Abstract
Purpose
Since the SARS-CoV-2 pandemic, countries are overwhelmed by critically ill Coronavirus disease 2019 (COVID-19) patients. As ICU capacity becomes limited we characterized critically ill COVID-19 patients in the Netherlands.
Methods
In this case series, COVID-19 patients admitted to the ICU of the Jeroen Bosch Hospital were included from March 9 to April 7, 2020. COVID-19 was confirmed by a positive result by a RT-PCR of a specimen collected by nasopharyngeal swab. Clinical data were extracted from medical records.
Results
The mean age of the 50 consecutively included critically ill COVID-19 patients was 65 ± 10 years, the mean BMI was 29 ± 4.7 and 66% were men. Seventy-eight percent of patients had ≥1 comorbidity, 34% had hypertension. Ninety-six percent of patients required mechanical ventilation and 80% were ventilated in prone position. Venous thromboembolism was recognized in 36% of patients. Seventy-four percent of patients survived and were successfully discharged from the ICU, the remaining 26% died (median follow up 86 days). The length of invasive ventilation in survivors was 15 days (IQR 12–31).
Conclusions
The survival rate of COVID-19 critically ill patients in our population is considerably better than previously reported. Thrombotic complications are commonly found and merit clinical attention.
Trial registration number: NL2020.07.04.01
Keywords: COVID-19, SARS- Cov-2., Critical care., Mortality., Venous Thromboembolism.
Abbreviations list: ARDS, Acute Respiratory Distress Syndrome; BMI, Body Mass Index; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; IBW, Ideal Body Weight; ICU, Intensive Care Unit; IQR, Interquartile range; LDH, Lactate dehydrogenases; NICE, National Intensive Care Evaluation; PEEP, Positive end-expiratory pressure; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SD, Standard deviation; SDD, Selective digestive decontamination; RT-PCR, Reverse-transcriptase-polymerase-chain-reaction; VTE, Venous thromboembolism
Highlights
-
•
Critically ill COVID-19 patients stress capacity of ICUs around the globe
-
•
Scientific data regarding their course of disease and outcome are needed
-
•
This case series investigated 50 COVID-19 ICU patients in the Netherlands
-
•
Venous thrombo-embolism is observed in 36% of patients and this merits attention
-
•
The survival rate is 74%, whereas the remaining 26% of patients died
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified mid-December 2019 in Wuhan, China, and is responsible for the development of coronavirus disease 2019 (COVID-19) [1,2]. Given its rapid global spread, over 10 million cases have been confirmed and COVID-19 has been responsible for at least 500,000 deaths by June 30, 2020 [3].
COVID-19 represents a severe respiratory illness characterized by fever, dry cough and dyspnoea [2]. Although the most infected individuals experience mild complaints, infected patients are frequently admitted to hospitals. More importantly, a significant proportion of the admitted patients develop acute respiratory failure and require management in intensive care units (ICUs) [2,4,5]. Several studies report prolonged treatment in ICU together with a high mortality in critically ill COVID-19 patients [[6], [7], [8]]. The need for prolonged intensive care treatment stresses health care facilities, as seen in China and the North of Italy [2,5].
In the Netherlands, the first confirmed COVID-19 case was reported on the 27th of February 2020 and was followed by an outbreak in the region of North Brabant [9]. Dutch hospitals increased their intensive care capacity and a national coordination center allocates ICU patients to hospitals across the country.
Scientific data regarding critically ill COVID-19 patient course of disease and outcomes are essential for the management of intensive care capacity and allocation of health care resources. This study provides a characterization of the COVID-19 critically ill patients admitted to the ICU in a large single-center teaching hospital in the Netherlands. Moreover, during the study venous thromboembolism (VTE) was increasingly recognized and found in a third of critically ill COVID-19 patients. As VTE may have important clinical consequences, this merits attention.
2. Methods
2.1. Study design, setting and participants
This single-center case series study was performed at the ICU of Jeroen Bosch hospital, ‘s-Hertogenbosch, the Netherlands. The Jeroen Bosch hospital is a 630 bed teaching hospital in the region of Noord-Brabant, the Netherlands. The hospital normally has 14–16 ICU beds serving approximately 300.000 residents of the ‘s-Hertogenbosch and Bommelerwaard area. During the outbreak our ICU capacity was doubled with a total of 25 ICU beds designated for both COVID-19 patients and non-COVID-19 patients.
