Abstract
SARS-CoV-2 (also known as COVID-19) has been an unprecedented challenge in many parts of the medical field with blood banking being no exception. COVID-19 has had a distinctly negative effect on our blood collection nationwide forcing blood banks, blood centers, and the US government to adopt new policies to adapt to a decreased blood supply as well as to protect our donors from COVID-19. These policies can be seen distinctly in patient blood management and blood bank operations. We are also faced with developing policies and procedures for a nontraditional therapy, convalescent plasma; its efficacy and safety is still not completely elucidated as of yet.
Keywords: COVID-19, Blood banking, Transfusion medicine, Blood shortage, Blood wastage, FDA donation policies, Convalescent plasma
Key points
-
•
COVID-19 has had a negative impact on blood collection.
-
•
The Centers for Disease Control, blood centers, and American Red Cross have developed new policies to protect donors and the blood supply.
-
•
Blood management has become more important with decreasing supply as well as management of blood bank personnel.
-
•
Convalescent plasma, although touted as a possible treatment, has limited literature on its efficacy.
Introduction
Within 3 months of the first diagnosed case, the outbreak of acute respiratory disease caused by the novel coronavirus (SARS-CoV-2), also known as COVID-19, rapidly grew into a global pandemic. The eruption of this virus, reportedly, may have been linked to a seafood and wildlife market in Wuhan, Hubei Province, China.1 , 2 Person-to-person spread is thought to occur via respiratory droplet contact (≤6 feet), skin contacts, and even the transmission through air while speaking. Despite the extraordinary universal attempts to limit the spread of this virus, new cases are diagnosed on a daily basis throughout the world and have dramatically affected, among other health care disciplines, the blood bank and transfusion medicine. Because of the growing prevalence and highly infectious nature of COVID-19, new policies and guidelines have started to develop in transfusion medicine practices. In this article the authors address the myriad of ways that COVID-19 has affected blood banking and transfusion medicine, including the safety of both blood donors and blood product recipients, the management and distribution of blood products during a pandemic, and the use of blood product–derived therapeutics.
Clinical manifestations of COVID-19
Common symptoms of COVID-19 infection, which typically appear within 2 to 14 days after exposure, include fever, sore throat, cough, shortness of breath, chills, muscle pain, headache, and sensory changes such as loss of smell or taste. Nausea, vomiting, diarrhea, skin rash, delirium, and dizziness have also been reported.3 , 4 In advanced cases, mild-to-severe lower respiratory tract infection can be seen and may progress to critical status, as a result of the cytokine storm, requiring intubation and mechanical ventilation. Acute respiratory failure and a widespread thromboembolic disease are also common in these critical cases.5 , 6 However, conservative estimates of 30% to as high as 96% of infected individuals may manifest mild to no symptoms, posing huge challenges in containing this pandemic crisis and protecting blood donors.7
In one of the earlier studies of COVID-19 patients, Guan and colleagues8 reported that in addition to the usual viral route of respiratory droplets, the virus could be transmitted by saliva, urine, and stool.9 Extracted data of 1099 patients with laboratory-confirmed COVID-19–related acute respiratory distress syndrome (ARDS) showed a predominant male gender (58%), median age of 47 years with most common symptoms of fever (88%) and cough (68%). The median incubation period was found to be 3 days with a range of 0 to 24 days. Only 1.2% of patients reported to have a direct contact with wildlife and 31% had been to Wuhan city, whereas the majority (72%) had contact with people from Wuhan city. At time of admission, ground-glass opacity was the typical (56.4%) radiological finding on chest computed tomography (CT). Interestingly, a significant number of severe cases were diagnosed by clinical symptoms and real-time reverse transcriptase polymerase chain reaction (RT-PCR) with normal radiological findings. Multivariate analysis revealed that severe pneumonia was an independent factor associated with either intensive care unit (ICU) admission, mechanical ventilation, or death (hazard ratio, 9.80; 95% confidence interval, 4.06–23.67).8
COVID-19 diagnosis
Ideally, testing every blood donor for COVID-19 would be the best practice; however, at least for the time being, this task cannot realistically be accomplished. To date, 7 recognized types of coronavirus strains that can infect humans have been identified, including Alpha coronavirus (229E and NL63) and Beta coronavirus (OC43 and HKU1). The rare but more severe types are called MERS-CoV, which lead to Middle East respiratory syndrome (MERS), and SARS-CoV, responsible for severe acute respiratory syndrome (SARS) endemic.10
Laboratory confirmation of COVID-19 infection is based on detection of unique sequences of viral RNA by RT-PCR. Sputum samples provide better detection than throat samples, whereas lower respiratory tract samples are superior to those from the upper respiratory tract.11 The presence of SARS-CoV-2 RNA in the blood is a marker of severe illness based on 113 studies.12 Additional laboratory findings in COVID-19 infection include lymphopenia (83%); neutrophilia; and elevated levels of serum alanine aminotransferase, aspartate aminotransferase, lactate dehydrogenase, C-reactive protein (CRP), ferritin, and D-dimer.13 Substantial increase in CRP, ferritin, and D-dimer levels were found to be associated with severe infection.14 In addition, significant association has been recognized between lymphopenia and high levels of D-dimer with mortality.15 , 16
Bilateral air-space consolidation is typically seen on chest radiograph; however, findings may be unremarkable early in the disease. Chest CT images usually demonstrate bilateral, peripheral ground glass opacities, which is nonspecific to COVID-19 infection.
