To the Editor: Autoimmune bullous diseases (AIBDs) are potentially life-threatening disorders requiring long-term immunomodulatory therapies.1 , 2 As the coronavirus disease 2019 (COVID-19) has emerged as a widespread public health emergency since December 2019,3 we have published recommendations for their management during the outbreak.4 However, there is currently only sparse evidence-based information on how the pandemic affects this special patient population, considering that AIBDs are rare conditions and that it is difficult to collect large cohorts. Therefore, a rapid systematic review of published cases has been conducted.
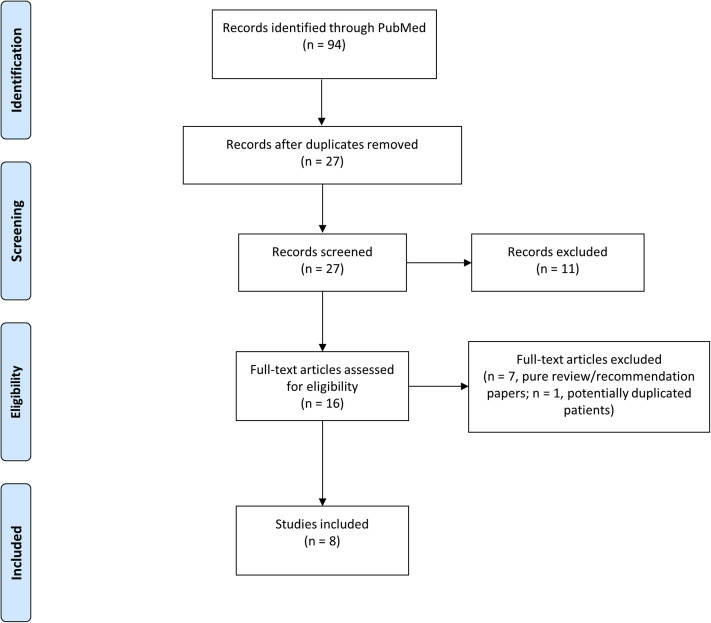
Literature was comprehensively screened using the PubMed database from inception to July 28, 2020. Search terms were “pemphigus” or “pemphigoid” or “bullous” or “blistering” combined with “COVID-19” or “SARS-CoV-2” or “coronavirus.” Inclusion criteria were English-language clinical and epidemiologic reports related to AIBD cases in association with the COVID-19 outbreak and indexed in the mentioned database. Pure review/recommendation articles and articles not related to both AIBDs and COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) were excluded. The main endpoints were the proportion of patients with AIBDs with COVID-19 symptoms and confirmed COVID-19 as well as the rate of related hospitalizations and deaths. Screening and review of articles were performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Fig 1 ).
Fig 1.
PRISMA flow diagram—inclusion of records.
Eight articles (7 case series and 1 case report) with a pooled total of 732 patients with AIBDs (211 pemphigus, 112 pemphigoid, 409 not specified) from 3 countries were included in the final analysis (Table I ). Except for 1 report, all information was collected via telephone/telemedicine visits that have been performed instead of in-person encounters during the COVID-19 outbreak. COVID-19 symptoms were reported in 70 (9.5%) patients, and in 16 (2.1%) patients the diagnosis was confirmed. Six (0.8%) patients had severe symptoms requiring hospitalization, and 3 (0.4%) died of COVID-19, all of whom were elderly people and/or had comorbidities. Only 3 (0.4%) patients were reported to have some limited control of their AIBD.
Table I.