The first 50 critically ill adults with a laboratory confirmed COVID-19 infection that required intensive care management were consecutively included from March 9, 2020 and followed up to June 20, 2020. A confirmed case was defined as a positive laboratory result on a reverse-transcriptase-polymerase-chain-reaction (RT-PCR) of a specimen collected by nasopharyngeal swab or, if this test was negative, a positive RT-PCR on lower respiratory tract specimens. All COVID-19 patients were treated with chloroquine and received cefuroxime in the context of selective digestive decontamination (SDD) and low-dose low-molecular weight heparins as thrombosis prophylaxis (unless patients used anticoagulation for other indications).
Since the Dutch national coordination center allocates ICU patients from hospitals in high endemic areas to hospitals in low endemic areas to equalize workload, many patients were transferred to other hospitals. Data on course of disease and treatment outcome was acquired by telephone contact with the treating intensivist or from the ICU discharge letter.
This study is conducted according to the principles of the Declaration of Helsinki (version Oct 2008) and in accordance with the Medical Research Involving Human Subjects Act (WMO) and other guidelines, regulations and acts. Given the observational nature of the study together with the societal impact informed consent was waived. The study was approved by the Jeroen Bosch Hospital local ethics committee (NL2020.07.04.01).
2.2. Data collection
Investigators reviewed electronic medical records, laboratory results and radiological examinations. The demographical data concern admission date, age, gender, body mass index (BMI), ideal body weight (IBW), smoking status and comorbidities. Data on symptoms, vital parameters, laboratory parameters and radiographic findings were extracted, as well as the course of disease on the ICU (e.g. the need for mechanical ventilation, renal replacement therapy and complications such as VTE. Outcome parameters concern survival, discharge, days of mechanical ventilation, length of stay in hospital and length of stay in ICU.
2.3. Statistical analysis
This observational case series aims to describe clinical characteristics and patient outcomes in critically ill COVID-19 patients admitted in the ICU and is thus is purely descriptive. No sample size calculation was performed and no formal analyses for statistical significance were performed. The continuous data are expressed as means with standard deviation (SD) or medians with interquartile ranges (IQR), as appropriate. The categorical data are expressed as counts and percentages. Analyses were performed with SPSS.
3. Results
Fifty critically ill COVID-19 patients were consecutively included from March 9, 2020 and followed up to June 20, 2020. The demographical and clinical characteristics are displayed in Table 1 . The mean age was 65 ± 10 (range 33–82 years); 66% of patients were men and the mean BMI was 29 ± 4.7. Twenty-two percent of the patients had no relevant comorbidities. The most common comorbidities were hypertension (34%), type II diabetes (14%), obstructive sleep apnea (12%) and ischemic heart disease (10%) (see Table 1). The mean duration of symptoms on admission to the hospital was 8.2 ± 3.5 days. The most common symptoms were shortness of breath (84%) and cough (82%). Upon presentation at the emergency department 53% of the patients had fever (see Table 2 ).
Table 1.
Patient demographics and comorbidities.
| Demographics | Patients (n = 50) |
|---|---|
| Age, years | 65 (10) (range 33–82) |
| Gender | |
| Male | 33 (66%) |
| Female | 17 (34%) |
| Body Mass Index | 29 (4,7) |
| Tobacco use | |
| Never smoker | 23/33 (70%) |
| Current smoker | 0 (0) |
| former smoker | 10/33 (30%) |
| Comorbidities | |
| No relevant comorbidities | 11 (22%) |
| Hypertension | 17 (34%) |
| Diabetes mellitus | |
| Type 1 | 0 (0%) |
| Type 2 | 8 (16%) |
| Cardiovascular disease | |
| Ischemic heart disease | 5 (10%) |
| Congestive heart failure | 0 (0%) |
| Rhythm disturbances | 2 (4%) |
| Respiratory disease | |
| Asthma | 3 (6%) |
| COPD | 1 (2%) |
| Other | 4 (8%) |
| Obstructive sleep apnea | 6 (12%) |
| Chronic kidney disease | 3 (6%) |
| Active malignancy | 2 (4%) |
| Immunosuppression | 3 (6%) |
Data are mean (SD) or n (%), unless otherwise specified.
Table 2.