Management of COVID-19
An effective COVID-19 vaccine would be the ultimate solution for all of the concerns surrounding blood bank industry. However, currently there is no vaccine available to protect against SARS-CoV-2. Likewise, no prophylactic therapy has yet been proved to be effective in patients who have been exposed to SARS-CoV-2 nor a clearly successful treatment of those who develop the infection.17 Patients who are confirmed positive for COVID-19 and present with mild symptoms are usually managed by self-isolation at home for up to 14 days, which is also the minimal deferral period recommended by many blood centers.7, 8, 9, 10 In advanced cases, hospitalization may be required for clinical observation and supportive management with fluid and oxygen resuscitations, anticoagulation, empirical antibiotics in case of a secondary infection, and nonsteroidal antiinflammatory agents in some cases. Critical cases may require ICU admission for possible intubation and mechanical ventilation. Corticosteroids and immunosuppressive agents are usually not recommended except when required for other indications or in a cytokine storm. Extracorporeal membrane oxygenation can be considered but is associated with a high mortality rate.
Complications of COVID-19 infection include pneumonia, respiratory failure, ARDS, sepsis and septic shock, cardiomyopathy and arrhythmia, acute kidney injury, bacterial infections, thromboembolism, gastrointestinal bleeding, polyneuropathy, and death.
The only means we have for reducing infection in the general population and protecting blood donors relies on recommended infection control. Measures, such as proper hand and environmental hygiene, and appropriate use of personal protective equipment along with maintaining the social distancing (at least 6 feet) are necessary to prevent COVID-19 spreading. Early detection, triage, and isolation of potentially infectious patients are also important considerations. A combination of these measures is the basis of current blood donation protocols, which will hopefully protect and maintain our blood supply.
Blood donation and blood products
There are multiple Federal Drug Administration (FDA) criteria a blood donor must meet before donating blood products. These parameters range from physical requirements, such as age, weight, temperature, blood pressure, and pulse, to a background check of the donor’s sexual, medical, and travel history. Any discrepancies or issues that arise during the interview process and the physical examination could temporarily or permanently defer the donor from the blood donation system.18
Following the start of the COVID-19 outbreak in the United States, additional screening questions and requirements were implemented. Although not standardized across all blood collection organizations, the American Red Cross implemented new deferral policies in February 2020 before regional and national shutdown. All donors with a recent travel history to China, Hong Kong, Macau, Iran, Italy, and South Korea were deferred for 28 days. Donors diagnosed or suspected to have COVID-19 or had contact with a COVID-19–positive patient were also deferred for 28 days despite the absence of any data or evidence as of yet that SARS-CoV-2 can be transmitted through blood products.19
Several measures were adopted by all blood donation centers and blood drives to prevent transmission of SARS-CoV-2. Measures included temperature screening for all donors and staff before entry into the donation centers, social distancing (>6 feet) when possible, disinfecting machines and surfaces between donations, having donors and staff wear face masks, use of hand sanitizer before and during the donation process, and increased spacing between beds. These preventative practices echoed the Centers of Disease Control (CDC) guidelines and were similarly implemented in other blood donation centers.20, 21, 22, 23
As these policies were put into place, blood donations began to decrease as the COVID-19 pandemic grew and blood drive cancellation increased. Regionally, one of the hospitals affected was University of Washington Medical Center, which reported a blood supply shortfall as early as February 29, 2020.24 Nationally blood collections dropped and in a press release by the American Red Cross on March 17, 2020, approximately 2700 Red Cross blood drives were canceled across the country, resulting in 86,000 fewer donations. Evidently, more than 80% of their usual blood supply comes from these blood drives.25 Of note, as per the American Association of Blood Banks (AABB), 33,000 units of blood are needed daily to meet patient need before the pandemic.26
As a result, hospitals needed to develop strategies to adapt to these blood supply shortages. Mitigation strategies that were proposed included additional criteria for transfusion orders review with more stringent guidelines. Splitting platelet units into 2 doses each were also considered to minimize platelet shortage. Hospitals increasingly adopted these measures over the course of a few weeks starting in March 2020.27
On April 2, 2020, the FDA issued new blood donation guidance to address the need for blood and blood components. They no longer required collections to be discarded due to errors in vital signs or donation intervals and added a 72-hour window to allow a donor to respond to questions about eligibility and component suitability.28 The FDA changed deferral guidelines and a previous guidance that deferred many donors for up to 12 months due to various reasons was revised to a deferral of 3 months (Table 1 ).29 In addition, donors who were previously permanently deferred between 1980 to 1986 due to spending more than 3 months in specified European countries were allowed to be reconsidered for donation. Exceptions and alternatives were also issued under 21 CFR 640.120(b) to address blood and blood component shortages.30
Table 1.
