Abstract
Objective
This study evaluated the potential efficacy of a novel approach to treat COVID-19 patients, using an oxygen-ozone (O2-O3) mixture, via a process called Oxygen-Ozone- Immunoceutical Therapy. The methodology met the criteria of a novel, promising approach to treat successfully elderly COVID-19 patients, particularly when hospitalized in intensive care units (ICUs) Experimental design: We investigated the therapeutic effect of 4 cycles of O2-O3 in 50 hospitalized COVID-19 subjects suffering from acute respiratory disease syndrome (ARDS), aged more than 60 years, all males and undergoing non invasive mechanical ventilation in ICUs.
Results
Following O2-O3 treatment a significant improvement in inflammation and oxygenation indexes occurred rapidly and within the first 9 days after the treatment, despite the expected 14–20 days. A significant reduction of inflammatory and thromboembolic markers (CRP, IL-6, D-dimer) was observed. Furthermore, amelioration in the major respiratory indexes, such as respiratory and gas exchange markers (SatO2%, PaO2/FiO2 ratio), was reported.
Conclusion
Our results show that O2-O3 treatment would be a promising therapy for COVID-19 patients. It leads patients to a fast recovery from ARDS via the improvement of major respiratory indexes and blood gas parameters, following a relatively short time of dispensed forced ventilation (about one to two weeks). This study may encourage the scientific community to further investigate and evaluate the proposed method for the treatment of COVID-19 patients.
Keywords: Ozone therapy, Immunoceutical, Oxygen, COVID-19, Pneumonia, D-dimer, IL-6
1. Introduction
The “new” coronavirus, SARS-CoV-2, the causative pathogen of COVID-19, rapidly spread worldwide with a significant mortality rate [1]. According to the World Health Organization (WHO), SARS-CoV-2 is characterized by a 14-days incubation period and despite this interval is typically reported for the previous SARS-CoV1, it is yet considered as the minimal window time where COVID-19 should initiate its symptomatology [2], [3], [4]. During the asymptomatic incubation time a quick global human-to-human transmission can occur, as infected people can easily escape simple detection methods such as body temperature. This hampers a rapid intervention to prevent severe exacerbations caused by COVID-19 and leading people to intensive care units (ICUs). Initially China, then followed by most of the countries worldwide, have put millions of people under lockdown to face at the rapid SARS-CoV2 outbreak and to prevent further spreading of the disease by non-hospitalized and mainly asymptomatic people. In symptomatic COVID-19 patients an exacerbation of the disease, notably characterized by a systemic disorder of vascular physiology, pulmonary function and immunity, rapidly occurs [5], [6]. Evidence-based medicine has recently developed a promising bulk of therapeutic approaches against this disease and research worldwide against COVID-19 is increasing its efforts. In this context, Hotchkiss and Opal introduced a new approach to fight virulent pathogens such as coronaviruses, via an immuno-modulation, a strategy they called “a new path” [7]. This should suggest that immuno-modulatory molecules, such as ozone, may counteract COVID-19 development. The authors actually addressed the possibility of tailoring targeted drugs to boost immune functions in patients with life-threatening infectious [7]. Therefore, molecules able to tune immunity whereas adjusting further physiological parameters are particularly attracting current pharmacology. This may be crucial for planning a novel immuno-therapeutic strategy to dampen the current COVID-19 outbreak, by modulating the immune response via molecules able to tune the cross talk between immunity and the lung microbiome or to directly affecting the virus life-cycle. A possibility may be offered by ozone.
In a letter to the Italian Society of Oxygen Ozone Therapy (SIOOT), dated March 24th 2020, the ISS, i.e. the Italian National Institute of Health, assessed a previously proposed treatment employing a mixture of oxygen and ozone (O2-O3) as effective to treat patients suffering from COVID-19. So far, only few interesting hypotheses were made to elucidate the potential mechanism of action of the Oxygen Ozone (O2-O3) therapy in the treatment of SARS-CoV2 infections and further research is needed [8], [9].
Despite the great attention devoted to the possibility of using ozone in the therapy of COVID-19, very few, quite anecdotic attempts to use ozone in COVID-19 treatment were made so far [10]. As far as we know, to date no cohort observational clinical study has been performed for this new therapeutic strategy, if we except the very recent pre-print from Hernandez et al., besides the use in only modest clinical COVID-19 cases [11]. Further clinical data are needed to assess if ozone can be helpful in restoring lung functionality and oxygenation in COVID-19 affected patients.
