6% | 69 | 55.6% | 0.923 | .337 |
| Education | ||||||
| <High school | 0 | 0.0% | 8 | 6.5% | 9.602 | .048 |
| High school graduate | 15 | 11.7% | 14 | 11.4% | ||
| Partial college | 68 | 53.1% | 54 | 43.9% | ||
| College graduate | 34 | 26.6% | 36 | 29.3% | ||
| Graduate/professional school | 11 | 8.6% | 11 | 8.9% | ||
| Employment | 13.258 | .010 | ||||
| >40 hr per week | 28 | 22.0% | 39 | 32.0% | ||
| <40 hr per week | 60 | 47.2% | 45 | 36.9% | ||
| Retired | 5 | 3.9% | 1 | 0.8% | ||
| Unemployed | 19 | 15.0% | 31 | 25.4% | ||
| Student | 15 | 11.8% | 6 | 4.9% | ||
| Income | 9.491 | .091 | ||||
| <$9,999 | 49 | 38.6% | 30 | 25.0% | ||
| $10,000–$14,999 | 15 | 11.8% | 15 | 12.5% | ||
| $15,000–$24,999 | 16 | 12.6% | 15 | 12.5% | ||
| $25,000–$34,999 | 10 | 7.9% | 15 | 12.5% | ||
| $35,000–$49,999 | 10 | 7.9% | 21 | 17.5% | ||
| >$50,000 | 27 | 21.3% | 24 | 20.0% | ||
| Marital status | 7.459 | .059 | ||||
| Single | 97 | 75.2% | 79 | 64.8% | ||
| Married | 22 | 17.1% | 22 | 18.0% | ||
| Separated/divorce/widowed | 8 | 6.2% | 11 | 9.0% | ||
| Cohabitating | 2 | 1.6% | 10 | 8.2% | ||
If variables were transformed to address nonnormality, the t-test results are based on the transformed variables, but the nontransformed means and SDs are reported. Items in bold are statistically significant at p < .05.
Catas catastrophizing; CS conditioning stimulus; NA Native American; NHW non-Hispanic white; PANAS Positive and Negative Affect Schedule; PCS Pain Catastrophizing Scale; PSS Perceived Stress Scale; SCL-90 Symptom Checklist 90; SD standard deviation; SF-36 Medical Outcomes Study 36-item Short Form Health Survey; STAI State Trait Anxiety Inventory.
Variable Conditioning and Control Variables
Psychological problems (GSI) and situation-specific catastrophizing in response to electric stimuli were positively skewed; thus, they were log-transformed. Dispositional catastrophizing, PANAS negative affect, PANAS positive affect, STAI state anxiety, log depression, log anxiety, log GSI, perceived stress, SF-36 subscales (Bodily Pain and General Health), pain ratings, and NFR magnitudes all had outliers, so they were winsorized.
After a thorough exploration of the pain and NFR dependent variables, it was found that four participants had all CPM baseline pain ratings at ceiling (≥95 NRS rating) or floor (≤5 NRS rating), so they were excluded from the analysis of CPM of pain to avoid biasing the results. There were no ceiling or floor effects for NFR. Individual stimulation trials for which the prestimulus baseline EMG exceeded 3 µV (due to excessive muscle tension) were excluded from NFR analysis (4.7% of 2,485 trials).
Given the group differences in background variables noted above, the following control variables were used: BMI, GSI, perceived stress, and SF-36 General Health. Sex and age were also entered as control variables due to research suggesting that they can affect the magnitude of CPM [17, 39–41]. Suprathreshold stimulus intensity was also entered as a control variable because it was individually calibrated to each individual. These six control variables were entered in all analyses. In multilevel growth models examining whether race moderated the effects of catastrophizing on CPM, pain ratings of the cold-water CS were also entered to control for the individual variability in CS pain experienced.
The Relationship Between Pain Catastrophizing and Pain Inhibition in NAs
CPM of pain
Results from the multilevel growth model of pain are reported in Table 3 and depicted in Fig. 2. To briefly summarize the findings, situation-specific catastrophizing was associated with greater inhibition of pain. Race did not moderate this relationship.
Table 3.
Results of multilevel growth curve analysis for CPM of pain ratings
| 95% Confidence interval | ||||
|---|---|---|---|---|
| Fixed effects | Estimate | SE | Lower | Upper |
| Intercept | 35.942* | 0.977 | 34.020 | 37.866 |
| Sex | 0.408 | 0.853 | −1.272 | 2.088 |
| Age | 0.131 | 0.070 | −0.007 | 0.270 |
| BMI | 0.158 | 0.216 | −0.267 | 0.583 |
| Log GSI (psych distress) | 32.052* | 14.885 | 2.736 | 61.367 |
| Perceived stress | −0.217 | 0.208 | −0.627 | 0.194 |
| SF−36 Gen Health | −0.152 | 0.086 | −0.321 | 0.017 |
| Stimulus number | 0.727* | 0.101 | 0.528 | 0.927 |
| Suprathreshold stimulus intensity | 0.358* | 0.071 | 0.219 | 0.498 |
| CS Pain | 0.335* | 0.048 | 0.242 | 0.429 |
| Race | −0.281 | 0.962 | −2.177 | 1.614 |
| Dispositional catastrophizing | 0.097 | 0.148 | −0.194 | 0.387 |
| SS Catastrophizing | −0.086 | 0.099 | −0.281 | 0.108 |
| CPM phase | −6.890* | 0.636 | −8.150 | −5.633 |
| Disp Catas × CPM Phase | −0.068 | 0.087 | −0.240 | 0.104 |
| SS Catas × CPM Phase | −0.120* | 0.050 | −0.220 | −0.021 |
| Race × CPM Phase | −0.570 | 0.603 | −1.762 | 0.622 |
| Race × Disp Catastrophizing | −0.259 | 0.135 | −0.524 | 0.006 |
| Race × SS Catastrophizing | −0.054 | 0.079 | −0.209 | 0.102 |
| Race × Disp Catas × CPM Phase | −0.012 | 0.087 | −0.185 | 0.160 |
| Race × SS Catas × CPM Phase | 0.050 | 0.050 | −0.050 | 0.149 |
| 95% Confidence interval | ||||
| Random effects | Estimate | SE | Lower | Upper |
| AR1 diagonal | 68.409* | 8.527 | 53.582 | 87.339 |
| AR1 rho | 0.651* | 0.049 | 0.545 | 0.736 |
| CPM phase intercept variance | 172.167* | 18.908 | 138.825 | 213.517 |
| Intercept and slope covariance | −38.710* | 10.024 | −58.356 | −19.064 |
| CPM phase slope variance | 49.274* | 8.960 | 34.502 | 70.372 |
Estimates are unstandardized relationships between the predictors and the dependent variable. Sex was coded −1 = male and 1 = female. Race was coded −1 = non-Hispanic white and 1 = Native American. AR1 = first-order autoregressive structure. Estimates in bolded text are significant at *p < .05.