Updated blood collection policies and Federal Drug Administration regulatory changes
| New Screening Measures and Changes | Deferral |
|---|---|
| Persons who traveled in COVID-19 endemic areas a | 14–28 da |
| Persons diagnosed with COVID-19, contact with people with the virus, and those suspected of having it a | |
| For male donors who would have been deferred for having sex with another man | From 12 mo to 3 mo |
| For female donors who would have been deferred for having sex with a man who had sex with another man | |
| For those with recent tattoos and piercings | |
| For those who have traveled to malaria-endemic areas (and are residents of malaria nonendemic countries): the agency is changing the recommended deferral period from 12 to 3 mo. In addition, the guidance provides notice of an alternate procedure that permits the collection of blood and blood components from such donors without a deferral period, provided the blood components are pathogen-reduced using an FDA-approved pathogen reduction device. | |
| For those who spent time in certain European countries or on military bases in Europe who were previously considered to have been exposed to a potential risk of transmission of Creutzfeldt-Jakob disease or variant Creutzfeldt-Jakob disease, the agency is eliminating the recommended deferrals and is recommending allowing reentry of these donors. | From indefinite deferral to no deferral |
Concurrently, the CDC and Centers for Medicare and Medicaid Services recommended rescheduling elective surgeries as needed and shifting elective urgent inpatient surgical procedures to outpatient settings when feasible. The American College of Surgeons similarly recommended the same guidelines.31 They stated surgeries should be reviewed with “a plan to minimize, postpone, or cancel electively scheduled operations, endoscopies, or other invasive procedures.”32 These policies were initiated by hospitals such as University of Washington Medical Center where elective surgeries and procedures were postponed starting March 7, 2020. During that time, blood usage and blood demand reached parity at Washington Medical Center.24 This decrease in elective surgery was echoed in many hospitals, with one report citing a 71.7% decrease in surgical volume.33
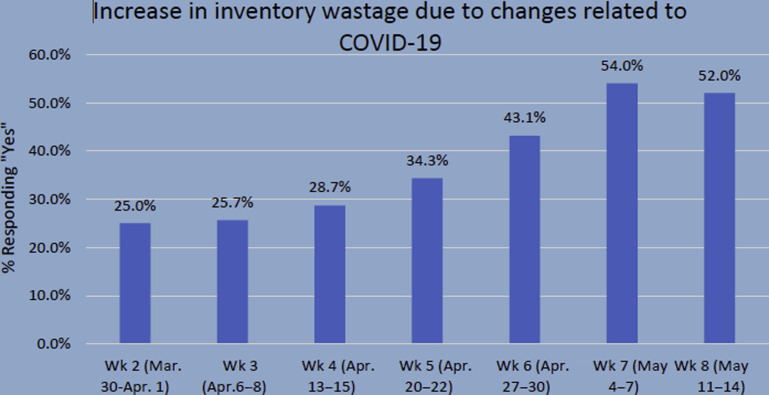
Nationally, elective surgery cancellation and blood mitigation strategies became ubiquitous as seen by the AABB survey. The week of March 23, 2020, most of the hospitals reported they were no longer performing elective surgeries, with only 10.6% of hospitals still conducting elective surgeries.34 Blood usage mitigation techniques as mentioned earlier became more prevalent.27 In fact, blood wastage increased the following week, March 30th, with 25% of hospitals reporting increased blood wastage due to cancellation of elective surgeries and nonurgent medical procedures.35 Wastage peaked the week of May 4 at 54% and the subsequent week, May 11, decreased to 52% as many hospitals started to resume elective procedures (Fig. 1 ).36 , 37
Fig. 1.
As elective surgical cases were canceled and blood utilization decreased in much of the United States, a trend of increased wastages was seen.
(From AABB. COVID-19 weekly hospital transfusion services survey: week 8 snapshot. Available at: http://www.aabb.org/research/hemovigilance/bloodsurvey/Docs/AABB-COVID-19-Impact-Survey-Snapshot-Week-8.pdf.)
Patient blood management during COVID-19 pandemic
General Principles
Blood transfusion is considered one of the most common hospital procedures performed in the United States. The safety of blood products and the appropriateness of transfusion are significant and timely issues. Over the last 3 decades, studies have shown that transfusion of one red blood cell (RBC) unit increases wound complications by 4%, hospital length of stay (LOS) by 1.5 days, and mortality by 0.9%.38, 39, 40 In nonbleeding patients, restricting blood transfusions by using a hemoglobin trigger of less than 7 g/dL significantly reduces cardiac events, rebleeding, bacterial infections, and total mortality.41 Other blood components carry similar risks. Plasma is frequently misused, and its benefits are overestimated particularly in nonbleeding patients. In a retrospective cohort study, Warner and colleagues42 found that prophylactic administration of plasma in the critically ill was not associated with improved clinical outcomes. Similar studies on prophylactic preprocedure platelet transfusion showed an increase in risk of thrombosis and mortality.43
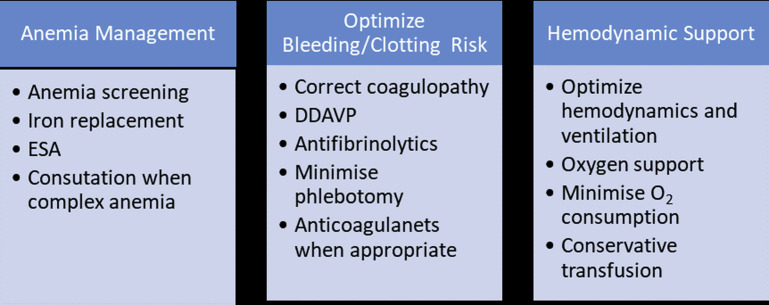
Patient blood management (PBM) is a multidisciplinary, evidence-based strategy in which the need for blood products is managed in order to provide better patient outcomes and appropriate stewardship of a limited resource while reducing health care costs (Table 2 ). The primary goal of PBM program is to ensure optimal decision support using evidence-based guidelines and transfusing the most appropriate blood products with a minimum dose required for the clinical situation.44 In addition, pharmaceutical products such as desmopressin and antifibrinolytics (such as Amicar or Tranexamic acid) have been shown to reduce bleeding.45, 46, 47, 48 Prothrombin complex concentrates and vitamin K may also be effective in warfarin reversal and correcting international normalized ratio.49, 50, 51 Iron supplements, oral or intravenous preparations, and erythropoiesis-stimulating agents are proved to be useful in repleting iron stores and thus increasing hemoglobin levels.52 PBM and bloodless medicine programs is now a priority throughout national and international health systems (Fig. 2 ).53 Hospitals and academic medical centers across the nation are beginning to develop bloodless medicine and PBM programs in response to the favorable evidence.
