Graphical abstract
Keywords: MERS-CoV, 4-Anilino-6-aminoquinazoline, Coronavirus, Inhibitor, Optimization
Abstract
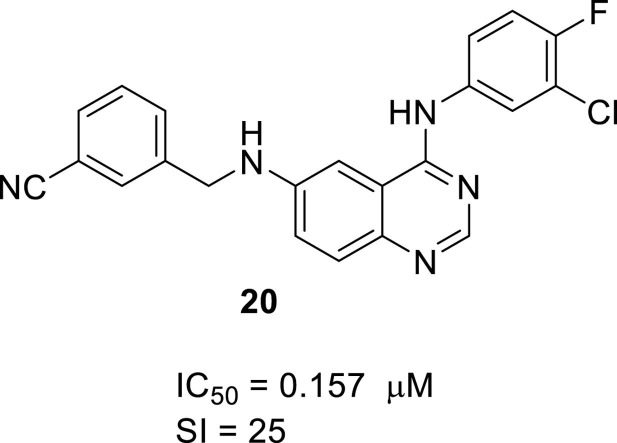
New therapies for treating coronaviruses are urgently needed. A series of 4-anilino-6-aminoquinazoline derivatives were synthesized and evaluated to show high anti-MERS-CoV activities. N4-(3-Chloro-4-fluorophenyl)-N6-(3-methoxybenzyl)quinazoline-4,6-diamine (1) has been identified in a random screen as a hit compound for inhibiting MERS-CoV infection. Throughout optimization process, compound 20 was found to exhibit high inhibitory effect (IC50 = 0.157 μM, SI = 25) with no cytotoxicity and moderate in vivo PK properties.
Coronaviruses (CoVs) are a group of positive-sense, single-stranded RNA viruses that cause severe respiratory diseases in a broad range of animal species, including humans.1, 2, 3 In 2003, one of the novel coronaviruses, severe acute respiratory syndrome CoV (SARS-CoV), caused a total of 8,422 cases of SARS with 916 deaths.4 The other novel coronavirus, Middle East respiratory syndrome CoV (MERS-CoV), has emerged in April 2012 and posed a serious threat to public health. As of 4 April 2020, a total of 2,494 human MERS-CoV infections with 858 deaths had been reported from 27 countries.5 Although MERS-CoV can cause primary infections from direct contact with animal reservoirs like camels,6 person-to-person transmission of this virus has mainly occurred in health-care facilities and family clusters.7, 8, 9 Recently outbreak of COVID-19, which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged in China and has spread to several other countries.
Drug repositioning for an FDA-approved compound library found that numerous compounds inhibited MERS-CoV infection. However, there are still no approved drugs for coronaviruses.10 Through high content screening (HCS) platform of Institut Pasteur Korea (IPK) using the Korea Chemical Bank (KCB),11 we found several novel compounds that can inhibit MERS-CoV infection.12 We found that N4-(3-chloro-4-fluorophenyl)-N6-(3-methoxybenzyl)quinazoline-4,6-diamine 1 was effective for inhibiting MERS-CoV infection. Quinazoline derivatives have previously been reported as potent inhibitors of the protein kinase of epidermal growth factor receptor (EGFR).13 Most of those compounds were approved as anticancer drugs, including Gefitinib,14 Lapatinib,15 Erlotinib,16 and Afatinib17. We thought that quinazoline compounds can exhibit good bioavailability and be easily extended to treatment of MERS-CoV infection. Here we report on the synthesis and biological effects of 4-anilino-6-aminoquinazoline derivatives.
The general synthetic route for 4-aminoquinazoline derivatives is described in Scheme 1 . 2-Amino-5-nitrobenzonitrile was reacted with dimethylformamide-dimethyl acetal (DMF-DMA) in toluene to give (E)-N′-(2-cyano-4-nitrophenyl)-N,N′-dimethylformimidamide 2. N,N-dimethylformamidine 2 was treated with acetic acid and anilines at 120 °C to produce 6-nitroquinazoline 3.18 Reduction of the nitro group of 3 was carried out using iron powder and NH4Cl in isopropyl alcohol and water at 100 °C to afford 6-aminoquinazoline 4. Aromatic amine 4 was reductive alkylated with aldehydes using NaBH(OAc)3 and trifluoroacetic acid in isopropyl acetate at 100 °C to give N-substituted quinazolines 5.
Scheme 1.
Synthesis of 6-substituted 4-anilinoquinazoline derivatives. Reagents and conditions: (a) DMF-DMA, toluene, 110 °C, 5 h; (b) ArNH2, AcOH, 120 °C, 7 h; (c) Fe, NH4Cl, i-PrOH/H2O (10/1), 110 °C/4h; (d) Aldehyde, NaBH(OAc)3, trifluoroacetic acid, i-PrOAc, 90 °C, 3 h; Acyl chloride, Et3N, dichloromethane, rt; or carboxylic acid, EDCI, i-Pr2NEt, DMF, rt.