BMI body mass index; CPM conditioned pain modulation CS conditioning stimulus; Disp dispositional pain catastrophizing;; GSI Global Severity Index of the Symptom Checklist 90; SE standard error of coefficient/estimate; SF-36 Medical Outcomes Study 36-item Short Form Health Survey; SS situation-specific pain catastrophizing.
Fig. 2.
Relationship between situation-specific pain catastrophizing (SS Catas) and conditioned pain modulation of (CPM) pain ratings. Results suggest that there was a significant interaction between SS catastrophizing and modulation of pain by the cold-water conditioning stimulus. Pain ratings were generally lower during conditioning phase (Cond) relative to the preconditioning baseline (Base) phase; however, the degree of pain inhibition increased with higher SS catastrophizing scores. p-values represent the significance level of the baseline versus conditioning comparison at the three levels of SS Catas (−1 SD, mean, +1 SD).
The model for pain was significantly different than the intercept-only model, ∆× 2 (degrees of freedom [df] = 22) = 608.404, p < .00001, suggesting that the model was statistically significant in predicting pain ratings. The main effect of CPM phase was significant (p < .001) and indicated that, on average, pain was reduced by 6.891 NRS points by the CS. However, this effect was moderated by situation-specific pain catastrophizing (p = .018) such that higher catastrophizing was associated with greater pain inhibition by the CS (Fig. 2). Simple CPM slopes for the three levels of situation-specific catastrophizing (−1 SD, mean, and +1 SD) were examined according to Preacher et al. [42]. All three demonstrated statistically significant inhibition of pain (ps < .001). Dispositional catastrophizing did not influence CPM of pain, nor did race (Table 3).
The main effect of psychological distress (SCL-90 GSI, p = .031) indicated that those with greater distress experienced more pain. The main effect of stimulus number (p < .001) indicated that there was significant sensitization of pain across the five stimulations in each CPM phase. The main effects of CS pain (p < .001) and suprathreshold stimulus intensity (p < .001) indicated that higher values on these variables were associated with more pain in response to electric stimulations.
Although the model predicted significant variance in the intercepts and slopes, there was still variance remaining as indicated by their significant random effect estimates (Table 3). Moreover, the significant estimate for the covariance between the intercept and slope of CPM phase underscores the need to control for the law of initial values (i.e, those that start out with higher pain ratings at baseline are more likely to have larger decreases in pain and vice versa).
CPM of NFR
Results from the multilevel growth model of NFR are reported in Table 4 and depicted in Fig. 3. To briefly summarize the findings, situation-specific catastrophizing was associated with reduced inhibition of NFR. Race did not moderate this relationship.
Table 4.
Results of multilevel growth curve analysis for CPM of nociceptive flexion reflex (NFR) magnitudes
| 95% Confidence interval | ||||
|---|---|---|---|---|
| Fixed effects | Estimate | SE | Lower | Upper |
| Intercept | 1.156* | 0.038 | 1.081 | 1.232 |
| Sex | −0.018 | 0.034 | −0.084 | 0.049 |
| Age | −0.002 | 0.003 | −0.007 | 0.003 |
| BMI | −0.004 | 0.009 | −0.021 | 0.012 |
| Log GSI (psych distress) | 0.338 | 0.593 | −0.830 | 1.506 |
| Perceived stress | 0.002 | 0.008 | −0.014 | 0.019 |
| SF−36 Gen Health | −0.001 | 0.003 | −0.008 | 0.005 |
| Stimulus number | −0.047* | 0.006 | −0.058 | −0.035 |
| Suprathreshold stimulus intensity | 0.012* | 0.003 | 0.007 | 0.018 |
| CS pain | −0.006* | 0.002 | −0.009 | −0.002 |
| Race | −0.010 | 0.038 | −0.085 | 0.064 |
| Dispositional catastrophizing | −0.010 | 0.006 | −0.022 | 0.001 |
| SS catastrophizing | −0.001 | 0.004 | −0.008 | 0.007 |
| CPM phase | −0.019 | 0.027 | −0.072 | 0.034 |
| Disp Catas × CPM Phase | 0.001 | 0.004 | −0.007 | 0.008 |
| SS Catas × CPM Phase | 0.006* | 0.002 | 0.001 | 0.010 |
| Race × CPM Phase | 0.022 | 0.027 | −0.031 | 0.075 |
| Race × Disp Catastrophizing | 0.003 | 0.005 | −0.008 | 0.013 |
| Race × SS Catastrophizing | 0.002 | 0.003 | −0.004 | 0.008 |
| Race × Disp Catas × CPM Phase | 0.000 | 0.004 | −0.008 | 0.007 |
| Race × SS Catas × CPM Phase | −0.001 | 0.002 | −0.006 | 0.003 |
| 95% Confidence interval | ||||
| Random effects | Estimate | SE | Lower | Upper |
| AR1 diagonal | 0.155* | 0.006 | 0.145 | 0.167 |
| AR1 rho | 0.071* | 0.030 | 0.012 | 0.129 |
| CPM phase intercept variance | 0.277* | 0.029 | 0.226 | 0.339 |
| Intercept and slope covariance | −0.059* | 0.016 | −0.091 | −0.027 |
| CPM phase slope variance | 0.096* | 0.016 | 0.070 | 0.132 |
Estimates are unstandardized relationships between the predictors and the dependent variable. Sex was coded −1 = male and 1 = female. Race was coded −1 = non-Hispanic White and 1 = Native American. AR1 = first-order autoregressive structure. Estimates in bolded text are significant at *p < .05.
BMI body mass index; CPM conditioned pain modulation; CS conditioning stimulus; Disp dispositional pain catastrophizing; GSI Global Severity Index of the Symptom Checklist 90; SE standard error of coefficient/estimate; SF-36 Medical Outcomes Study 36-item Short Form Health Survey; SS situation-specific pain catastrophizing.