This case series study has the purpose to evaluate for the first time the clinical impact of O2-O3 treatment in COVID-19 affected hospitalized patients, focusing on the pulmonary recovery and immune response following the described therapy.
2. Materials and methods
2.1. Subjects recruitment and co-morbidity
In this case series study, a number of 50 SARS-COV2 on 78 positive elderly patients was considered. Patients undergoing ICU hospitalization for COVID-19, showing ARDS and interstitial pneumonia at chest computer tomography (CT), were considered eligible to be enrolled in the study, 48 of them completed the study, 2 died. All subjects (mean age 75 yrs ± 11.4 SD, all males) agreed and signed an informed consent.
Patient statistical stratification of the many different co-morbidities included 4%: no co-morbidity, 24%: 1 co-morbidity (obesity), 36%: 2 co-morbidities, (obesity with arterial hypertension), 32%:2 co-morbidities (type 2 diabetes, obesity), 2%: three co-morbidities (type 2 diabetes, obesity, hypertension), 2%: others (COPD). The percentage of co-morbidities present in the sample was comparable to the co-morbidity stratification in the resident population of elderly people in Lombardy.
2.2. Sample size
Sample size was calculated for one study group vs the whole population and as an endpoint a binomial (dichotomous) approach, following an anticipated incidence of 27.85% (positive outcome) on the population of Lombardy and at least 50% of positive outcome in the study population, with power = 80%, alpha error = 0.05, beta error = 0.2. In this case the minimal number of samples was 34 (SPSS v 24.0), following previously reported methodological recommendations [12].
2.3. Inclusion criteria
The hospitalized patients underwent routine nasal-pharyngeal swabbing for SARS-CoV2 RT-qPCR (TaqMan2019-nCoV assay kit v1, Thermo-Fisher on Applied Biosystem 7500), according to standardized protocols and WHO guidelines [13]. O2-O3 treatment was included in the consensus routine therapy for COVID-19. Major inclusion (eligibility) criteria were represented by people with concomitant pneumonia and COVID-19 double positivity. All subjects were all male and older than 60 years, in order to achieve a homogeneous sampling regarding age. Some patients might have pharmaceutical untreated and/or compensated metabolic co-morbidities, whereas patients having diabetes were monitored for glucose levels [14].
2.4. Exclusion criteria
Major exclusion criteria were patients suffering from pulmonary or respiratory acute and/or severe syndrome without being positive for SARS-CoV2, age younger than 60 years old, undergoing tracheotomy and other highly critical invasive approaches in the intensive care units (ICUs) and suffering from severe cardiovascular pathologies. Due to the very critical condition associated with invasive tracheotomy, we decided to exclude these patients from O2-O3 treatment, as we cannot be ensured about possible exacerbating side effects in this circumstance.
2.5. Blood specimens and analysis
Patients underwent a set of laboratory tests throughout their O2-O3 treatment. K3-EDTA Vacutainer whole blood specimens, Na-citrate plasma and serum samples were collected and processed in the local hospital laboratories for whole blood analysis (WBCs, RBCs, PLTS, hemoglobin, hematocrit and leukocyte formula), C-reactive protein (CRP), IL6 (R&D Hu-Quantikine® ELISA kit), pro-calcitonin (PCT, R&D DY8350-05 ELISA kit), D-dimer (Human D2D Elabscience® ELISA kit), liver and kidney major functional markers and gas exchange/pH parameters (blood gas analysis, pH, peripheral partial pressures O2 and CO2, acidic components (H2CO3 — and lactate)), ratio arterial concentration of O2 to the O2 inspiration fraction (PaO2/FiO2). Major parameters indicating significant changes in patients’ physiology during the O2-O3 treatment, were collected and further described.
2.6. Patients therapy and clinical outcomes (endpoints)
Patients were initially treated with the therapeutic panel shown in Table 1A . Because of COVID-19, these patients presented a severe impairment of pulmonary functionality and oxygen saturation percentage and underwent non invasive ventilation. These subjects represented the majority (86%) of all subjects included in this study, whereas the rest (11%) underwent invasive ventilation (endotracheal intubation) and 3% unexpected tracheotomy, therefore excluded from the treatment. The therapy panel to treat COVID-19 was suggested and recommended according the most recent clinical and pharmacological experience with SARS-CoV2-caused pneumonia [15]. O2-O3 treatment was introduced to have as major goals the following endpoints:: a) reduction in the inflammation burst (markers of inflammation CRP ≤ 30%, IL-6 ≤ 25%,), b) decrease of D-dimer (≤35%), c) increase in the parameters of respiratory functions and oxygen exchange (O2 saturation % ≥ 10%, PaO2/FiO2 ≥ 6%).