Table 2.
University of Rochester Medical Center evidence-based transfusion guideline
| Product | Clinical Indication | Transfusion Trigger |
|---|---|---|
| Red blood cells | Anemia | Hct <21%; Hgb <7 g/dL |
| Anemia with acute coronary syndromes | Hct <24%; Hgb <8 g/dL | |
| Platelets | High risk of bleeding | Platelet count <10,000 |
| Fever or sepsis | Platelet count<20,000 | |
| Acute bleeding | Platelet count<50,000 | |
| Intracranial hemorrhage | Platelet count<100,000 | |
| Documented platelet dysfunction | Per platelet function test | |
| Plasma | Urgent need for warfarin reversal | INR >1.7 |
| Clinical coagulopathy | Based on relevant laboratory and TEG values | |
| Acute bleeding | To maintain the RBC to plasma ratio of our MTP | |
| Plasma exchange for TTP | ||
| Factor V or XI deficiency | ||
| Cryoprecipitate | Low fibrinogen level | <150 and bleeding |
| Documented dysfibrinogenemia | Clinically significant bleeding without obvious causation | |
| Uremic coagulopathy unresponsive to DDAVP | ||
| Factor VIII deficiency |
Abbreviations: DDAVP, desmopressin; Hct, hematocrit; Hgb, hemoglobin; INR, international normalized ratio; MTP, massive transfusion policy; TEG, thromboelastography; TTP, thrombotic thrombocytopenic purpura.
Fig. 2.
Patient blood management strategies.
Patient blood management strategies used in COVID-19 pandemic
During the 2020 COVID-19 pandemic, blood supply shortages were observed worldwide, including the United States. The major blood suppliers and hospitals across the country issued emergency pleas for donations and reports significant numbers of blood drives were canceled due to school and workplace closures, which resulted in thousands of fewer blood donations typically collected.19 In Beijing, China during the 2003 SARS epidemic, blood products shortages necessitated importation of blood products from other Chinese provinces to supply needs for clinical use in patients.54 , 55 Similarly, the COVID-19 pandemic has had a major ripple effect on the number of eligible blood donors, blood supply, and on blood safety.
Therefore, PBM strategies are imperative in order to manage shortages during natural disaster or disease pandemics as well as long-term socioeconomic effects following these crises. Implementation of the most recent evidence-supported transfusion guidelines and eliminating unnecessary transfusions are considered the main goals of PBM programs during major disasters. Some effective strategies are as follows:
-
•
Evaluation of appropriateness of transfusion orders and further discussion with clinical team if needed.
-
•
Use of other pharmaceutical products such as desmopressin, antifibrinolytics, vitamin K, prothrombin complex concentrates, or intravenous iron if appropriate.
-
•
Blood-sparing strategies during surgery such as implementation of normovolemic or hemodilution measures or usage of cell salvage.
-
•
Staff education and open communication is imperative.
Challenges of managing resumption of normal hospital surgical occupancy
Although the demand for blood products during the COVID-19 pandemic has decreased due to postponement of elective surgeries, hospitals must have an emergency blood management plan. This plan should be in place and ready to be implemented during and following any natural crisis in order to maintain sustainability of a safe blood supply. As hospitals resume elective surgery and see increases in non-COVID-19 admissions, long-term shortages beyond the pandemic peak persist. At this time, blood collection centers and donors are still required to avoid large gatherings and follow all safety measures. Thus, a significant blood supply shortage may be present beyond the pandemic peak. In addition, due to the prolonged incubation period (up to 14 days) of SARS-CoV-2 and the potential of asymptomatic carriers, recruitment of blood donors as well as maintaining safety in the blood collection process remains a major concern.
Effect of COVID-19 pandemic on transfusion service and blood bank operations
Being prepared to face a pandemic ensures that blood products are available to those patients requiring transfusion support, and a robust plan helps to protect the safety and health of the transfusion service professionals needed to perform testing and prepare blood products.56 The effects of any pandemic on transfusion service and blood bank operations are 2-fold the work force and the blood supply.
Although many employees were encouraged or required to work remotely during the COVID-19 pandemic, this is not an option for laboratory professionals working in an academic medical center transfusion service and blood bank. To provide necessary transfusion support and to prepare and modify blood products, blood bank employees must be physically present in the laboratory at all times. Thus, for employees’ safety and to prevent the spread of infection, repeated cleaning and disinfection of the work environment should be undertaken in conjunction with universal masking of employees and meticulous handwashing. To promote social distancing, separating staff by off-setting shift hours across the operation will more evenly distribute the staff across a 24-hour period. Depending on the physical layout of the work area it may be possible to set up workstations in different areas of the laboratory so that the staff is physically separated from each other as much as possible. That being said, plans must be in place to continue operations if multiple staff members become infected and cannot work. Because of the extensive regulations surrounding blood bank testing and competency requirements, cross-training technologists from other areas in the clinical laboratory on short notice tends not to be a viable option. A plan must be in place at the institutional level to postpone elective surgeries and other elective procedures that rely on transfusion support if staffing levels become critically low due to employee absenteeism and quarantine.