Fig. 3.
Relationship between situation-specific pain catastrophizing (SS Catas) and conditioned pain modulation (CPM) of nociceptive flexion reflex (NFR) magnitudes (a correlate of spinal nociception). Results suggest that there was a significant interaction between SS catastrophizing and modulation of NFR by the cold-water conditioning stimulus. For persons reporting low SS catastrophizing, NFRs were significantly lower during the conditioning phase (Cond) relative to the preconditioning baseline (Base) phase. However, as catastrophizing increased, the degree of NFR inhibition was attenuated. For persons with high SS catastrophizing, the NFR started to facilitate. p-values represent the significance level of the baseline versus conditioning comparison at the three levels of SS catas (−1 SD, mean, +1 SD).
The final model was significantly different than the intercept-only model, ∆× 2 (df = 22) = 188.475, p < .00001, suggesting that the model was statistically significant in predicting NFR magnitudes. There was a significant interaction of situation-specific catastrophizing and CPM Phase (p = .013) such that higher catastrophizing was associated with less inhibition of NFR by the CS (Fig. 3). Simple CPM slopes for the three levels of situation-specific catastrophizing (−1 SD, mean, and +1 SD) graphed in Fig 3 were examined according to Preacher et al. [42]. Only persons at −1 SD of situational-specific catastrophizing showed significant inhibition of NFR (p = .009). Dispositional catastrophizing did not influence CPM of NFR, nor did race.
The main effect of stimulus number (p < .001) indicated that there was significant habituation of NFR magnitudes across the five stimulations in each CPM phase. The main effect of CS pain (p = .002) indicated that higher CS pain was associated with lower NFRs. By contrast, the main effect of suprathreshold stimulus intensity (p < .001) indicated that the higher the stimulus intensity, the larger the NFRs were in general.
Although the model predicted significant variance in the intercepts and slopes, there was still significant variance in NFR remaining as indicated by their significant random effect estimates (Table 4). Moreover, the significant estimate for the covariance between the intercept and slope of CPM phase underscores the need to control for the law of initial values.
Does Situation-Specific Catastrophizing Provide an Indirect (Mediated) Pathway Between NAs and CPM?
As Fig. 4 shows, the bootstrapped test of the indirect effect (a × b pathway) indicates that situation-specific catastrophizing mediated the relationships between race and CPM-related pain inhibition and CPM-related NFR inhibition (i.e., the bootstrapped 95% confidence intervals [CIs] for the indirect effects did not contain zero). This means that NAs experienced more situation-specific catastrophizing, which, in turn, led to greater inhibition of pain (Panel A) and less inhibition of NFR (Panel B).
Fig. 4.
Unstandardized regression coefficients for the relationship between race (non-Hispanic White [NHW] = 0, Native American [NA] = 1) and conditioned pain modulation (CPM) of pain (Panel A) and CPM of the nociceptive flexion reflex (NFR: Panel B) as mediated by situation-specific catastrophizing during the cold-water conditioning stimulus. Pain inhibition and NFR inhibition variables were change scores (pain/NFR during conditioning minus pain/NFR during baseline); thus, negative values indicate inhibition, whereas positive values indicate facilitation. All models controlled for: biological sex, age, body mass index, perceived stress, general health perception, and suprathreshold stimulus intensity. These models suggest that NAs experience greater situation-specific catastrophizing, which leads to greater inhibition of pain and less inhibition of NFR. *p < .05. **p < .01
Exploratory Analysis: Does CS Pain Mediate the Relationship Between Situation-Specific Catastrophizing and CPM?
As Fig. 5 shows, the bootstrapped test of the indirect effect (a × b pathway) indicates that CS pain mediated the relationship between situation-specific catastrophizing and CPM-related inhibition of pain (the bootstrapped 95% CI did not contain 0). This means that as more situation-specific catastrophizing was experienced, the CS resulted in more pain, which, in turn, led to greater CPM-related inhibition of pain (Fig. 5; Panel A). By contrast, CS pain did not mediate the relationship between situation-specific catastrophizing and CPM-related inhibition of NFR (Fig. 5; Panel B). This was apparently due to the lack of a relationship between CS pain and inhibition of NFR (b path). These analyses were also conducted with race as a moderator of the a path and the b path in the models, but race was not a significant moderator of either path in either model (all ps > .19).
Fig. 5.
Unstandardized regression coefficients for the relationship between situation-specific catastrophizing and conditioned pain modulation (CPM) of pain (Panel A) and CPM of the nociceptive flexion reflex (NFR; Panel B) as mediated by cold-water pain (CPM conditioning stimulus). Pain inhibition and NFR inhibition variables were change scores (pain/NFR during conditioning minus pain/NFR during baseline); thus, negative values indicate inhibition, whereas positive values indicate facilitation. All models controlled for biological sex, age, body mass index, perceived stress, general health perception, and suprathreshold stimulus intensity. These models suggest that the effect of situation-specific catastrophizing on CPM of pain, but not NFR, is mediated by the painfulness of the conditioning stimulus. *p < .05. **p < .01
Do Results Vary With Situation-Specific Pain Catastrophizing in Response to Electric Stimuli?
To determine if our results were specific to situation-specific catastrophizing during the CS, all analyses were conducted again substituting situation-specific catastrophizing to the electric stimuli variable for situation-specific catastrophizing to the CS. For the multilevel growth curves, results were similar, except that situation-specific catastrophizing about electric pain no longer moderated the effect of CPM of pain (p = .086) but did continue to moderate the effect for CPM of NFR (p = .010). Moreover, situation-specific catastrophizing about electric pain also exerted main effects on electric pain (p < .001) and NFR (p = .008) such that higher catastrophizing was associated with higher overall electric pain and lower overall NFRs.
For the models examining whether situation-specific catastrophizing served as a mediator between race and CPM, the indirect effect was no longer significant for CPM of pain (indirect effect = −.21; bootstrapped 95% CI: −.81, .06) or for CPM of NFR (indirect effect = .01; bootstrapped 95% CI: −.002, .04). However, for the models examining whether CS pain served as a mediator between situation-specific catastrophizing and CPM, the conclusions were the same. The indirect effect was significant for CPM of pain (indirect effect = −1.51; bootstrapped 95% CI: −2.72, −.06) but not for CPM of NFR (indirect effect = .009; bootstrapped 95% CI: −.04, .06).