Table 1A.
Elective pharmacological therapy used in the study.
|
The O2O3 therapy in COVID-19 patients was carried on according to the protocol used by SIOOT [8]. The treatment requires an ozone generator, a medical grade compressed oxygen, a syringe and a certified bag with an intravenous cannula for ozone therapy via auto-hemotherapy. A maximal volume of 200 ml of blood was withdrawn from any patient and collected in a CE certified SANO3 bag, then immediately treated with 45 μg/ml O2-O3 mixture (Multioxygen Medical 95 CPS) and reintroduced into the circulatory blood directly [16].
Ozone therapy can be considered as extremely safe, with a reported complication rate of only 0.7 adverse events every 100,000 treatments (0.0007%), usually lasting 30 min [8]. It is also very cost effective [17]. Regularly peripheral blood samples were withdrawn from veni-puncture and samples were analyzed for blood gases. Within the first week of hospitalization, patients subjected to a non invasive ventilation, were suggested to undergo an O2-O3 treatment and time 0 started with the first treatment. O2-O3, treatment lasted from three to five (median = four) cycles with 100–200 ml (median = 125 ml, CI95 = 45–215 ml) of O2-O3 once a day for 5 consecutive days (dosage: 45 μg/ml) with a Multioxygen Medical 95 CPS in CE certified SANO3 bag. Then oxygen saturation indexes were evaluated.
2.7. Device
The device Multioxygen® Medical 95 (Rome, Italy) is an outpatient unit for O2-O3 therapy, which allows operators to customize the gas mixture according to the clinical request. The equipment is for medical and/or research use only. The O2-O3 mixture generator is managed by a microprocessor, which guarantees the precision of the delivery, once the O3-O2 mixture amount is selected by the operator. It is possible to customize the treatment by selecting the ozone concentration in a continuous range from 1 to 100 μg of O3. The ozone concentration can also be further modulated by varying the oxygen percentage of the mixture. The treatment can be performed by only expert and highly trained medical personnel and can be carried out in all conditions of use, especially for topical treatments.
2.8. Statistics
Data means ± SD and CI95 whenever indicated were used for statistics. A p < 0.05 value of significance from non-parametric tests (Wilcoxon rank test, following a Shapiro-Wilk test) was calculated for each matched group. Analysis of covariance (ANCOVA) for two independent samples was performed to remove confounding effects from other therapeutic drugs that were administered during the O2-O3 treatment. Effect size calculation using the Cohen d test was also applied. Data were calculated with SPSS Vs. 24 and plotted with Sigma plot Vs.13.
3. Results
Fundamentally, a great heterogeneity of investigated parameters to follow up the ability of our O2-O3 therapy approach to treat COVID-19 patients was observed. A limitation of the study is represented by a relatively small group of patients, despite the sample size minimal for a positive Cohen d test was 34, Yet, the investigation included a limited range of ages, and moreover the exclusion of female subjects and the possible interference, though prevented, of statistical confounders on the observed markers from other therapies. Despite these flaws, the most encouraging outcome is the ability of O2-O3 treatment to reduce significantly (p = 0.0023) the recovery of normal functional pulmonary parameters in less than 10 days (median 13.45 ± 2.33 days), respect to the expected, estimated values for hospitalization in our structures (22.13 ± 3.44 days). Table 1B shows O2-O3 treatment effects observed on a selected group of parameters evaluated in the present study following an ANCOVA test. Ozone effect on the increase in leukocytes was not significant, whereas two major inflammatory markers, i.e. CRP and IL-6, were markedly reduced, i.e. of about 48.15% (covariance = 9576.177, p = 0.0167), and 86.17% (covariance = 9113.337, p = 0.0275), respectively. Reductions were observed also for bacterial-mediated inflammation markers (pro-calcitonin, PCT) and fasting glucose, despite the hypoglycemic effect of O2-O3 treatment was non-significant (p > 0.05, see Table 1B. Among the observed effects of O2-O3 treatment, it is noteworthy the increase in the percentage of O2 saturation (SatO2%) = + 13,26%, p < 0.001,. This result has been further evaluated considering that Hb values and arterial pressures were almost unchanged during the O2-O3 treatment and that the ratio PaO2/FiO2 increased following the treatment, just irrespective of the modest increase (+4,5%) following noninvasive mechanical ventilation (ANCOVA p = 0.00456), so reporting a possible recovery of normal respiratory functions mainly due to O2-O3 treatment (see also Fig. 1 )
Table 1B.