The second concern that transfusion services and blood banks may experience during a pandemic is the blood products supply. Blood collection mainly depends on volunteer blood donors at collection centers. During such a crisis blood donation can be greatly reduced as a result of donors becoming infected, unable to donate, or avoiding public gatherings.57 Several conservation options should be considered by the transfusion service in order to conserve a rapidly dwindling blood supply along with additional measures promoted by a PBM program, when available.
-
•
The blood type of trauma patients should be determined as rapidly as possible so that transfusion can be performed using type-specific RBC, thereby conserving the supply of group “O” RBC, a universal donor type. Group “O−” RBC should be reserved for women of childbearing age (<50 years) and female children. All other group “O” individuals should receive group “O+” RBC.
-
•
If platelet availability is constrained, units can be split into 2 doses. At the authors’ facility they found that one-half unit of platelets is sufficient to provide clinical benefit to most patients. However, based on the patient’s clinical condition, a full dose can be transfused if required.
-
•
Because of decreased blood utilization as a result of elective procedures withholding, reducing standing orders and managing blood product standing orders with the blood supplier is essential to minimize waste, particularly in multisite hospital systems with transfusion services located at each hospital. Transferring RBC to the highest transfusion volume facility in the health care system could be an option as well to reduce wastage.
-
•
Inventory levels of reagents and supplies must be closely monitored. The transfusion service must work closely with the supply chain to ensure that critical reagents and supplies are available throughout the pandemic to perform critical testing. This may involve placing orders to bring levels to a level sufficient to perform testing for 3 months or more if availability or delivery could potentially be a problem.
-
•
To assist the blood supplier, facilities could host additional blood drives either at the facility itself (if there is sufficient room to ensure adequate social distancing) or supporting drives in a larger venue (such as a mall closed due to the pandemic or a government building not currently or minimally occupied).
-
•
If the hospital blood bank is FDA registered or licensed, the collection of convalescent plasma could be undertaken to provide a possible course of treatment either alone or in combination with other treatments to infected patients.58
Although blood conservation measures may be necessary, there likely will be a decrease in demand for RBC resulting from canceling elective surgical procedures. In the COVID-19 pandemic a reduction in blood orders from our blood supplier reduced spending at our facility by approximately 50%. Blood bank serologic testing was reduced by approximately 30% during the same time period but without the associated decrease in budget due to the advanced purchase of reagents and supplies. Close monitoring of testing volumes, blood product purchases, and waste is important to determine how the pandemic will affect cost projections.
Convalescent plasma
Blood-Based Therapeutics for COVID-19
Unlike other subspecialties in pathology and laboratory medicine, transfusion medicine/blood banking is almost exclusively focused on therapeutics, including hands on treatment of patients. Thus it is understandable that physicians, nurses, and medical technologists in this area of medicine have been focused on possible treatment approaches for COVID-19. In the absence of effective and safe antiviral treatments, the strategy of transfusing convalescent plasma has long attracted interest and has been used in treatment of infectious disease, most recently in viral diseases.59 The interest is due to the abundant evidence that humoral immunity plays a role in resolution of viral infection and prevention of reinfection after primary infection or vaccination. Convalescent plasma has a long history but almost no quality evidence for its efficacy and safety, as its use has often been reserved for last ditch efforts in desperately ill patients. In addition, much of the history of its use antedates the recognition that randomized trials are needed for ultimate proof of efficacy and safety due to the highly variable course of many illnesses, including COVID-19.60
In the current pandemic, early case reports of convalescent plasma usage reported that some patients who were seriously ill, requiring ICU care and mechanical ventilation,61 cleared virus more rapidly than expected and made sufficient recovery and shorter LOS. Thousands of units of plasma, almost all untested for antiviral neutralizing titers, have been transfused in the United States using FDA-approved emergency investigational new drug and expanded access protocols.62 Many small randomized trials and a few larger multicenter trials are underway a few months into the epidemic in the United States. These include some trials in patients with critical illness, using primary endpoints for efficacy such as ability to be weaned from mechanical ventilation, discharge from hospital, and survival. Other trials target prevention of infection in exposed individuals or prevention of hospitalization in newly SARS-CoV-2 RNA–positive individuals with mild or moderate symptoms. In most cases, given the uncertainty of whether antibody is protective, no specific titer or neutralizing titer of antibody in the plasma is specified. In other trials, high titers of antibody are required (eg, reactive at >1:320). Donors must meet standard FDA and state safety requirements for blood donation and be either RNA negative or greater than 28 days past clinical recovery in order to donate.63 Donation can be by manual plasmapheresis or machine apheresis, with the former considerably less efficient but less costly.