Does the Relationship Between CPM of Pain and CPM of NFR Differ between NAs and NHWs?
Given that there was a divergence in the modulatory effect of CPM of pain and CPM of NFR, we explored whether this was similar in NAs and NHWs. A regression analysis was conducted with CPM of pain as the criterion and CPM of NFR (mean centered), race, and the interaction of CPM of NFR and race as predictors. No predictor was significant: BCPMNFR = −1.86, p = .31, Brace = −1.80, p = .12, Binteraction = 4.23, p = .12. Moreover, the model only explained 2% of the variance (R2 = .0201) and was nonsignificant (p = .18). Thus, the modulation of pain and NFR were independent of one another and the relationship was similar in the two groups.
Discussion
The present study investigated the relationship between pain catastrophizing (dispositional and situation specific) and CPM of pain and NFR in healthy, pain-free NHW and NA participants from the OK-SNAP. It was predicted that pain catastrophizing would reduce the efficacy of CPM and that this effect would be stronger in NAs. We also examined whether situation-specific catastrophizing might serve as an indirect pathway (mediator) between NA race and CPM efficiency. Finally, because we measured situation-specific catastrophizing in response to the painful CS, we examined whether any relationship between situation-specific catastrophizing and CPM was mediated by the CS pain. There were several key findings from the study: (a) dispositional catastrophizing did not influence CPM of pain or CPM of NFR, (b) greater situation-specific catastrophizing reduced CPM-related NFR inhibition (as predicted) but improved CPM-related pain inhibition, (c) CS pain mediated the relationship between situation-specific catastrophizing and CPM of pain but not the relationship with CPM of NFR, (d) race did not moderate the direct or indirect relationships between situation-specific catastrophizing and CPM, and (e) situation-specific catastrophizing about the CS pain (but not about electric pain) mediated the relationship between NA race and CPM of pain and CPM of NFR. These findings are discussed below.
The Effects of Dispositional Versus Situation-Specific Pain Catastrophizing on CPM
Pain catastrophizing is one of the strongest psychological predictors of pain and pain-related outcomes, including pain perception, analgesic use, length of hospital stay, pain-related disability, and supraspinal responses to noxious stimulation [13, 14, 43–45]. Research over the past 15 years has found that the strength of the relationship between catastrophizing and pain is influenced by the type of pain catastrophizing that is measured. The traditional, dispositional (trait-like) measure of pain catastrophizing asks individuals to reflect back and report on their catastrophic thoughts to painful experiences in general, whereas situation-specific (state-like) measures of pain catastrophizing ask individuals to report on the catastrophic thoughts they had during a specific painful event. Although research has shown a relationship between pain and both catastrophizing types, the relationship is stronger for situation-specific catastrophizing, particularly, for experimental pain [14, 46–50]. Results from the current study support this.
Dispositional catastrophizing was not related to pain, NFR, CPM of pain, or CPM of NFR, yet situation-specific catastrophizing was associated with CPM of pain and CPM of NFR. Ostensibly, this is because situation-specific catastrophizing assesses the actual level of rumination, helplessness, and pain magnification the person experiences during the painful event, and these cognitions are what, theoretically, impact pain processing. By contrast, dispositional measures assess the overall level of catastrophic thoughts a person has across multiple situations. Not surprisingly, this may not reflect the cognitions going on during a specific painful event. Consistent with this, studies generally find a correlation around r = .50 for the two measures, even though they often use the same 13 items of the Pain Catastrophizing Scale [14]. In the present study, the correlation between dispositional catastrophizing and situation-specific catastrophizing was r = .37 (during CS pain) and r = .47 (during electric pain). Given this, future research should examine whether dispositional catastrophizing only influences pain outcomes when situation-specific catastrophizing is activated by the context as would be predicted by trait-activation theory [51].
Can Catastrophizing Improve CPM of Pain but Impair CPM of NFR?
We found that situation-specific catastrophizing decreased CPM-related NFR inhibition but, at the same time, it increased CPM-related pain inhibition. This is surprising, because pain catastrophizing is generally associated with negative pain outcomes, and better CPM-related pain inhibition is believed to be a resiliency factor that protects against chronic pain [52]. Shedding light on this paradox, a follow-up analysis found that this catastrophizing–CPM relationship was mediated by the pain evoked by the cold-water CS. Specifically, situation-specific catastrophizing increased the pain from the CS, which, in turn, increased CPM-related pain inhibition. Thus, it appears that, if catastrophic cognitions increase the painfulness of the CS, it improves the resulting pain inhibition, at least, in healthy participants. This is consistent with the evidence that the intensity of the CS influences the degree of CPM-related pain inhibition [6]. Moreover, a study by Nir et al. [53] found that if experimental cognitive manipulations are used to change the perceived painfulness of the CS (even though the CS intensity is kept constant), this influences CPM-related pain inhibition (less CS pain = less CPM-related pain inhibition).
Alternatively, situation-specific catastrophizing may have increased CPM-related pain inhibition indirectly by shifting attentional resources away from the electric test stimulus, thus, decreasing electric pain via distraction. Consistent with this, a study by Moont et al. [54] found that distraction during a CPM task further inhibited pain above and beyond CPM-related pain inhibition. Thus, those who catastrophized more may have attended more to the cold-water pain than the electric pain resulting in what appeared to be greater CPM-related pain inhibition. Unfortunately, we did not assess attention to resolve this issue. But, the fact that situation-specific catastrophizing to the electric stimulations (when substituted for catastrophizing to the CS in the analyses) did not moderate CPM of pain suggests that this is a possibility that is worth examining in future research.
To our knowledge, only one other study has found that catastrophizing led to greater CPM-related pain inhibition [19]. However, that study assessed CPM during naltrexone administration (an opioid antagonist), so it is not clear whether our results can be compared to theirs. Future studies are needed to determine whether the relationship between catastrophizing and improved CPM of pain can be replicated. If so, it will also be important to determine the circumstances under which catastrophizing leads to better endogenous inhibition of pain.