Symptoms and vital signs on admission.
| Symptoms | Patients (n = 50) |
|---|---|
| Duration of symptoms (days) | 8,2 (3,5) |
| Cough | |
| No cough | 2/38 (5%) |
| Dry | 23/38 (61%) |
| Productive | 13/38 (34%) |
| Gastro intestinal symptoms | |
| Yes | 17/34 (50%) |
| No | 17/34 (50%) |
| Headache | |
| Yes | 12/30 (40%) |
| No | 16/30 (53%) |
| Muscle strain | |
| Yes | 13/26 (50%) |
| No | 13/26 (50%) |
| Shortness of breath | |
| Yes | 42/47 (89%) |
| No | 5/47 (11%) |
| Exhaustion | |
| Yes | 20/25 (80%) |
| No | 5/25 (20%) |
| Vital signs | |
| Temperature > 38 °C | 25/47 (53%) |
| Heart rate > 100 beats per min | 29/47 (62%) |
| Respiratory rate > 20 breaths per min | 37/45 (82%) |
Data are mean (SD) or n/total n of patients with available data (%).
Bilateral pulmonary infiltrates were seen on 82% of the chest radiographs, see Table 3 . Only 4 patients had no pulmonary infiltrates on the chest radiograph at admission to the hospital. The patients laboratory investigation showed a lymphocytopenia in 63% of patients. Neutrophil-to-lymphocyte ratios were high in this cohort, median 6.4 (IQR 4.8–8.8) at admission to the hospital. C-reactive protein (CRP) levels were also strongly increased with a median of 145 mg/L (IQR 125–191), whereas procalcitonin concentrations were generally low with a median of 0.22 ng/mL (IQR 0.12–0.53). Notably, all patients had increased lactate dehydrogenases (LDH) and increased D-dimer levels. Also increased ferritin levels were observed, median 1200 ng/L (IQR 748–1750) (see Table 3).
Table 3.
Radiographic and laboratory findings on admission.
| Radiographic findings | ||
|---|---|---|
| Chest radiograph | No infiltrates | 4 (8%) |
| Unilateral infiltrates | 4 (8%) | |
| Bilateral infiltrates | 41 (82%) | |
| Pleural effusion | 0 | |
| Signs of congestive heart failure | 0 | |
| Consistent with COVID-19a | 16 (32%) | |
| Laboratory findings | ||
|---|---|---|
| White cell count | < 4 × 109/L | 2/47 (4%) |
| 4–10 × 109/L | 37/47 (79%) | |
| ≥ 10 × 109/L | 8/47 (17%) | |
| Lymphocyte count | < 1,0 × 109/L | 29/46 (63%) |
| 1,0–3,5 × 109/L | 17/46 (37%) | |
| Neutrophil count | < 1,5 × 109/L | 1/46 (2%) |
| 1,5–7,5 × 109/L | 36/46 (65%) | |
| ≥ 7,5 × 109/L | 15/46 (33%) | |
| Neutrophil-to-lymphocyte ratio | 6.4 (4.8–8.8) | |
| Platelet count | < 150 × 109/L | 6/47 (13%) |
| 150–400 × 109/L | 39/47 (83%) | |
| ≥ 400 × 109/L | 2/47 (4%) | |
| CRP, mg/L | 145 (125–191) | |
| Procalcitonin, ng/mL | 0,22 (0,12 - 0,53) | |
| Ferritin ug/mL | 1200 (748–1750) | |
| D-dimer | < 0,5 mg/L | 0 |
| 0,5–4,0 mg/L | 21/27 (78%) | |
| ≥ 4,00 mg/L | 6/27 (22%) | |
| CK | 0–144 U/L | 23/37 (62%) |
| ≥ 144 U/L | 14/37 (38%) | |
| ASAT, U/L | 54 (26–81) | |
| ALAT, U/L | 54 (26–84) | |
| LD | < 249 U/L | 0 |
| 250–500 U/L | 25/39 (64%) | |
| 500–1000 U/L | 13/39 (33%) | |
| ≥ 1000 U/L | 1/39 (3%) |
Data are n (%), n/total n of patients with available data (%) or median (IQR) unless otherwise specified. (CRP: C-reactive protein; CK: creatin kinase; ASAT: aspartate aminotransferase; ALAT: alanine aminotransferase; LD: lactate dehydrogenase).
According to the radiologist.
All patients were admitted to the ICU due to acute hypoxemic respiratory failure in absence of hemodynamic instability. Of the 50 admitted patients, 49 were in need of invasive or non-invasive respiratory support (98%)(see Table 4 ). Endotracheal intubation and mechanical ventilation was required in 47 of the 49 patients (94%), and 2 patients were be successfully managed with non-invasive ventilation with a high flow oxygen cannula. Two patients initially treated with a high flow oxygen cannula deteriorated and later required invasive mechanical ventilation.
Table 4.