Major changes of clinical and laboratory data on O2-O3 treated patients before and after treatment (CI95 ranges).
| parameter | before | after | p (*) | |
|---|---|---|---|---|
| Temperature | 99.86–101.66 °F | 96.8–97.7 °F | ↓ | P > 0.05 |
| Heart rate (min–max) | 90–100 | 70–75 | ↓ | P > 0.05 |
| Sat O2 % | 80–85% | 90–95% | ↑ | P < 0.001 |
| Fasting glucose mg/dL |
200–250 | 120–150 | ↓ | P > 0.05 |
| ALT (IU/L) | 90–100 | 40–50 | ↓ | P < 0.05 |
| Creatinine mg/dL |
1,5–3.0 | 1.0–1,3 | ↓ | P > 0.05 |
| Leukocytes number/μl |
3,000–4,000 | ≥ 5,500 | ↑ | P > 0.05 |
| CRP mg/dL |
10–15 | ≤ 5.0 | ↓ | P < 0.01 |
| IL6 (IU/L) |
25–660 | ≤ 100 | ↓ | P < 0.001 |
| LDH IU/L |
300–350 | ≤ 300 | ↓ | P < 0.05 |
| Procalcitonin ng/ml |
≤ 0.5 ng/ml | ≤ 0.05 ng/ml | ↓ | P < 0.05 |
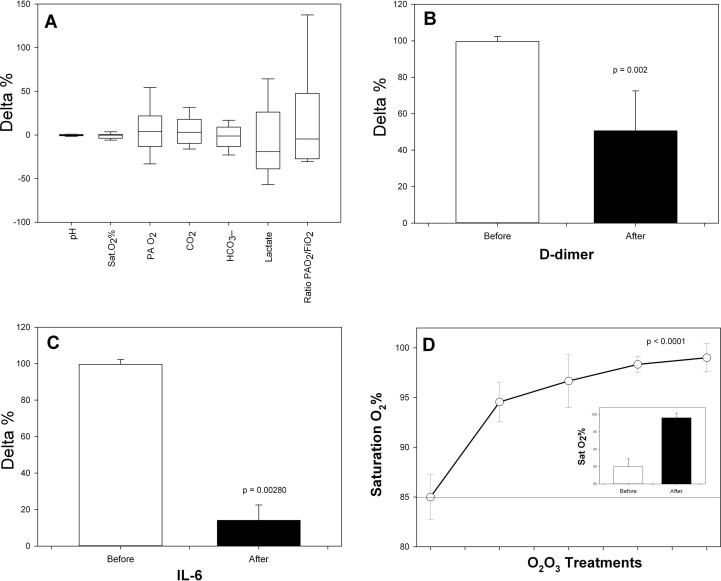
Fig. 1.
(A) Box plots of the delta percentage (delta%) of major blood gas analysis and respiratory gaseous indexes before and after the last O2-O3 treatment [(last – before)/before]*100; (B) Delta % of D-dimer (ng/ml) before and after the last O2-O3treatment [(last – before)/before]*100 t; (C) Delta % of IL-6 (IU/L) before and after the last O2-O3 treatment [(last – before)/before]*100; (D) Time course of Sat O2 % in respect to the different treatments points. In the small panel the delta % of cumulated data from before and from the last performed treatment (8.6 ± 1.4 days of treatment, Wilcoxon p = 0.000234). Statistics SPSS v 24.0. Plots with Sigma Plot v 13.0.