Summary of published reports relating to COVID-19 and autoimmune bullous disease cases
| Study type/origin | Patient population | Demographic characteristics | Comorbidities | Treatment | COVID-19 | Course of AIBD | Other main findings |
|---|---|---|---|---|---|---|---|
| Case series/Italy | 371 with AIBD (NS) | 202 women and 169 men Mean age: 55.8 y Disease duration NR |
NR | Azathioprine, cyclophosphamide, cyclosporine, dapsone, doxycycline, mycophenolate mofetil, methotrexate, prednisone/prednisolone, rituximab (n = 12), topical corticosteroids | Symptoms: 47 patients with COVID-19–related symptoms (NS) Confirmed diagnosis by PCR: 3 patients (NS) Hospitalization/death: NR |
NR | None of the patients had discontinued the prescribed therapies on their own accord or because urged by their general practitioners; none of the 12 patients actively on rituximab had COVID-19–related symptoms or positive testing results. |
| Case series/Italy | 30 with BP, 9 with pemphigus (NS), and 4 with MMP | Pemphigus: 1 woman Age: 65 y Disease duration: 40 mo Other patients NR |
NR | Mycophenolate mofetil (n = 1); other NR | Symptoms: 1 patient with nausea, fever, anorexia, and asthenia; other patients NR Confirmed diagnosis by PCR: 1 patient (woman, 65 y) with pemphigus receiving mycophenolate mofetil Hospitalization/death: NR |
Remission in the patient with confirmed COVID-19, some posterior tongue discomfort; other patients NR | — |
| Case series/Italy | 5 with BP and 4 with pemphigus (NS) | 7 women and 2 men Age range: 60-83 y (mean: 71.1 y) Disease duration NR |
NR | Prednisone (n = 9), topical corticosteroids (n = 6), rituximab (n = 1) | Symptoms: 8 patients with good general status Confirmed diagnosis by PCR: 1 patient (NS) Hospitalization/death: NR |
Stable disease (all) | — |
| Case series/Italy | 62 with BP and 31 with PV | BP: 28 women and 34 men Age range: 52-98 y (mean: 78.6 y); Disease duration: 1-123 mo PV: 14 women and 17 men Age: 19-95 y (mean: 62.5 y) Disease duration: 5-411 mo |
BP: diabetes (n = 22), hypertension (n = 17), neurologic/psychiatric diseases (n = 14), cardiovascular diseases (n = 21), chronic kidney failure (n = 6), dyslipidemia (n = 9), neoplasia (n = 4) PV: diabetes (n = 3), hypertension (n = 1), neurologic/psychiatric diseases (n = 5), cardiovascular diseases (n = 2), dyslipidemia (n = 1), neoplasia (n = 2) |
BP: azathioprine (n = 4), doxycycline (n = 6), systemic corticosteroids (<20 mg/d prednisone equivalent; n = 52) PV: azathioprine (n = 3), cyclophosphamide (n = 4), dapsone (n = 2), rituximab (n = 1), systemic corticosteroids (<20 mg/d prednisone equivalent; n = 22) |
Symptoms (BP): 6 patients with mild/moderate symptoms (eg, flu-like symptoms, cough, low-grade fever, and/or anosmia/ageusia); 4 patients with severe symptoms (eg, pneumonia with respiratory failure) Confirmed diagnosis by PCR (BP): 4 patients Hospitalization/death (BP): 4 and 3 (mean age, 85 y; all 3 with severe cognitive impairment) patients, respectively Symptoms (PV): 6 patients with mild/moderate symptoms (eg, flu-like symptoms, cough, low-grade fever, and/or anosmia/ageusia); 1 patient with severe symptoms (NS) Confirmed diagnosis by PCR (PV): 1 patient (69 y; previous breast cancer) Hospitalization/death (PV): 1 and 0 patients, respectively |
Remission (all) | Main risk factor of developing suspected COVID-19 symptoms was contact between the patient and an individual with known/suspected COVID-19; longer disease duration was more frequently associated with suspected COVID-19 symptomatic patients. |
| Case series/China | 38 with AIBD (NS) | NR | NR | NR | NR | Mild disease (all) | 17 patients discontinued their therapy; 21 patients admitted that they were worried about COVID-19 infection. |
| Case report/Iran | 1 with MMP | 1 man Age: 43 y Disease duration: 4 mo |