CS Pain Does Not Mediate the Relationship Between Catastrophizing and CPM of NFR
Interestingly, CS pain did not mediate the relationship between situation-specific catastrophizing and CPM of NFR. This, combined with the fact that situation-specific catastrophizing led to less CPM-related NFR inhibition but more CPM-related pain inhibition, points to the notion that CPM of pain and CPM of NFR are controlled by separate mechanisms. Indeed, an imaging study by Piché et al. [55] found that CPM modulation of pain was associated with a circuit involving the orbitofrontal cortex, posterior cingulate cortex (PCC), anterior cingulate cortex (ACC), subgenual cingulate cortex, anterior insula, amygdala, parahippocampal gyrus (PHG), and medial prefrontal cortex. By contrast, CPM modulation of NFR was associated with a circuit involving the primary somatosensory cortex, paracentral lobule, supplementary motor area, ACC, PCC, PHG, prefrontal cortex, and connections with the pons, PAG, and RVM. Thus, CPM modulation of pain involves a cortico-cortical circuit, whereas CPM modulation of NFR involves a corticospinal circuit. In light of this, it may be said that the divergent effects of situation-specific catastrophizing on CPM of pain versus CPM of NFR may be explained by separate neural circuitries. Future studies should examine whether catastrophizing correlates with CPM-related activation in supraspinal areas.
What Confers Risk for Chronic Pain?: CPM of Pain versus CPM of NFR
Studies have found that less CPM-related pain inhibition is associated with chronic pain (for a review, see [9]). However, most of this work has been correlational and cross-sectional. That said, two studies have used CPM of pain to prospectively predict chronic pain. In a study of 62 patients, Yarnitsky et al. [12] demonstrated that CPM of heat pain (assessed before thoracotomy) could be used to predict postsurgical chronic pain (defined as pain ratings ~29 weeks after surgery, which were >20 out of 100 rating). This was true even after controlling for sex, age, daily opiate dosage, type of surgery, and pain threshold. Similarly, Wilder-Smith et al. [11] followed 20 patients undergoing abdominal surgery and found that CPM of electric and pressure pain was associated with postoperative pain. Thus, these studies provide evidence that CPM of pain can assess risk for future chronic pain.
NFR assesses spinal nociception; thus, CPM of NFR is a more direct measure of descending, brain-to-spinal cord inhibitory processes. Despite this, relatively few laboratories assess CPM of NFR, perhaps, because it requires more time to administer and greater technical proficiency. Therefore, we are unaware of any published study linking CPM of NFR prospectively to chronic pain. However, preliminary data from OK-SNAP follow-up screens suggest that CPM of NFR may be a better predictor of chronic pain onset than CPM of electric pain. Of the 73% of OK-SNAP participants that have responded to follow-ups, 16% have developed chronic pain (defined as persistent pain lasting ≥3 months, which does not remit at subsequent follow-up screens). After controlling for age, BMI, sex, perceptions of health (at enrollment), and psychological symptoms (at enrollment), CPM of NFR, but not CPM of pain, predicted chronic pain onset [56]. Given this, our findings suggest that situation-specific pain catastrophizing may disengage the inhibition of spinal nociception, thereby, increasing the risk of chronic pain in the future. CPM of electric pain (as we have assessed it) may not predict pain risk. These hypotheses can be addressed more fully when all OK-SNAP follow-up data are collected.
It is also worth pointing out that this study provides some of the first evidence that catastrophizing can influence spinal nociception. Several studies have failed to find a correlation between catastrophizing (dispositional or situational) and NFR threshold [31, 48, 49, 57, 58], temporal summation of NFR [31], and emotional modulation of NFR [59]. Moreover, experimental interventions to reduce catastrophizing do not cause changes in NFR that are mediated by catastrophizing [60, 61]. The present study suggests that situation-specific catastrophizing may influence descending modulation of spinal nociception via CPM-related processes.
Implications for Chronic Pain Risk in NAs
The first paper from OK-SNAP reporting on primary outcomes found that NAs report greater situation-specific catastrophizing during painful events when compared to NHWs [21]. Given that pain catastrophizing can promote and/or maintain chronic pain [62–64], we believe that this might place NAs at risk for pain by causing a vicious pain-promoting cycle (pain→catastrophizing→pain). The present findings extend these results to show that situation-specific catastrophizing increases the efficiency of pain inhibition and decreases the efficiency of NFR inhibition. Although these effects were not moderated by race, situation-specific catastrophizing in response to the CS served as an indirect pathway between NA race and CPM outcomes. These findings have several implications.
First, the lack of moderation by race indicates that the effects of situation-specific pain catastrophizing on endogenous pain inhibition do not pose a unique pain risk pathway for NAs. Thus, this is not a mechanism that is stronger, or only occurs, in NAs. Rather, situation-specific pain catastrophizing had a similar effect on CPM for both NAs and NHWs. Notably, this may be true for situation-specific catastrophizing in general because the same conclusion was found when situation-specific catastrophizing in response to electric pain was used in the model predicting CPM of NFR.
Despite this, the tendency for NAs to experience greater situation-specific catastrophizing may contribute to their pain disparity. We found that situation-specific catastrophizing to the cold-water CS provided an indirect effect linking race to CPM (Fig. 4). NAs were more likely to experience situation-specific catastrophizing, which, in turn, led to greater pain inhibition but less NFR inhibition. As noted earlier, we found that CPM of NFR, but not CPM of electric pain, predicts chronic pain onset [56]. This leads to the second implication of our findings: NAs are at a greater risk for developing chronic pain due to the tendency to catastrophize more, which, in turn, disrupts descending inhibition of spinal nociception (i.e., NA race→situation-specific catastrophizing→reduced inhibition of NFR→increased chronic pain risk). As a result, treatments that reduce pain catastrophizing may be particularly helpful for NA patients because they may help promote descending inhibition [65–68].
It is not clear why situation-specific catastrophizing to electric pain did not serve as a mediator between race and CPM, but it may be due to the degree (or level) of catastrophizing that was evoked. An examination of the means in Table 2 shows that catastrophizing was twice as high in response to the CS pain as electric pain. Perhaps higher levels of situational catastrophizing are necessary to influence CPM-related modulation. Alternatively, catastrophizing during the CS pain may be the necessary component to influence CPM-related inhibition.