Fig. 1A shows the variability expressed in box plots as delta percentage (Δ%) (the difference % between post- and pre- O2-O3 treatment) with means and their CI95 of the blood gas analysis and functional gas-exchange (respiratory) indexes. The largest variability can be observed for serum lactate (mmol/L) (mean = − 8,220% ± 39.91 SD) and the (PaO2/FiO2Ratio, mean = +13.812% ± 58.897 SD). A very interesting result is the significant reduction of D-dimer in patients undergoing at least three consecutive treatments with O2-O3. In these patients, circulating D-dimer lowered of about 50.617% ± 21.904 SD respect to the level reported before treatment (p = 0.002) (Fig. 1B). O2-O3 treatment reduced dramatically also systemic inflammation, by dampening the level of serum IL-6 (- 86.17% ± 33.44 SD, Fig. 1C) and restoring the percentage of O2 saturation throughout the whole treatment (Fig. 1D). The Δ% delta percentage of Sat O2 %, adjusted for the possible statistical confounders, increased of about one sixth following 8.6 ± 1.4 days of treatment respect to the starting condition (ANCOVA test, p = 0.00543) (panel inside Fig. 1D).
4. Discussions
Data show that O2-O3 treatment can improve COVID-19 patients’ clinical condition towards their quite complete recovery in a very modest time course, boosting many gas exchange parameters in subjects undergoing forced non invasive ventilation in ICUs. This brought patients quite rapidly towards a normal respiratory physiology, reducing the inflammatory, ischemic and thromboembolic impact and ultimately resulting in a quite full recovery of parameters of O2 saturation percentages (O2Sat%). A possible explanation for the action of O2-O3 might encompass macro-effects due to the subject’s response to ischemia/reperfusion injury or thromboembolic mechanisms, possibly occurring in COVID-19-caused pneumonia. However, as such explanation should include the possible effect of further therapy with which O2-O3 treatment would be associated, a much direct molecular effect of ozone must be considered to shed light on the evidence here reported. This direct action, to be further demonstrated with a larger group of patients, should involve an improvement in the host’s immune response, particularly if inflammation is properly dampened.
Actually, further mechanisms could be implicated, to elucidate the effect of O2-O3 treatment in COVID-19.
A possibility may come from the induction of Nrf2-mediated blocking of the fusion of SARS-CoV2 S protein with the ACE2 receptor [18]. Coronaviruses possess abundant cysteines in their spike proteins, which may be easily and safely targeted by ozone as a pro-axidant molecule. As cysteine residues are also abundant in conserved motifs of the viral envelope proteins, essential for viral cell entry, they are also a target for palmitoylation. Ozone’s ability to effectively inactivate cysteine dependent proteins was described recently as an ozonide attack on cysteine-dependent papain [18]. It is believed that enzymes could be inactivated by oxidizing the active sulfhydryl group to sulfenate or sulfenic acid. These mechanisms are able to directly dampen SARS-CoV2 infection and to result in a more rapid recovery from pneumonia. Furthermore, coronavirus spike proteins are also rich in tryptophan, which likewise cysteine is very vulnerable for oxidation [3]. So, it is possible to argue about a direct effect of ozone on the viral biology of SARS-CoV2.
Furthermore, the interesting result about D-dimer expands the debate about the role of our O2-O3 treatment in thromboembolism, or the co-morbid vascular and thrombotic disorders associated with COVID-19, as D-dimer is usually increased during COVID-19 and highly modulated in the presence of ozone [19], [20]. This may be a further intriguing cause for which ozone is efficacious in our study.
Also, we are persuaded that O2-O3 acts on the nitric oxide (NO) and iNOS signaling pathways. NO dampens the viral replication of SARS-CoV2, particularly by inhibiting the palmitoylation of the S (spike) protein, so preventing virus attachment to the ACE2 receptor [21], [22]. Here it should also be mentioned that ozone increases the expression of the induced nitric oxide synthase (iNOS), the main enzyme producing NO, in rat type II pneumocytes [23]. Moreover, the action of O2-O3 on NLRP3 inflammasome should be also considered, probably it causes an effect on IL-6 [24].
Finally, we are currently investigating if one of the major causative mechanism in COVID-19 is actually related to thromboembolic phenomena, our observed reduction in markers such as D-dimer appears to be encouraging evidence supporting the issue, despite the fact that ischemia/reperfusion injury (HRI), was a much more stressed issue in the research about ozone treatment [25], [26].
The role of HRI in this context should be better highlighted. Ozone was evaluated in aortic dissection models where following ozonation of whole peripheral blood, some authors reported that the median levels of PO2 and SatO2 were increased in samples with ozone concentrations at 40 μg/mL, 80 μg/mL and 160 μg/mL in both patients with aortic dissection and in the control group, (p < 0.05), compared with samples exposed to 0 μg/mL ozone. Ozone, in this circumstance does not damage blood cells and erythrocytes [27].