The antiviral activities of the synthesized compounds in Vero cells were determined by immunofluorescence assay (IFA). Vero cells were infected with a korean clinical MERS-CoV isolates and the inhibitory concentration (IC50) and cytotoxic concentration (CC50) values of the compounds were calculated by nonlinear regression analysis.11 Molecules with higher selective index (SI) were considered as active molecules (Fig 1 ).
Fig. 1.
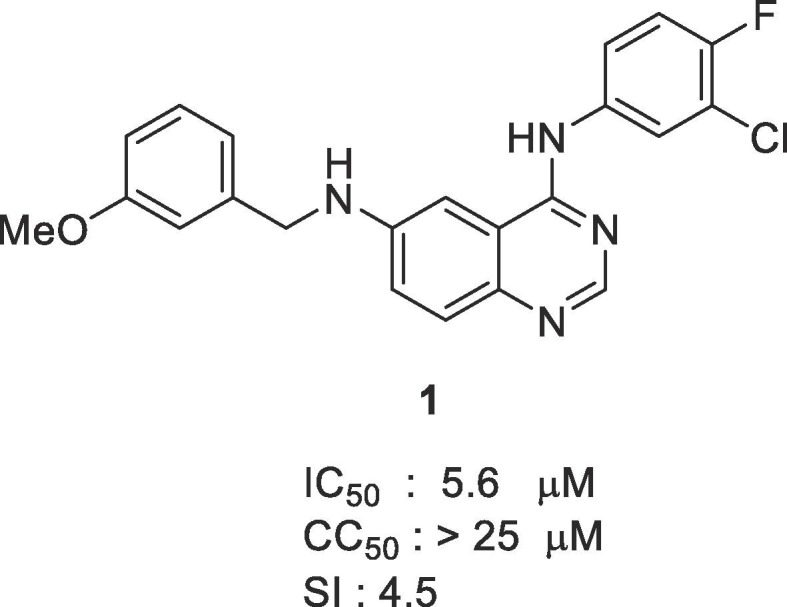
Hit against MERS-CoV from a KCB library screen.
Our preliminary structure activity relationship (SAR) studies surrounding 1 were carried out by introducing halide groups on phenyl ring of 4-anilino group (Table 1 ). 4-Bromo substituent (6) maintained the potency. 4-Fluoro (7), 3-chloro (8), 4-cyano (9), and 3-acetyl (10) reduced potency compared to 1. Interestingly, 4-trifluoromethyl (11) can slightly improve the potency. In the next phase optimization, we observed the effects of the substituents of 6 position of quinazoline ring, having fixed with 3-chloro-4-fluoro and 4-trifluoromethyl substituents on phenyl ring at 4 position (Table 2 ).
Table 1.
Antiviral effect and toxicity of 4-anilino groups of hit compound 1.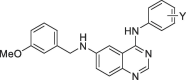
| Compound | Y | IC50(μM)a | CC50(μM)b | SIc |
|---|---|---|---|---|
| 1 | 3-Cl,4-F | 5.6 | >25 | 4.5 |
| 6 | 4-Br | 7.4 | >25 | 3.4 |
| 7 | 4-F | 22.7 | >25 | 1.1 |
| 8 | 3-Cl | >25 | 23.0 | 1.0 |
| 9 | 4-CN | >25 | >25 | 1.0 |
| 10 | 3-COCH3 | >25 | >25 | 1.0 |
| 11 | 4-CF3 | 1.8 | >25 | 1.0 |
IC50 and CC50 were derived from the results of at least two dependent experiment in Vero cells infected with MERS-CoV.
SI(selective index) = CC50/IC50 for inhibiting MERS-CoV infection.
Table 2.