There are many unresolved issues concerning convalescent plasma efficacy and safety, for example,59 does antibody clearance of virus lead to clinical improvement in moderately to severely ill patients?,60 does antibody prevent clinical deterioration in recently infected, asymptomatic or mildly symptomatic patients?, and61 are there mid- to longer-term consequences of transfusing allogeneic plasma? Many statements have been made that allogeneic plasma transfusion is a common therapy with only rare acute complications (acute lung injury, volume overload, hemolysis, anaphylaxis, etc.). However, recent observational literature in critically ill patients demonstrate that allogeneic plasma transfusion is associated with nosocomial bacterial infection,64 organ failure, and thrombosis.65 The role of transfusing ABO “compatible plasma,” an accepted practice, in worsening the risk of bleeding,66 infection/sepsis,67 organ failure,67 and mortality66 is not proved, but transfusion of ABO compatible, but not identical, plasma is likely not immunologically neutral. Immune complexes between antibody and soluble antigen form after transfusion and, at least in model systems, can activate monocytes, interfere with platelet and coagulation factor function, and may injure endothelial cells.68
A likely more efficacious and safer product than convalescent plasma being considered for use in COVID-19 disease and its prevention is hyperimmune immunoglobulin G (IgG). Intravenous IgG (IVIgG) made from multiple donors with detectable titers of anti-SARS-CoV-2 antibody would be expected to be more potent and carry fewer risks than convalescent allogeneic plasma that has not been processed.69 However, it will be many months before such products are routinely available and much less proven effective and safe in patients. One clear cut benefit of IVIgG is a reduced risk of some adverse immune effects (acute lung injury, hemolysis, organ failure) because no single donor is heavily represented. Infectious disease transmission, although uncommon, after plasma that has been tested for HIV, hepatitis C, etc. is in general not a risk with use of IVIgG preparations. Both convalescent plasma and hyperimmune IgG may carry risks of antibody enhancement of viral infection.70
Finally, although not a blood product, humanized monoclonals to proteins and glycoproteins that are necessary for viral entry and replication hold some promise as preventive and therapeutic strategies in COVID-19.
Blood safety during COVID-19
Steps Taken to Protect Blood Supply
In general, blood donors must be healthy on the day of donation and meet existing FDA donor screening measures. Typically, these measures should prevent individuals with any respiratory symptoms or infection from donation. Donors are also instructed to contact the blood collection center if any signs or symptoms developed within the next few days of donation. The collected blood or blood components will then be discarded and any distributed products will be recalled. Nevertheless, to date no transfusion-transmitted COVID-19 cases have been reported.
Risk of blood products contamination with COVID-19
Overall, respiratory viruses are not known to be transmitted via blood transfusions. Thus far, there are no reported cases of transfusion-transmitted COVID-19 nor any type of the other coronaviruses. As a precaution measure to the blood safety, particularly after the rapid increase in COVID-19 infection rates in China, Chang and colleagues71 screened all donations collected at the Wuhan Blood Center over 2 months (January through March 2020). RT-PCR testing for SARS-CoV-2 RNA was performed on pools of 6 to 8 plasma samples. Out of the screened 2430 donations, one donor tested positive for SARS-CoV-2. This donor was positive for COVID-19 previously and was quarantined appropriately in a cabin hospital in Wuhan until 2 consecutive negative throat swab results 3 days apart were obtained. At the time of donation, the donor displayed no symptoms. However, his plasma SARS-CoV-2 was still detectable. Later on, in a retrospective testing of 4995 donations collected between December 2019 and January 2020, plasma samples of 3 more healthy donors from Wuhan were also found to be positive for SARS-CoV-2. However, specific IgG and IgM against SARS-CoV-2 by enzyme-linked immunosorbent assay were negative, indicating the possibility of infection in the early stage. The investigator concluded that because of the asymptomatic COVID-19 cases, screening donors for SARS-CoV-2 will be critical to ensure blood safety.
Hence, to date there are no FDA requirements in place to screen blood donors or test blood components for COVID-19. Individuals with COVID-19 infection do not meet the blood donation guidelines.
Acknowledgments
Disclosure
Dr. Majed A. Refaai has received consulting fees and/or research funding from CSL Behring, Octapharma, Bayer, Instrumentation Laboratory, and iLine microsystems and has received speaking fees from CSL Behring.The other Authors have nothing to disclose.
References
- 1.Wu F., Zhao S., Yu B., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.Sultan S., Altayar O., Siddique S.M., et al. AGA Institute rapid review of the gastrointestinal and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology. 2020;159(1):320–334.e27. doi: 10.1053/j.gastro.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronavirus (COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/index.html Available at: Accessed May 28, 2020.
- 7.Oran D.P., Topol E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review [published online ahead of print, 2020 Jun 3] Ann Intern Med. 2020 doi: 10.7326/M20-3012. M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nomoto H., Ishikane M., Katagiri D., et al. Cautious handling of urine from moderate to severe COVID-19 patients. Am J Infect Control. 2020;48(8):969–971. doi: 10.1016/j.ajic.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killerby M.E., Biggs H.M., Haynes A., et al. Human coronavirus circulation in the United States 2014-2017. J Clin Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W., Lan Y., Yuan X., et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9(1):469–473. doi: 10.1080/22221751.2020.1732837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascarella G., Strumia A., Piliego C., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu T., Zhang J., Yang Y., et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12(7):e12421. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan L., Wang Q., Zhang D., et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jean S.S., Lee P.I., Hsueh P.R. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020;53(3):436–443. doi: 10.1016/j.jmii.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CFR - code of federal regulations title 21. 2019. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=630.10 Available at: Accessed May 15, 2020.
- 19.What to know about the coronavirus and blood donation. 2020. https://www.redcrossblood.org/donate-blood/dlp/coronavirus--covid-19--and-blood-donation.html Available at: Accessed May 15, 2020.
- 20.Coronavirus and blood donation. 2020. https://www.mbc.org/coronavirus-blood-donation/ Available at: Accessed May 15, 2020.
- 21.COVID-19 and the blood supply. 2020. https://www.carterbloodcare.org/covid-19-and-the-blood-supply/ Available at: Accessed May 15, 2020.
- 22.COVID Info. 2020. https://www.vitalant.org/COVID-Info Available at: Accessed May 15, 2020.
- 23.COVID-19 response. 2020. https://www.bloodcenter.org/donate/donor/covid19-response/ Available at: Accessed May 15, 2020.