ICU treatment, complications and outcomes.
| Therapies and complications. | |
| High flow nasal cannula | 4/50 (8%) |
| Invasive mechanical ventilation | 47/50 (94%) |
| Ventilation in prone position | 35/44 (80%) |
| ECMO | 1/46 (2%) |
| RRT | 12/49 (24%) |
| Highest PEEP, cm H2O | 14 (12–16) |
| Highest FiO2, % | 70 (60–90) |
| Percutaneous tracheostomy | 15/38 (39%) |
| Evidence of co-infection during ICU treatment | |
| Bacterial | 13/50 (26%) |
| Fungal | 2/50 (4%) |
| Other | 1/50(2%) |
| Catheter related blood stream infections | 4/50 (8%) |
| VTE | 18/50 (36%) |
| Peak SOFA score | 9,5 (6–11) |
| Outcomes | |
|---|---|
| Length of stay in ICU (days) | |
| Survivors | 19 (11–34) |
| Non-survivors | 10 (6–22) |
| Duration of mechanical ventilation (days) | |
| Survivors | 15 (12−31) |
| Non-survivors | 10 (6–21) |
| Survival | 37/50 (74%) |
| Length of follow up (survivors, days) | 86 (82–94) |
Data are n/total n of patients with available data (%) or median (IQR) unless otherwise specified. (ECMO: extracorporeal membrane oxygenation; RRT: renal replacement therapy; VTE: venous thromboembolism).
Among the mechanically ventilated patients, 80% required ventilation in prone position during their stay in ICU according to our local protocol. The median highest positive end-expiratory pressure (PEEP) recorded was 14 (IQR 12–16) cm H2O, and the median highest FiO2 recorded was 0.7 (IQR 0.6–0.9) during mechanical ventilation. Data on renal replacement therapy was available for 49 patients, 12 (24%) of which required renal replacement therapy (10 continuous venovenous hemofiltration, 2 intermittent hemodialysis). Bacterial co-infections, defined as signs of infection requiring treatment during the ICU admission, were found in 26% of the patients and mostly concerned secondary infections, such as ventilator-associated pneumonia. In 8% of patients catheter related blood stream infections were observed. The median peak SOFA score for our cohort was 9.5 (IQR 6–11), thereby representing a severely compromised population.
During the study period VTE were increasingly recognized. Eighteen out of 50 patients developed VTEs (36%). In 13 patients pulmonary embolism was found, in 5 patients catheter related thrombi were observed, one patient developed a deep venous thrombosis of the forearm and one patient developed a spontaneous jugular vein thrombus (see Table 4). These complications occurred despite of the thrombosis prophylaxis that is generally administered to ICU patients.
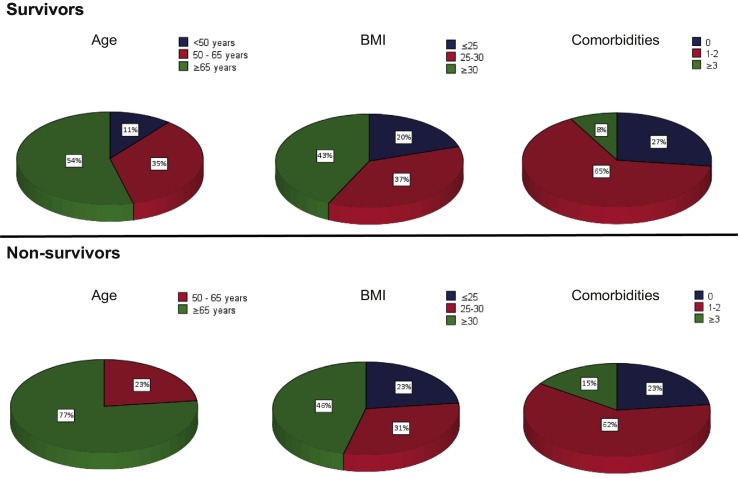
As of June 20, 2020, 37 of the 50 patients (74%) survived and were successfully discharged from the hospital, the remaining 26% of patients died. The median length of invasive mechanical ventilation in survivors was 15 days (IQR 12–31), whereas the median length of invasive mechanical ventilation in non survivors was 10 days (IQR 6–21). The length of stay in ICU was 19 days (IQR 11–34) for survivors, compared to 10 days (6–22) for non-survivors. The median follow up for patients who survived is 86 days (see Table 4). Moreover, all patients had a follow up of ≥10 weeks. Compared with survivors, non-survivors are generally older, while no clear differences in their BMI and the number of comorbidities were observed (see Fig. 1 ). Moreover, no difference was found in neutrophil-to-lymphocyte ratio in survivors versus non-survivors (data not shown).