5. Conclusion
This is a case series study where we showed that, despite the relative small number of cases, ozone, as part of the O2-O3 therapy, may reduce the impact and possible exacerbation caused by COVID-19 related pneumonia in elderly hospitalized patients undergoing non invasive ventilation in a time significantly shorter that the expected mean from other therapies. Obviously, this evidence needs to be reappraised and further confirmed. Limitation of the study are represented by criticism in the homogeneity of patients, based on their co-morbidities and by the existence of confounding variables, which are quite impossible to remove in a case series study. Notably, our evidence suggests that a systemic oxygen ozone therapy can be considered as a novel and effective method to immune-modulate host response against SARS-CoV2. Because of the relatively small group of patients, the effects described here need to be further assessed. The use of this new therapy in combination with other treatment options like the usage of special drugs such as hydroxychloroquine or Remdesivir®, may be recommended in COVID-19 patients. We are aware of that further studies on a larger collective are requested to confirm our hypothesis. We encourage our colleagues to find confirmation of our approach, also immediately in combination with their current treatment regimen, in order to generate more evidence that O2O3 therapy works synergistically and effectively in controlling and treating COVID-19 pneumonia.
CRediT authorship contribution statement
Marianno Franzini: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft. Luigi Valdenassi: Investigation, Resources, Visualization. Giovanni Ricevuti: Investigation, Resources, Visualization. Salvatore Chirumbolo: Data curation, Formal analysis, Supervision, Validation, Writing - original draft, Writing - review & editing. Markus Depfenhart: Methodology, Visualization, Writing - review & editing. Dario Bertossi: Data curation, Methodology, Supervision, Validation. Umberto Tirelli: Software, Visualization.
Acknowledgement
We acknowledge and thank the Società Italiana di Ossigeno Ozono Terapia (SIIOT) for its fundamental contribution in this research work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2020.106879.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The Incubation Period of Coronavirus Disease 2019 (COVID-19) From Publicly Reported Confirmed Cases: Estimation and Application. Ann. Intern. Med. 2020;172(9):577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu D., Wu T., Liu Q., Yang Z. The SARS-CoV-2 outbreak: What we know [published online ahead of print, 2020 Mar 12] Int. J. Infect. Dis. 2020;94:44–48. doi: 10.1016/j.ijid.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K., Trilling M., Lu M., Dittmer U., Yang D. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS‐CoV, MERS‐CoV, and 2019‐nCoV. J Med Virol. 2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorbello M., El‐Boghdadly K., Di Giacinto I., Cataldo R., Esposito C., Falcetta S., Merli G., Cortese G., Corso R.M., Bressan F., Pintaudi S., Greif R., Donati A., Petrini F. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75(6):724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 7.Phimister E.G., Hotchkiss R.S., Opal S.M. Activating Immunity to Fight a Foe — A New Path. N Engl J Med. 2020;382(13):1270–1272. doi: 10.1056/NEJMcibr1917242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (O2–O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4059–4061. doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- 9.S. Chirumbolo, G. Bjørklund. The bimodal SARS-CoV2 as an effect of environmental and allergic causes J Allergy Clin Immunology 2020 in press, DOI:https://doi.org/10.1016/j.jaci.2020.05.011. [DOI] [PMC free article] [PubMed]
- 10.Hernández A., Papadakos P.J., Torres A. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19 [published online ahead of print, 2020 Apr 14]. Dos terapias conocidas podrían ser efectivas como adyuvantes en el paciente critic infectado por COVID-19 [published online ahead of print, 2020 Apr 14] Rev. Esp. Anestesiol Reanim. 2020;S0034–9356(20):30075-X. doi: 10.1016/j.redar.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.A., Hernandez, V., Montserrat, A., Pablos, F., Vlas, P., J., Papadokos, D., N., Wijeeysundera, M., Vives. Ozone therapy for patients with SARS-CoV2 pneumonia: a single-center prospective cohort study MedRXiv doi: https://doi.org/10.1101/2020.06.03.20117994, June 12, 2020.