Diabetes, hypertension, and benign prostatic hypertrophy | Intravenous immunoglobulins (5 × 30 g), mycophenolate mofetil (2 g/d), prednisolone (up to 50 mg/d), rituximab (4 × 500 mg) | Symptoms: fever, chills, malaise, dry cough, dyspnea, dizziness, decreased oxygen saturation, pneumonia, and laboratory test result abnormalities (ie, lymphopenia, positive CRP, and high LDH and ESR) Confirmed diagnosis: yes (by spiral chest CT scan; PCR negative) Hospitalization/death: yes and no, respectively |
Initial disease progression, but improvement with intravenous immunoglobulins | Improvement of both MMP and COVID-19 with adjuvant intravenous immunoglobulins |
| Case series/Italy | 10 with BP | 6 women and 4 men Median age: 68.5 y Disease duration: NR |
Diabetes, hypertension, malignancy (n = 3) | Azathioprine (n = 3), systemic corticosteroids (n = 8), topical corticosteroids (n = 8) | NR | Mild to moderate grade (n = 7) and severe grade (n = 3) of the disease; almost all patients (n = 8) had good control of their disease | — |
| Case series/Iran | 167 with PV | Mean age: 48.6 y Sex and disease duration NR for most patients |
NR | Rituximab (all), corticosteroids and other immunosuppressants (NS; n = 165) | Symptoms: 4 patients with fever, nausea, vomiting, myalgia, dry cough, and/or dyspnea Confirmed diagnosis by CT scan: 5 patients, 1 of whom was asymptomatic (4 women and 1 man; mean age, 41.8 y; all without a past medical history) Hospitalization/death: NR |
None of the patients with confirmed COVID-19 experienced disease recurrence | 45 (26.9%) patients received rituximab within 1 year of the pandemic, and none of them developed COVID-19; 150 (89.8%) patients adhered to home quarantine, and all used face masks in public places. |
AIBD, Autoimmune bullous disease; BP, bullous pemphigoid; CRP, C-reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; MMP, mucous membrane pemphigoid; mo, month; NR, not reported; NS, not specified; PCR, polymerase chain reaction; PV, pemphigus vulgaris, y, year.
Due to the journal's policy, full references of the respective studies cannot be displayed due to limitation in citation numbers.
Although old age and certain comorbidities such as hypertension and diabetes represent well-described risk factors for complicated COVID-19, the role of immunosuppression remains controversial.3 , 5 Taking into account that approximately 15% of COVID-19 cases are of a severe nature in the general population and that the overall mortality rate associated with COVID-19 is 1.5% to 3.6%,3 this preliminary systematic analysis suggests that patients with AIBDs receiving immunomodulatory therapies are basically not at increased risk of severe or fatal COVID-19. This assumption, which may potentially be at least partly associated with enhanced disease prevention in this patient cohort, is in line with previous reports about other immunosuppressed patient populations during the present and past coronavirus outbreaks.5 The results further indicate that the pandemic generally does not seem to negatively affect the course of AIBDs. Therefore, although surveillance of and precautions for this particular patient group must remain, delays or obstructions in important immunomodulatory treatment should be avoided during the pandemic. Nevertheless, data from this brief systematic review need to be interpreted with caution until more comprehensive investigations such as international registries further define the influence of COVID-19 on patients with AIBDs.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: not applicable.
Reprints not available from the authors.
References
- 1.Schmidt E., Kasperkiewicz M., Joly P. Pemphigus. Lancet. 2019;394:882–894. doi: 10.1016/S0140-6736(19)31778-7. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 3.Sharma R., Agarwal M., Gupta M., et al. In: Coronavirus Disease 2019 (COVID-19). Medical Virology: From Pathogenesis to Disease Control. Saxena S., editor. Springer; Singapore: 2020. Clinical characteristics and differential clinical diagnosis of novel coronavirus disease 2019 (COVID-19) pp. 55–70. [Google Scholar]
- 4.Kasperkiewicz M., Schmidt E., Fairley J.A., et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020;34:e302–e303. doi: 10.1111/jdv.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]