Structure-Activity Relation of Derivatives of 1 and 11.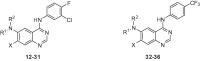
| Compound | X | R1 | R2 | IC50(μM)a | CC50(μM)b | SIc |
|---|---|---|---|---|---|---|
| 1 | H | 3-CH3O-PhCH2 | H | 5.6 | >25 | 4.5 |
| 12 | H | 2-CH3O-PhCH2 | H | 3.8 | >25 | 7 |
| 13 | H | 2-OH-PhCH2 | H | 3.6 | >25 | 7 |
| 14 | H | 3,4-F2-PhCH2 | H | 4.6 | 23.0 | 5 |
| 15 | H | 4-F-PhCH2 | H | 4.6 | 14.4 | 3 |
| 16 | H | 2-NO2-PhCH2 | H | 3.3 | >25 | 8 |
| 17 | H | 3-NO2-PhCH2 | H | 6.1 | >25 | 4 |
| 18 | H | 4-NO2-PhCH2 | H | 2.7 | >25 | 8 |
| 19 | H | 2-CN-PhCH2 | H | >25 | >25 | 1 |
| 20 | H | 3-CN-PhCH2 | H | 0.157 ± 0.002 | 3.59 ± 1.1 | 25 |
| 21 | H | 4-CN-PhCH2 | H | 4.8 | >25 | 5 |
| 22 | H | 3-H2NCO-PhCH2 | H | >25 | >25 | 1 |
| 23 | H | 3-CF3-PhCH2 | H | 2.3 | 9.2 | 4 |
| 24 | H | n-butyl | n-butyl | >25 | >25 | 1 |
| 25 | H | cyclohexyl | H | 15 | >25 | 1 |
| 26 | H | CH3CO | H | >25 | >25 | 1 |
| 27 | H | 3-CN-PhCO | H | >25 | >25 | 1 |
| 28 | H | 3-CN-trans-cynnamoyl | H | >25 | >25 | 1 |
| 29 | OMe | 3-CN-PhCH2 | H | 5.8 | 13.2 | 2 |
| 30 | OH | 3-CN-PhCH2 | H | >25 | >25 | 1 |
| 11 | H | 3-CH3O-PhCH2 | H | 1.8 | >25 | 1 |
| 31 | H | 2-CN-PhCH2 | H | >25 | >25 | 1 |
| 32 | H | 3-CN-PhCH2 | H | 3.6 | >25 | 7 |
| 33 | H | 4-CN-PhCH2 | H | 4.1 | >25 | 6 |
| 34 | H | 3-NO2-PhCH2 | H | 3.7 | >25 | 7 |
| 35 | H | 4-NO2-PhCH2 | H | 3.3 | >25 | 8 |
IC50 and CC50 were derived from the results of at least two dependent experiment in Vero cells infected with MERS-CoV.
SI (selective index) = CC50/IC50 for inhibiting MERS-CoV infection.
First, substituent effects at 6 position of 1 having 3-chloro-4-fluoro aniline at 4 position were evaluated. Changing 3-methoxybenzyl amine to 2-methoxybenzyl amine group 12 showed similar activity (IC50 = 3.8 μM). Introducing 2-hydroxybenzyl amine at 6 position of quinazoline ring 13 was tolerated (IC50 = 3.6 μM). 3,4-Difluoro (14) and 4-fluoro (15) compounds gave similar activities (IC50 = 4.6 μM). We next explored the effects of electron-withdrawing groups. Compounds with nitro groups (16 to 18) showed similar inhibitory effects (IC50 = 3.3, 6.1 and 2.7 μM, respectively). Compound with 2-cyanobenzyl amine at 6 position (19) showed no inhibitory effect, whereas 4-cyanoebnzyl amine (21) functionality was tolerated (IC50 = 4.8 μM). Gratifyingly, 3-cyanobenzyl amine analogue 20 resulted in significant higher activity (IC50 = 0.157 μM). We tested other electron-withdrawing groups at meta position of benzyl amine substituent. 3-Amidobenzyl amine analogue 22 was detrimental for activity but trifluoromethyl substituent at 3 position 23 retained the inhibitory effect (IC50 = 2.3 μM). Then benzyl amine substituents at 6 position were changed to aliphatic amines and amides (24–28). Aliphatic amine substituents, such as di-n-butyl (24) and cyclohexyl (25), showed little inhibitory effects and amide groups (26–28) were detrimental. We introduced several substituents at 7 position of 20. Methoxy at 7 position gave a little decreased activity (29, IC50 = 5.8 μM) but hydroxy (30) showed no inhibitory effects.
The antiviral effects of derivatives at 6 position of 11 with 4-trifluoromethyl aniline group at 4 position were also examined. Most of substituents were well tolerated. 3-Cyanobenzyl (32), 4-cyanobenzyl (33), 3-nitrobenzyl (34) and 4-nitrobenzyl (35) showed similar activities (IC50 = 3.3 to 4.1 μM) to 11. However, 2-cyanobenzyl (31) substituent exhibited decreased potency.
Considering activity and cytotoxicity, 20 was thought to be the best compounds for anti-MERS drug and evaluated for its hERG, metabolic stability, cytotoxicity (Table 3 ). Our optimized lead compound 20 was found to show no hERG binding and have good microsomal stability in both mouse and human. Compound 20 showed no cytotoxicity toward Vero (not infected with MERS-CoV) as well as toward HFL-1, L929 NIH 3 T3 and CHO-K1 cell lines, which shows that potential interaction between compound 20 and viruses might affect the cell viability. The pharmacokinetic parameters of 20 were evaluated in rats by intravenous (i.v.) and oral (p.o.) routes at 2 and 5 mg/kg, respectively (Table 4 ). 20 showed reasonable oral bioavailability (21%).