- 12.Pourhoseingholi M.A., Vahedi M., Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol. Hepatol. Bed. Bench. 2013;6(1):14–17. [PMC free article] [PubMed] [Google Scholar]
- 13.Lan P.T., Cuong H.Q., Linh H.T., Hieu N.T., Anh N.H., Ton T., Dong T.C., Thao V.T., Tuoi D.T.H., Tuan N.D., Kim Loan H.T., Long N.T., Thang C.M., Hai N.D., Thao N.T.T. Development of standardized specimens with known concentrations for severe acute respiratory syndrome coronavirus 2 Realtime-RT-PCR testing validation. Bull. World Health Organ. E-pub. April 2020;20 doi: 10.2471/BLT.20.259630. [Preprint] [DOI] [Google Scholar]
- 14.Marfella R., Paolisso P., Sardu C., Bergamaschi L., D’Angelo E.C., Barbieri M., Rizzo M.R., Messina V., Maggi P., Coppola N., Pizzi C., Biffi M., Viale P., Galiè N., Paolisso G. Negative impact of hyperglycaemia on tocilizumab therapy in Covid-19 patients [published online ahead of print, 2020 May 21]. Diabetes Metab. S1262. 2020;3636(20):30082–30083. doi: 10.1016/j.diabet.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn D.G., Shin H.J., Kim M.H. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19) J. Microbiol. Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elvis A.M., Ekta J.S. Ozone therapy: A clinical review. J. Nat. Sci. Biol. Med. 2011;2(1):66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.V., Simonetti, V., Quagliariello, M., Franzini, R.V., Iaffaioli, N., Maurea, L., Valdenassi. Ozone Exerts Cytoprotective and Anti-Inflammatory Effects in Cardiomyocytes and Skin Fibroblasts after Incubation with Doxorubicin. Evid Based Complement Alternat Med. 2019 (2019) 2169103. Published 2019 Nov 18. doi:10.1155/2019/2169103H. [DOI] [PMC free article] [PubMed]
- 18.Zhang J.M., Penninger Y., Li N., Zhong A.S., Slutsky Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potentialtherapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection [published online ahead of print, 2020 Mar 16] Clin. Chem. LabMed. 2020 doi: 10.1515/cclm-2020-0188. /j/cclm.ahead-of-print/cclm-2020-0188/cclm-2020-0188.xml. [DOI] [PubMed] [Google Scholar]
- 20.Kahle J.I., Neas L.M., Devlin R.B. Interaction effects of temperature and ozone on lung function and markers of systemic inflammation, coagulation, and fibrinolysis: a crossover study of healthy young volunteers. Environ Health Perspect. 2015;123(4):310–316. doi: 10.1289/ehp.1307986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akerström S., Gunalan V., Keng C.T., Tan Y.J., Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the Sprotein are affected. Virology. 2009;395(1):1–9. doi: 10.1016/j.virol.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robba C., Robba C., Battaglini D., Ball L., Patroniti L.N., Loconte M., Brunetti I., Vena A., Giacobbe D., Bassetti M., Rocco P.R.M., Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19, Respiratory Physiology and amp. Neurobiology. 2020 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Punjabi C.J., Laskin J.D., Pendino K.J., Goller N.L., Durham S.K., Laskin D.L. Production of nitric oxide by rat type II pneumocytes: increased expression of inducible nitric oxide synthase following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11(2):165–172. doi: 10.1165/ajrcmb.11.2.7519435. [DOI] [PubMed] [Google Scholar]
- 24.Rowen R.J. Ozone and oxidation therapies as a solution to the emerging crisis in infectious disease management: a review of current knowledge and experience. Med Gas Res. 2019;9(4):232–237. doi: 10.4103/2045-9912.273962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagai M., Bocci V. Mechanisms of Action Involved in Ozone Therapy: Is healinginduced via a mild oxidative stress? Med Gas Res. 2011;1:29. doi: 10.1186/2045-9912-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.S. Safari, M. Mehrani, M. Yousefifard. Pulmonary Thromboembolism as a Potential Cause of Clinical Deterioration in COVID-19 Patients; a Commentary. Arch AcadEmerg Med. 8(1) (2020) e52. Published 2020 Apr 19. [PMC free article] [PubMed]
- 27.Deng L., Meng W., Li D., Qiu D., Wang S., Liu H. The effect of ozone on hypoxia, hemolysis and morphological change of blood from patients with aortic dissection (AD): a preliminary in vitro experiment of ozonated autohemotherapy for treating AD. Am. J. Transl. Res. 2018;10(6):1829–1840. Published 2018 Jun 15. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.