- 24.Pagano M.B., Hess J.R., Tsang H.C., et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID-19) pandemic affecting Washington State. Transfusion. 2020;60(5):908–911. doi: 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 25.American red cross faces severe blood shortage as coronavirus outbreak threatens availability of nation’s supply. 2020. https://www.redcross.org/about-us/news-and-events/press-release/2020/american-red-cross-faces-severe-blood-shortage-as-coronavirus-outbreak-threatens-availability-of-nations-supply.html Available at: Accessed May 15, 2020.
- 26.Message to blood donors during the COVID-19 pandemic. AABB: Bethesda, MD; 2020. p. 2. [Google Scholar]
- 27.COVID-19 impact on hospital practices: week 1-4 survey snapshot. AABB: Bethesda, MD; 2020. Available at: http://www.aabb.org/research/hemovigilance/bloodsurvey/Docs/AABB-COVID-19-Impact-Survey-Snapshot-Week-1-4.pdf. Accessed May 15, 2020. [Google Scholar]
- 28.Alternative Procedures for Blood and Blood Components during the COVID-19 Public Health Emergency. U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. p. 8. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alternative-procedures-blood-and-blood-components-during-covid-19-public-health-emergency. Accessed May 15, 2020.
- 29.Coronavirus (COVID-19) update: FDA provides updated guidance to address the urgent need for blood during the pandemic. 2020. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-updated-guidance-address-urgent-need-blood-during-pandemic Available at: Accessed May 15, 2020.
- 30.Alternative procedures for blood and blood components during the COVID-19 public health emergency. 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alternative-procedures-blood-and-blood-components-during-covid-19-public-health-emergency Available at: Accessed May 15, 2020.
- 31.Healthcare facilities: preparing for community transmission. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-hcf.html Available at: Accessed May 15, 2020.
- 32.COVID-19: recommendations for management of elective surgical procedures. 2020. https://www.facs.org/covid-19/clinical-guidance/elective-surgery Available at: Accessed May 15, 2020.
- 33.Hemingway J.F., Singh N., Starnes B.W. Emerging practice patterns in vascular surgery during the COVID-19 pandemic. J Vasc Surg. 2020;72(2):396–402. doi: 10.1016/j.jvs.2020.04.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AABB survey COVID-19 impact on care of patients requiring transfusion: week 1 snapshot. 2020. http://www.aabb.org/research/hemovigilance/bloodsurvey/Docs/AABB-COVID-19-Impact-Survey-Snapshot-Week-1.pdf 1. Available at: Accessed May 16, 2020.
- 35.AABB survey COVID-19 impact on care of patients requiring transfusion: week 2 snapshot. 2020. http://www.aabb.org/research/hemovigilance/bloodsurvey/Docs/AABB-COVID-19-Impact-Survey-Snapshot-Week-2.pdf 1. Available at: Accessed May 16, 2020.
- 36.AABB COVID-19 weekly hospital transfusion services survey: week 7 snapshot. 2020. http://www.aabb.org/research/hemovigilance/bloodsurvey/Docs/AABB-COVID-19-Impact-Survey-Snapshot-Week-7.pdf 1. Available at: Accessed May 16, 2020.
- 37.AABB COVID-19 weekly hospital transfusion services survey: week 8 snapshot. 2020. http://www.aabb.org/research/hemovigilance/bloodsurvey/Docs/AABB-COVID-19-Impact-Survey-Snapshot-Week-8.pdf 1. Available at: Accessed May 16, 2020.
- 38.Ferraris V.A., Davenport D.L., Saha S.P., et al. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147(1):49–55. doi: 10.1001/archsurg.2011.790. [DOI] [PubMed] [Google Scholar]
- 39.Bernard A.C., Davenport D.L., Chang P.K., et al. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208(5):931–937. doi: 10.1016/j.jamcollsurg.2008.11.019. 937.e1-2; [discussion: 938–9] [DOI] [PubMed] [Google Scholar]
- 40.Ferraris V.A., Ferraris V.A., Brown J.R., et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 41.Hajjar L.A., Vincent J.L., Galas F.R., et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 42.Warner M.A., Chandran A., Jenkins G., et al. Prophylactic plasma transfusion is not associated with decreased red blood cell requirements in critically ill patients. Anesth Analg. 2017;124(5):1636–1643. doi: 10.1213/ANE.0000000000001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt A.E., Henrichs K.F., Kirkley S.A., et al. Prophylactic preprocedure platelet transfusion is associated with increased risk of thrombosis and mortality. Am J Clin Pathol. 2017;149(1):87–94. doi: 10.1093/ajcp/aqx151. [DOI] [PubMed] [Google Scholar]
- 44.Mehra T., Seifert B., Bravo-Reiter S., et al. Implementation of a patient blood management monitoring and feedback program significantly reduces transfusions and costs. Transfusion. 2015;55(12):2807–2815. doi: 10.1111/trf.13260. [DOI] [PubMed] [Google Scholar]
- 45.Twum-Barimah E., Abdelgadir I., Gordon M., et al. Systematic review with meta-analysis: the efficacy of tranexamic acid in upper gastrointestinal bleeding. Aliment Pharmacol Ther. 2020;51(11):1004–1013. doi: 10.1111/apt.15761. [DOI] [PubMed] [Google Scholar]
- 46.Myles P.S., Smith J.A., Forbes A., et al. Tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376(2):136–148. doi: 10.1056/NEJMoa1606424. [DOI] [PubMed] [Google Scholar]
- 47.Dunn C.J., Goa K.L. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005–1032. doi: 10.2165/00003495-199957060-00017. [DOI] [PubMed] [Google Scholar]