Fig. 1.
Characteristics of survivors versus non-survivors.
4. Discussion
Critically ill COVID-19 patients admitted to our ICU in the Netherlands with a follow up of 2.5 months show a survival rate of 74%, whereas the remaining 26% of patients died. All patients were admitted to the ICU due to acute hypoxemic respiratory failure. The median length of mechanical ventilation was 15 days for survivors, compared to 10 days for non-survivors. Mechanically ventilated patients required high levels of support with a median highest recorded FiO2 of 0.7 (IQR 0.6–0.9) and median highest recorded PEEP of 14 cm H2O (IQR 12–16). Moreover, 80% of patients were ventilated in prone position during their ICU stay.
The mortality rate observed in this study is considerably lower than previously reported. Yang et al. reported 61.5% mortality in a similar sized study performed in China [7]. Two studies performed in the United States report a mortality of 67% and 50%, respectively [8,10]. These studies included a broader age range of patients and their findings may be at least partially explained by a worse prognosis in older patients. A recent report by Grasselli et al. including 1591 COVID-19 positive ICU patients from Italy showed a similar mortality rate of 26%, however, 58% of patients still received active treatment in ICU at the time of publication [6]. The mortality rates may be subject to various factors. First, countries that were affected early in the pandemic were overwhelmed by this previously unknown disease and may have experienced issues with allocation of healthcare resources [2,5]. After early warnings from our colleagues, the level of preparedness in the Netherlands was high, shown by the increased ICU capacity on a national level and the Dutch system of patient allocation across the country.
Second, in the Netherlands a stringent patient selection for ICU admission is generally applied, hence the data may be subject to patient selection. Patients with important comorbidities, a poor functional status or frailty are less frequently admitted to the ICU [11]. This is supported by data from the Dutch surveillance registry, National Intensive Care Evaluation (NICE). In their last report, the overall mortality of COVID-19 critically ill patients in the Netherlands was 31%, in a similar patient group in terms of age, gender and comorbidities [12]. In comparison to previous studies, our study has a significantly longer follow up and, additionally, prolonged ICU treatment may be pursued in patients with a better prognostic perspective.
In the early stages of the pandemic little was known on the clinical course of the disease, whereas over time more became known and different treatment- and supportive strategies were proposed. Below, several factors that may have improved clinical outcome from a medical perspective are discussed.
COVID-19 in critically ill patients is characterized by severe hypoxemia that has been attributed to the development of Acute Respiratory Distress Syndrome (ARDS) [6,[13], [14]]. A substantial proportion of our patients were ventilated in prone position according to our local protocol (based on the ARDSnet low tidal volume protocol) [13,15]. These lung-protective ventilatory strategies may improve patient survival in COVID-19, although this has not yet been formally established.
Interestingly, most mechanically ventilated COVID-19 patients had a discrepantly good lung compliance [16]. Reports of pulmonary embolism in critically ill COVID-19 patients gained attention and during the study period VTE indeed were increasingly recognized. VTE were found in over a third of the critically ill COVID-19 patients and probably VTE have been overlooked in earlier cases. A recent publication that systematically investigated VTE in critically ill COVID-19 patients reports an incidence of 31% [17]. Inflammation and coagulation are closely linked biological systems [18,19]. The risk of VTE is higher during episodes of increased inflammation as has been shown in several clinical settings [[20], [21], [22]]. In critically ill COVID-19 patients, the risk for VTE is particularly high and more emphasis should be placed on the early recognition of thrombotic complications since VTE have important clinical consequences and may improve patient survival.
Another explanation for the discrepantly compliant lung may be the development of bradykinin-dependent local lung angioedema [23]. Van de Veerdonk et al. suggest that that blocking of bradykinin type 1 and bradykinin receptor type 2 can have beneficial effects in critically ill COVID-19 patients [[23]]. Further research is needed to increase our understanding of the underlying mechanisms and to address the role of bradykinin in COVID-19.
Lastly, it has been suggested that the administration of corticosteroids may be beneficial in critically ill COVID-19 patients [24]. In the late phase of our study several patients were treated with corticosteroids in the presence of a hyperinflammatory profile without signs of infection. There has been a long-standing questionable relationship with the use of corticosteroids in critically ill patients and well-designed studies are needed to address its role.