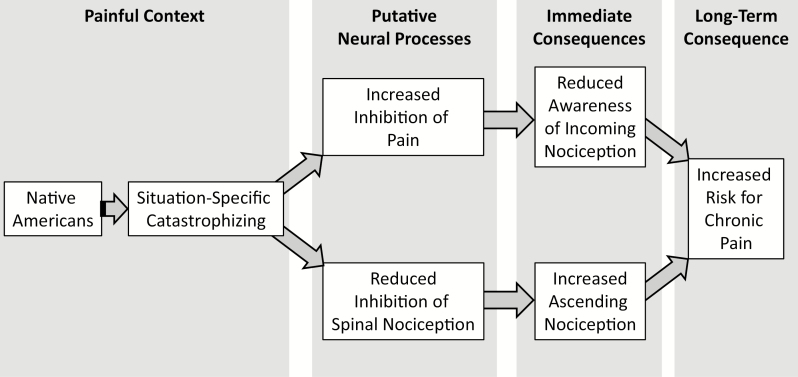
Although CPM of NFR may be a better predictor of chronic pain onset, it is nonetheless interesting that greater situation-specific catastrophizing to the CS promoted greater CPM-related pain inhibition in NAs. As noted, more-efficient CPM of pain is believed to be a resiliency factor that protects against chronic pain development [52]. However, for NAs that engage in situation-specific catastrophizing, this appears to cause a disconnect between the incoming pain signal at the spinal level and the subsequent pain perception that is registered. Our results indicate that situation-specific catastrophizing disengages descending inhibition of incoming nociception at the spinal level at the same time that pain is dampened. This could lead to an increased barrage of ascending nociception that is not experienced as painful. Indeed, the perception of pain normally serves as a warning that protects against injury, promotes learning to avoid future injury, and encourages recuperative behaviors after injury [69, 70]. Without the pain that normally accompanies an incoming nociceptive barrage to serve as a warning, there may not be a motivation to terminate the noxious event or to seek treatment following an injury. The result could be the third implication of these findings: NAs may be at risk for chronic pain because pain perception is dampened at the same time that inhibition of spinal nociception is disrupted (Fig. 6). Therefore, incoming nociception at the spinal (and, perhaps, supraspinal) level is left unchecked to result in problems like central sensitization [71]. At the same time, pain perception is dampened, reducing the probability of normally adaptive responses to the incoming nociception. Future research is needed to verify this assertion and to examine whether supraspinal nociception (e.g., measured from pain evoked potentials or functional magnetic resonance imaging of the pain neuromatrix) is modulated in parallel with pain perception or with NFR.
Fig. 6.
Model depicting one hypothetical pathway for chronic pain risk in Native Americans.
As a final point, dispositional catastrophizing did not differ between NAs and NHWs. This underscores the importance of assessing both dispositional and situation-specific catastrophizing to assess risk factors for pain in NAs.
Strengths and Limitations
This study had a number of strengths. It is one of the largest studies of racial/ethnic differences in pain processing to date; thus, it provided adequate power to examine these issues. Both dispositional and situation-specific catastrophizing were assessed, as well as endogenous modulation of pain and NFR (a physiologic indicator of spinal nociception). Moreover, multilevel growth curves were used to model CPM-related changes in pain/NFR, and state-of-the art bootstrapped mediation analyses were conducted. Nonetheless, a few limitations should be noted.
In order to study risk factors that might predispose persons to future chronic pain, only healthy, pain-free participants were recruited to rule out that any group differences noted were due to disease severity or racial/ethnic disparities in treatment. Therefore, our findings may not generalize to participants with chronic pain or other significant clinical issues. Second, there were a number of participants enrolled in OK-SNAP that did not attend or complete the CPM testing day. This could limit generalizability of our findings. Fortunately, there were a few group differences between those that completed and those that did not (Table 1). Nonetheless, our results may not generalize to older participants with fewer psychological symptoms and less bodily pain. Third, although care was taken to recruit participants with similar background characteristics (e.g., psychological/physical health and SES), there were a few group differences that had to be controlled statistically. Statistical control is not as good as experimental control and added parameters to our models. However, sensitivity analyses to assess the adequacy of our sample size included these parameters to ensure that models were not underpowered. Fourth, we found that NAs were more likely to engage in situation-specific catastrophizing, which appears to place them at risk for future chronic pain. At this time it is not clear why situation-specific catastrophizing is higher in NAs, so future research needs to address this issue. Fifth, CPM was one of the last pain tasks that were administered on the testing day. Although we required breaks in between tasks to minimize carryover, it is possible that results may have differed had CPM been assessed before other tasks. Sixth, our NA participants were mostly from northeastern Oklahoma where most NAs are not reservation dwelling. Our findings may not generalize to NAs from other geographical regions. And finally, endogenous inhibition represents only one potential risk factor for chronic pain. Indeed, pain amplification processes (e.g., temporal summation) are also known to play a role [52]. Although pain amplification indices were assessed in the parent study, we did not include them in the current analyses so that we could focus on the intricacies of endogenous inhibition in NAs. A future paper will examine the relationship between race, catastrophizing, and temporal summation.
Summary
This study found that situation-specific (i.e., catastrophic thoughts during a specific painful event), but not dispositional, pain catastrophizing influenced CPM of pain and CPM of NFR. As predicted, greater catastrophic thoughts impaired inhibition of spinal nociception (NFR); however, it led to an unexpected increase in pain inhibition. This counterintuitive effect on CPM of pain was at least partly explained by pain in response to the CS. Specifically, greater catastrophizing about the CS led to more CS pain, which, in turn, led to greater CPM-related pain inhibition. These relationships have particular importance for NAs because NAs engaged in more situation-specific catastrophizing than the NHW group. This, in turn, led to not only greater disengagement of descending inhibition of NFR but also more pain inhibition for NAs. Together, this could represent a mechanistic pathway for chronic pain risk in NAs.
Acknowledgments
Funding: Research reported was supported by the National Institute On Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD007807. E.L., S.P., and Y.G. were supported by the National Science Foundation Graduate Research Fellowship Program. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation.
Compliance with Ethical Standards
Authors’ Statement of Conflict of Interest and Adherence to Ethical Standards The authors report no conflicts of interest.
Authors’ Contributions T. A. T. analyzed the data and co-wrote the manuscript for publication. J. L. R. served as the primary investigator, designed the study, analyzed the data for publication, and contributed to writing the manuscript. B. L. K., M. F. P., E. W. L., S. P., C. A. S., N. H., Y. M. G., M. J. D., and F. H. collected data and contributed to writing the manuscript. J. O. S. helped design the study, served as co-investigator, and helped write the manuscript.