- 48.Lim C.C., Tan H.Z., Tan C.S., et al. Desmopressin acetate (DDAVP) to prevent bleeding in percutaneous kidney biopsy: a systematic review. Intern Med J. 2020 doi: 10.1111/imj.14774. [DOI] [PubMed] [Google Scholar]
- 49.Refaai M.A., Kothari T.H., Straub S., et al. Four-factor Prothrombin complex concentrate reduces time to procedure in vitamin K antagonist-treated patients experiencing gastrointestinal bleeding: a post hoc analysis of two randomized controlled trials. Emerg Med Int. 2017;2017:8024356. doi: 10.1155/2017/8024356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Polito N.B., Kanouse E., Jones C.M.C., et al. Effect of vitamin K administration on rate of warfarin reversal. Transfusion. 2019;59(4):1202–1208. doi: 10.1111/trf.15146. [DOI] [PubMed] [Google Scholar]
- 51.Mazur H., Young S., McGraw M., et al. 471: efficacy and safety of 4F-PCC VS. FFP for warfarin reversal in emergent surgery/invasive procedure. Crit Care Med. 2019;47(1):216. [Google Scholar]
- 52.Cho B.C., Serini J., Zorrilla-Vaca A., et al. Impact of preoperative erythropoietin on allogeneic blood transfusions in surgical patients: results from a systematic review and meta-analysis. Anesth Analg. 2019;128(5):981–992. doi: 10.1213/ANE.0000000000004005. [DOI] [PubMed] [Google Scholar]
- 53.Tokin C., Almeda J., Jain S., et al. Blood-management programs: a clinical and administrative model with program implementation strategies. Perm J. 2009;13(1):18–28. doi: 10.7812/tpp/08-029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai X., Ren M., Chen F., et al. Blood transfusion during the COVID-19 outbreak. Blood Transfus. 2020;18(2):79–82. doi: 10.2450/2020.0076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raturi M., Kusum A. The active role of a blood center in outpacing the transfusion transmission of COVID-19. Transfus Clin Biol. 2020;27(2):96–97. doi: 10.1016/j.tracli.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.AABB Interorganizational Task Force on Pandemic Influenza and the Blood Supply, in Pandemic influenza issues outline. AABB. p. 16. Bethesda, MD;2020.
- 57.Maintaining a Safe and Adequate Blood Supply during Pandemic Influenza, in Guidelines for Blood Transfusion Services. World Health Organization (WHO). Geneva, Switzerland;2011.
- 58.Roback J.D., Guarner J. Convalescent Plasma to Treat COVID-19: Possibilities and Challenges [published online ahead of print, 2020 Mar 27] JAMA. 2020 doi: 10.1001/jama.2020.4940. [DOI] [PubMed] [Google Scholar]
- 59.Bloch E.M., Shoham S., Casadevall A., et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130(6):2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dzik S. COVID-19 Convalescent Plasma: Now Is the Time for Better Science [published online ahead of print, 2020 Apr 23]. Transfus Med Rev. 2020;S0887-7963(20)30026-2. 10.1016/j.tmrv.2020.04.002. [DOI] [PMC free article] [PubMed]
- 61.Zeng F., Chen X., Deng G. Convalescent plasma for patients with COVID-19. Proc Natl Acad Sci U S A. 2020;117(23):12528. doi: 10.1073/pnas.2006961117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joyner M. 2020. https://www.uscovidplasma.org/#workflow Available at: Accessed May 22, 2020.
- 63.2020. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/donate-covid-19-plasma Available at:
- 64.Subramanian A., Berbari E.F., Brown M.J., et al. Plasma transfusion is associated with postoperative infectious complications following esophageal resection surgery: a retrospective cohort study. J Cardiothorac Vasc Anesth. 2012;26(4):569–574. doi: 10.1053/j.jvca.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Bence CM, Traynor MD Jr, Polites SF, et al. The incidence of venous thromboembolism in children following colorectal resection for inflammatory bowel disease: A multi-center study [published online ahead of print, 2020 Feb 20]. J Pediatr Surg. 2020;S0022-3468(20)30121-4. 10.1016/j.jpedsurg.2020.02.020. [DOI] [PubMed]
- 66.Refaai M.A., Fialkow L.B., Heal J.M., et al. An association of ABO non-identical platelet and cryoprecipitate transfusions with altered red cell transfusion needs in surgical patients. Vox Sang. 2011;101(1):55–60. doi: 10.1111/j.1423-0410.2010.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Inaba K., Branco B.C., Rhee P., et al. Impact of ABO-identical vs ABO-compatible nonidentical plasma transfusion in trauma patients. Arch Surg. 2010;145(9):899–906. doi: 10.1001/archsurg.2010.175. [DOI] [PubMed] [Google Scholar]
- 68.Refaai M.A., Cahill C., Masel D., et al. Is it time to reconsider the concepts of "universal donor" and "ABO compatible" transfusions? Anesth Analg. 2018;126(6):2135–2138. doi: 10.1213/ANE.0000000000002600. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen A.A., Habiballah S.B., Platt C.D., et al. Immunoglobulins in the treatment of COVID-19 infection: proceed with caution! Clin Immunol. 2020;216:108459. doi: 10.1016/j.clim.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu L., Wei Q., Lin Q., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019;4(4):e123158. doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang L., Zhao L., Gong H., et al. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020;26(7):1631–1633. doi: 10.3201/eid2607.200839. [DOI] [PMC free article] [PubMed] [Google Scholar]