Table 3.
Result of hERG, Microsomal stability, cytotoxicity of 20.
| Compound | hERG (μM) | MSa |
Cytotoxicity (μM)b |
|||||
|---|---|---|---|---|---|---|---|---|
| ma | ha | Veroc | HFL-1 | L929 | NIH 3T3 | CHO-K1 | ||
| 20 | >50 | 60.7 | 56.4 | >100 | >100 | >100 | >100 | >100 |
% original compound remained after 30 min incubation.
Cell information. Vero: African green monkey kidney cell line, HFL-1: human embryonic lung cell line, L929: NCTC clone 929, mouse fibroblast cell line, NIH 3T3: mouse embryonic fibroblast cell line, CHO-K1: Chinese hamster ovary cell line.
Vero cell was not infected with MERS-CoV.
Table 4.
In vivo pharmacokinetic profiles in rat of 20.
| Parameters* | I.V., 2 mg/kg | P.O., 5 mg/kg |
|---|---|---|
| Tmax (h) | NA** | 2.0 |
| Cmax (μg/h) | NA | 0.2 |
| T1/2 (h) | 5.9 | 5.5 |
| AUC (μg·h/mL) | 1.11 | 0.57 |
| CL (L/h/kg) | 1.73 | NA |
| Vss (L/Kg) | 6.3 | NA |
| Ft (%) | NA | 21 |
All results are the mean of experiments using three rats.
NA: not applicable.
In conclusion, we have synthesized a series of 4-anilino-6-aminoquinazoline derivatives and most of the compounds showed anti-MERS-CoV activity in Vero cell. The best compound among the derivatives was 20, which had the form of quinazoline with 3-Chloro-4-fluoroaniline at 4 position and 3-cyanobenzyl amine at 6 position. Compound 20 showed high anti-MERS-CoV activity (IC50 = 0.157 μM, SI = 25) with no cytotoxicity and moderate in vivo PK property. Further studies on additional SAR and pharmacological investigation of these compounds are currently underway.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The chemical library used in this study was kindly provided by Korea Chemical Bank (http://www.chembank.org/) of Korea Research Institute of Chemical Technology. This work was supported by a grant of National Research Council of Science & Technology (NST) by the Korean government (MSIP) (No. CRC-16-01-KRICT).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bmcl.2020.127472.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Woo P.C., Lau S.K., Huang Y. Exp Biol Med. 2009;234:1117. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 2.Chan J.F., Li K.S., To K.K. J Infect. 2012;65:477. doi: 10.1016/j.jinf.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan J.F., Lau S.K., Woo P.C. J Formos Med Assoc. 2013;112:372. doi: 10.1016/j.jfma.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO). Summary table of SARS cases by country, 1 November 2002 - 7 August 2003. https://www.who.int/csr/sars/country/2003_08_15/en/ [online].
- 5.World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV). https://www.who.int/emergencies/mers-cov/2020_04_04/en/ [online].
- 6.Azhar E.I., El-Kafrawy S.A., Farraj S.A. N Eng J Med. 2014;370:2499. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 7.Zumla A., Chan J.F., Azhar E.I. Nat Rev Drug Discov. 2016;15:327. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Assiri A., McGeer A., Perl T.M. N Eng J Med. 2013;369:407. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Memish Z.A., Zumla A.I., Al-Hakeem R.F. N Eng J Med. 2013;368:2487. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 10.Liang R., Wang L., Zhang N. Viruses. 2018;10:721. doi: 10.3390/v10120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruz D.J., Bonotto R.M., Gomes R.G. PLoS Negl Trop Dis. 2013;7:2471. doi: 10.1371/journal.pntd.0002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Yoon J.H., Lee J., Lee J.Y. Bull Korean Chem Soc. 2019;40:906. doi: 10.1002/bkcs.11832. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yoon J.H., Lee J.Y., Lee J. Bioorg Med Chem Lett. 2019;29 [Google Scholar]
- 13.Li H.-Q., Li D.-D., Lu X. Bioorg Med Chem Lett. 2012;20:317. [Google Scholar]
- 14.Lynch T.J., Bell D.W., Sordella R. N Engl J Med. 2004;350:2129. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 15.Wood E.R., Truesdale A.T., McDonald O.B. Cancer Res. 2004;64:6652. doi: 10.1158/0008-5472.CAN-04-1168. [DOI] [PubMed] [Google Scholar]
- 16.Raymond E., Faivre S., Armand J.P. Drugs. 2000;60:15. doi: 10.2165/00003495-200060001-00002. [DOI] [PubMed] [Google Scholar]
- 17.Vavalà T. Clin Pharmacol. 2017;9:147. doi: 10.2147/CPAA.S112715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandregowda V., Rao G.V., Reddy G.C. Org Proc Res Dev. 2007;11:813. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.