Ethical Approval All procedures for this study were approved by the institutional review boards of the Cherokee Nation, University of Tulsa, and the Indian Health Service Oklahoma City Area Office.
Informed Consent Prior to study participation, participants were given an overview of all procedures and provided verbal and written consent.
References
- 1. Pincus T, Burton AK, Vogel S, Field AP. A systematic review of psychological factors as predictors of chronicity/disability in prospective cohorts of low back pain. Spine (Phila Pa 1976). 2002;27:E109–E120. [DOI] [PubMed] [Google Scholar]
- 2. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. [DOI] [PubMed] [Google Scholar]
- 3. Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, eds. Textbook of Pain. Philadelphia, PA: Elsevier/Churchill Livingstone; 2006:125–143. [Google Scholar]
- 4. Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. [DOI] [PubMed] [Google Scholar]
- 5. Yarnitsky D. Conditioned pain modulation (the diffuse noxious inhibitory control-like effect): Its relevance for acute and chronic pain states. Curr Opin Anaesthesiol. 2010;23:611–615. [DOI] [PubMed] [Google Scholar]
- 6. Le Bars D, Willer J. Diffuse noxious inhibitory controls (DNIC). In: Bushnell MC, Basbaum AI, eds. The Senses: A Comprehensive Reference (Vol 5: Pain). Amsterdam, The Netherlands: Elsevier Academic Press; 2008:763–773. [Google Scholar]
- 7. Kirwilliam SS, Derbyshire SW. Increased bias to report heat or pain following emotional priming of pain-related fear. Pain. 2008;137:60–65. [DOI] [PubMed] [Google Scholar]
- 8. Robinson ME, Myers CD, Sadler IJ, Riley JL III, Kvaal SA, Geisser ME. Bias effects in three common self-report pain assessment measures. Clin J Pain. 1997;13:74–81. [DOI] [PubMed] [Google Scholar]
- 9. Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain. 2015;156 (suppl 1):S24–S31. [DOI] [PubMed] [Google Scholar]
- 10. Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J Pain. 2012;13:936–944. [DOI] [PubMed] [Google Scholar]
- 11. Wilder-Smith OH, Schreyer T, Scheffer GJ, Arendt-Nielsen L. Patients with chronic pain after abdominal surgery show less preoperative endogenous pain inhibition and more postoperative hyperalgesia: A pilot study. J Pain Palliat Care Pharmacother. 2010;24:119–128. [DOI] [PubMed] [Google Scholar]
- 12. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. [DOI] [PubMed] [Google Scholar]
- 13. Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- 14. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: A critical review. Expert Rev Neurother. 2009;9:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weissman-Fogel I, Sprecher E, Pud D. Effects of catastrophizing on pain perception and pain modulation. Exp Brain Res. 2008;186:79–85. [DOI] [PubMed] [Google Scholar]
- 16. Goodin BR, McGuire L, Allshouse M, et al. Associations between catastrophizing and endogenous pain-inhibitory processes: Sex differences. J Pain. 2009;10:180–190. [DOI] [PubMed] [Google Scholar]
- 17. Martel MO, Wasan AD, Edwards RR. Sex differences in the stability of conditioned pain modulation (CPM) among patients with chronic pain. Pain Med. 2013;14:1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grosen K, Vase L, Pilegaard HK, Pfeiffer-Jensen M, Drewes AM. Conditioned pain modulation and situational pain catastrophizing as preoperative predictors of pain following chest wall surgery: A prospective observational cohort study. PLoS One. 2014;9:e90185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. King CD, Goodin B, Kindler LL, et al. Reduction of conditioned pain modulation in humans by naltrexone: An exploratory study of the effects of pain catastrophizing. J Behav Med. 2013;36:315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barnes PM, Adams PF, Powell-Griner E.. Health Characteristics of the American Indian or Alaska Native Adult Population: United States, 2004–2008. Vol. 20 Hyattesville, MD: U.S. Department of Health and Human Services, National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 21. Rhudy JL, Lannon EW, Kuhn BL, et al. Sensory, affective, and catastrophizing reactions to multiple stimulus modalities: Results from the Oklahoma Study of Native American Pain Risk. J Pain. 2019;20:965–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rhudy JL, Lannon EW, Kuhn BL, et al. Assessing peripheral fibers, pain sensitivity, central sensitization, and descending inhibition in Native Americans: Main findings from the Oklahoma Study of Native American Pain Risk. Pain. 2020;161:388–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 24. Bebbington P, Nayani T. The psychosis screening questionnaire. Int J Methods Psychiatr Res. 1995;5:11–19. [Google Scholar]
- 25. Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. [DOI] [PubMed] [Google Scholar]
- 26. Spielberger CD. State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 27. Derogatis LR. SCL-90-R: Symptom Checklist-90-R: Administration, Scoring & Procedures Manual. Minneapolis, MN.: National Computer Systems; 1994. [Google Scholar]
- 28. Cohen S, Kamarck T, Mermelstein R.. Perceived Stress Scale. Menlo Park, CA: Mind Garden; 1994. [Google Scholar]
- 29. Ware JE, Snow KK, Kosinski M, Gandek B.. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute New England Medical Center; 1993. [Google Scholar]
- 30. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: Development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 31. Rhudy JL, Martin SL, Terry EL, et al. Pain catastrophizing is related to temporal summation of pain but not temporal summation of the nociceptive flexion reflex. Pain. 2011;152:794–801. [DOI] [PubMed] [Google Scholar]
- 32. Rhudy JL, France CR. Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain. 2007;128:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice AS. Reliability of conditioned pain modulation: A systematic review. Pain. 2016;157:2410–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sandrini G, Serrao M, Rossi P, Romaniello A, Cruccu G, Willer JC. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. [DOI] [PubMed] [Google Scholar]
- 35. Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE. Psychophysiological responses to pain: Further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiology. 2009;46:939–948. [DOI] [PubMed] [Google Scholar]
- 36. Wilcox RR. Understanding and Applying Basic Statistical Methods Using R. Hoboken, NJ: John Wiley & Sons; 2016. [Google Scholar]