This study has several limitations. First, the relatively small number of included patients from a single-center hospital may not broadly reflect critically ill COVID-19 patients. Second, due to the allocation of patients over ICUs across the country some cases had incomplete documentation concerning laboratory values and course of disease, but not clinical outcome.
In conclusion, critically ill COVID-19 patients admitted to the ICU have severe acute hypoxemic respiratory failure and many patients require prolonged mechanical ventilation with high levels of support. Nevertheless, the survival rate of COVID-19 critically ill patients in our population is 74%. Thrombotic complications were observed in a third of critically ill COVID-19 patients and this merits clinical attention.
Financial/non-financial disclosures
There was no funding for this investigator-initiated trial.
Declaration of Competing Interest
Authors declare no conflicts of interests.
Acknowledgements
F.A. and L.M. are the guarantors of the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Li Q., Guan X., Wu P. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO reports . 2020. Coronavirus disease 2019 (COVID-19) Situation Report – 162. [Google Scholar]
- 4.Murk J.L., van de Biggelaar R., Stohr J., Verweij J., Buiting A., Wittens S. Dutch. [The first 100 admitted COVID-19 patients in the Elisabeth-Tweesteden hospital] NTVG. 2020;164:D5002. [PubMed] [Google Scholar]
- 5.Grasselli G., Pesenti A., Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545–1546. doi: 10.1001/jama.2020.4031. [DOI] [PubMed] [Google Scholar]
- 6.Grasselli G., Zangrillo A., Zanella A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arentz M., Yim E., Klaff L. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. 2020;323(16):1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alderweireld C.E.A., Buiting A.G.M., Murk J.L.A.N., Berrevoets M.A.H., van Kasteren M.E.E. COVID-19; patient zero in the Netherlands. NTVG. 2020;164:D4962. [PubMed] [Google Scholar]
- 10.Bhatraju P.K., Ghassemieh B.J., Nichols M. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidet B., de Lange D.W., Flaatten H. Should this elderly patient be admitted to the ICU? Intensive Care Med. 2018;44:1926–1928. doi: 10.1007/s00134-018-5054-7. [DOI] [PubMed] [Google Scholar]
- 12.Nationale Intensive Care Evaluatie (NICE) COVID-19 in Dutch Intensive Care Units; Patient Characteristics and Outcomes, version 2020-06-29. 2020. https://www.stichting-nice.nl/COVID_report.pdf [Date accessed June 30, 2020]
- 13.Sefinition Task Forse A.R.D.S., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 14.Brower R.G., Lanken P.N., MacIntyre N. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 15.Guerin C., Reignier J., Richard J.C. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 16.Mauri T., Lazzeri M., Bellani G., Zanella A., Grasselli G. Respiratory mechanics to understand ARDS and guide mechanical ventilation. Physiol Meas. 2017;38:R280–H303. doi: 10.1088/1361-6579/aa9052. [DOI] [PubMed] [Google Scholar]
- 17.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020 Apr 10 doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esmon C.T. The interactions between inflammation and coagulation. Br J Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 19.Saghazadeh A., Rezaei N. Inflammation as a cause of venous thromboembolism. Crit Rev Oncol Hematol. 2016;99:272–285. doi: 10.1016/j.critrevonc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Aleva F.E., Voets L., Simons S.O., de Mast Q., van der Ven A., Heijdra Y.F. Prevalence and localization of pulmonary embolism in unexplained acute exacerbations of COPD: a systematic review and meta-analysis. Chest. 2017;151:544–554. doi: 10.1016/j.chest.2016.07.034. [DOI] [PubMed] [Google Scholar]
- 21.Ribeiro D.D., Lijfering W.M., Van Hylckama Vlieg A., Rosendaal F.R., Cannegieter S.C. Pneumonia and risk of venous thrombosis: results from the MEGA study. J Thromb Haemost. 2012;10:1179–1182. doi: 10.1111/j.1538-7836.2012.04732.x. [DOI] [PubMed] [Google Scholar]
- 22.Grimnes G., Isaksen T., Tichelaar Y., Braekkan S.K., Hansen J.B. Acute infection as a trigger for incident venous thromboembolism: results from a population-based case-crossover study. Res Pract Thromb Haemost. 2018;2:85–92. doi: 10.1002/rth2.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van de Veerdonk F., Netea M.G., van Deuren M., van der Meer J.W., de Mast Q., Bruggeman R.J. Kinins and cytokines in COVID-19: a comprehensive pathophysiological approach. Preprints. 2020 doi: 10.20944/preprints202004.0023.v1. [DOI] [Google Scholar]
- 24.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020 Mar 13 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]