- 37. Benjamin LS. Statistical treatment of the law of initial values (LIV) in autonomic research: A review and recommendation. Psychosom Med. 1963;25:556–566. [DOI] [PubMed] [Google Scholar]
- 38. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression Based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 39. Larivière M, Goffaux P, Marchand S, Julien N. Changes in pain perception and descending inhibitory controls start at middle age in healthy adults. Clin J Pain. 2007;23:506–510. [DOI] [PubMed] [Google Scholar]
- 40. Edwards RR. Age-related differences in endogenous pain modulation: A comparison of diffuse noxious inhibitory controls in healthy older and younger adults. Diss Abstr Int. 2003;63:4956. [DOI] [PubMed] [Google Scholar]
- 41. Bulls HW, Freeman EL, Anderson AJ, Robbins MT, Ness TJ, Goodin BR. Sex differences in experimental measures of pain sensitivity and endogenous pain inhibition. J Pain Res. 2015;8:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 43. Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LM. Psychological aspects of persistent pain: Current state of the science. J Pain. 2004;5:195–211. [DOI] [PubMed] [Google Scholar]
- 44. Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. [DOI] [PubMed] [Google Scholar]
- 45. Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–843. [DOI] [PubMed] [Google Scholar]
- 46. Dixon KE, Thorn BE, Ward LC. An evaluation of sex differences in psychological and physiological responses to experimentally-induced pain: A path analytic description. Pain. 2004;112:188–196. [DOI] [PubMed] [Google Scholar]
- 47. Edwards RR, Campbell CM, Fillingim RB. Catastrophizing and experimental pain sensitivity: only in vivo reports of catastrophic cognitions correlate with pain responses. J Pain. 2005;6:338–339. [DOI] [PubMed] [Google Scholar]
- 48. Rhudy JL, Maynard LJ, Russell JL. Does in vivo catastrophizing engage descending modulation of spinal nociception? J Pain. 2007;8:325–333. [DOI] [PubMed] [Google Scholar]
- 49. Rhudy JL, France CR, Bartley EJ, Williams AE, McCabe KM, Russell JL. Does pain catastrophizing moderate the relationship between spinal nociceptive processes and pain sensitivity? J Pain. 2009;10:860–869. [DOI] [PubMed] [Google Scholar]
- 50. Edwards RR, Smith MT, Stonerock G, Haythornthwaite JA. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. [DOI] [PubMed] [Google Scholar]
- 51. Tett RP, Burnett DD. A personality trait-based interactionist model of job performance. J Appl Psychol. 2003;88:500–517. [DOI] [PubMed] [Google Scholar]
- 52. Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: Between pro- and antinociception. Pain. 2014;155:663–665. [DOI] [PubMed] [Google Scholar]
- 53. Nir RR, Yarnitsky D, Honigman L, Granot M. Cognitive manipulation targeted at decreasing the conditioning pain perception reduces the efficacy of conditioned pain modulation. Pain. 2012;153:170–176. [DOI] [PubMed] [Google Scholar]
- 54. Moont R, Pud D, Sprecher E, Sharvit G, Yarnitsky D. “Pain inhibits pain” mechanisms: Is pain modulation simply due to distraction? Pain. 2010;150:113–120. [DOI] [PubMed] [Google Scholar]
- 55. Piché M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci. 2009;29:14236–14246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huber FA, Kuhn BL, Lannon EW, et al. Less efficient endogenous inhibition of spinal nociception predicts chronic pain onset: a prospective analysis from the Oklahoma Study of Native American Pain Risk (OK-SNAP). J Pain. 2019;20:S40. [Google Scholar]
- 57. France CR, France JL, al’Absi M, Ring C, McIntyre D. Catastrophizing is related to pain ratings, but not nociceptive flexion reflex threshold. Pain. 2002;99:459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. France CR, Keefe FJ, Emery CF, et al. Laboratory pain perception and clinical pain in post-menopausal women and age-matched men with osteoarthritis: relationship to pain coping and hormonal status. Pain. 2004;112:274–281. [DOI] [PubMed] [Google Scholar]
- 59. Bartley EJ, Rhudy JL. The influence of pain catastrophizing on experimentally induced emotion and emotional modulation of nociception. J Pain. 2008;9:388–396. [DOI] [PubMed] [Google Scholar]
- 60. Terry EL, Thompson KA, Rhudy JL. Experimental reduction of pain catastrophizing modulates pain report but not spinal nociception as verified by mediation analyses. Pain. 2015;156:1477–1488. [DOI] [PubMed] [Google Scholar]
- 61. Terry EL, Thompson KA, Rhudy JL. Does pain catastrophizing contribute to threat-evoked amplification of pain and spinal nociception? Pain. 2016;157:456–465. [DOI] [PubMed] [Google Scholar]
- 62. Campbell CM, McCauley L, Bounds SC, et al. Changes in pain catastrophizing predict later changes in fibromyalgia clinical and experimental pain report: Cross-lagged panel analyses of dispositional and situational catastrophizing. Arthritis Res Ther. 2012;14:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sturgeon JA, Zautra AJ. State and trait pain catastrophizing and emotional health in rheumatoid arthritis. Ann Behav Med. 2013;45:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Racine M, Moulin DE, Nielson WR, et al. The reciprocal associations between catastrophizing and pain outcomes in patients being treated for neuropathic pain: A cross-lagged panel analysis study. Pain. 2016;157:1946–1953. [DOI] [PubMed] [Google Scholar]
- 65. Darnall BD, Sturgeon JA, Kao MC, Hah JM, Mackey SC. From catastrophizing to recovery: A pilot study of a single-session treatment for pain catastrophizing. J Pain Res. 2014;7:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. [DOI] [PubMed] [Google Scholar]
- 67. Jensen MP, Turner JA, Romano JM. Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol. 2001;69:655–662. [DOI] [PubMed] [Google Scholar]
- 68. Thorn BE, Boothby JL, Sullivan MJL. Targeted treatment of catastrophizing for the management of chronic pain. Cogn Behav Pract. 2002;9:127–138. [Google Scholar]
- 69. Donaldson GW, Chapman CR, Nakamura Y, Bradshaw DH, Jacobson RC, Chapman CN. Pain and the defense response: Structural equation modeling reveals a coordinated psychophysiological response to increasing painful stimulation. Pain. 2003;102:97–108. [DOI] [PubMed] [Google Scholar]
- 70. Walters ET. Injury-related behavior and neuronal plasticity: An evolutionary perspective on sensitization, hyperalgesia, and analgesia. Int Rev Neurobiol. 1994;36:325–427. [DOI] [PubMed] [Google Scholar]
- 71. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]