Abstract
Objectives
To evaluate the diagnostic and prognostic performance of CT in patients referred for COVID19 suspicion to a French university hospital, depending on symptoms and date of onset.
Methods
From March 1st to March 28th, 214 patients having both chest CT scan and reverse transcriptase polymerase chain reaction (RT- PCT) within 24 h were retrospectively evaluated. Sensitivity, specificity, negative and positive predictive values of first and expert readings were calculated together with inter reader agreement, with results of RT-PCR as standard of reference and according to symptoms and onset date. Patient characteristics and disease extent on CT were correlated to short-term outcome (death or intubation at 3 weeks follow-up).
Results
Of the 214 patients (119 men, mean age 59 ± 19 years), 129 had at least one positive RT-PCR result. Sensitivity, specificity, negative and positive predictive values were 79 % (95 % CI: 71–86 %), 84 %(74–91 %), 72 %(63–81 %) and 88 % (81–93 %) for initial CT reading and 81 %(74–88 %), 91 % (82–96 %), 76 % (67–84 %) and 93 % (87–97 %), for expert reading, with strong inter-reader agreement (kappa index: 0.89). Considering the 123 patients with symptoms for more than 5 days, the corresponding figures were 90 %, 78 %, 80 % and 89 % for initial reading and 93 %, 88 %, 86 % and 94 % for the expert. Disease extent exceeded 25 % for 68 % and 26 % of severe and non-severe patients, respectively (p < 0.001).
Conclusion
CT sensitivity increased after 5 days of symptoms. A disease extent > 25 % was associated with poorer outcome.
Abbreviations: COVID-19, Coronavirus Disease 2019; CRP, C-reactive protein; CT, computed tomography; GGOs, ground glass opacities; RT-PCR, reverse transcription polymerase chain reaction; SARS-Cov-2, severe acute respiratory syndrome coronavirus 2
Keywords: COVID, Multidetector computed tomography, Diagnostic tests, Routine, Prognosis
1. Introduction
Since the outbreak of SARS-Cov-2 by the end of 2019, a new coronavirus responsible for coronavirus disease 2019 (COVID-19), the epidemic has spread throughout the world, and has caused more than 200 000 deaths worldwide whereas nearly 3 million people have contracted the virus as of April 26th. The high mortality rate is explained by the severity of the pneumonia (COVID-19 pneumonia) caused by the virus.
The standard of reference for confirming COVID-19 relies on microbiological tests such as reverse-transcriptase polymerase chain reaction (RT-PCR) or sequencing. However, these tests might not be available in an emergency setting and their turnaround time is several hours. Moreover, RT-PCR can be falsely negative when the viral load is insufficient or the virus not anymore present in the throat but in the lower respiratory tract.
Computed tomography (CT) has been used as an important complement to RT-PCR for diagnosing COVID-19 pneumonia in the current epidemic context, due to its rapidity, large availability and high sensitivity for diagnosis COVID-19 pneumonia.
Numerous reports have described the typical features of COVID-19 pneumonia, which is characterized by a predominance of ground glass opacities (GGOs), admixed with areas of focal consolidation, with a bilateral distribution and subpleural predominance [[1], [2], [3], [4], [5], [6], [7], [8]].
Several phases of the disease have been described. Pan et al. classified the evolution of lung abnormalities into four stages according to time periods, with an early phase (0−4days), a progressive stage (5–8 days) then a peak (9–13 days) and absorption (≥ 14 days) phase [3]. Salehi et al. also reported that CT findings in the intermediate stage of the disease were characterized by an increase in the number and size of GGOs [9]. Liang et al. reported that pure GGO decreased while linear opacities, consistent with an organizing pneumonia pattern, increased over time [10].
However, most reports come from China and it is not clear whether the CT manifestations of COVID-19 pneumonia are exactly the same in European patients or whether the respective performances of CT and RT-PCR are comparable. Thus, the purpose of our study was to evaluate the diagnostic and prognostic performance of CT in patients referred for COVID-19 suspicion to a French university hospital, depending on symptoms and date of onset, as well as factors associated to poor outcomes in our specific population.
2. Materials and methods
2.1. Patients
This single center retrospective study, conducted at (Blinded for review) University hospital, was approved by our local ethics committee (approval number: AAA-2020-08010) which waived the need for patients’ consent. We evaluated all patients presenting at the emergency department of our hospital who were referred for a computed tomography (CT) examination of the chest for a suspicion of COVID-19 pneumonia, between March 1st and March 28th. The inclusion criteria were the availability of clinical information regarding the date of onset and nature of clinical symptoms and availability of RT-PCR results. Exclusion criteria were major artifacts on CT and prior knowledge of RT-PCT positivity at time of CT examination.
2.2. Clinical and biological data collection
In addition to patient demographic characteristics and comorbidities, the nature and date of onset of clinical symptoms were retrieved form the electronic medical records, as well as date of discharge and information regarding clinical severity defined as intubation and/or death within the 3 weeks following CT examination. Blood test results including CRP, neutrophil and lymphocyte counts were collected together with the number and results of RT-PCR assay.
2.3. Computed tomography acquisitions
CT acquisitions had been performed on a multidetector CT unit (SOMATOM Definition Edge Siemens Healthineer, Erlangen, Germany). Patients were placed in supine position with arms extended above the head, whenever possible. Images were acquired in inspiration breath-hold, with the following parameters: tube voltage of 100 kVp, pitch value of 1.2 and 0.33 gantry rotation time. Automatic tube current modulation was systematically used with a quality reference tube current of 90 mAs. Collimation was 2 × 32 × 0.6 mm (z-flying focal spot). Images were reconstructed with a 1-mm section thickness and 0.8-mm increments, using a sinogram-affirmed iterative reconstruction (SAFIRE, Siemens Healthineer) with a strength level of 3, with two reconstruction filters (i30f and i70f).
CT acquisitions were all performed without contrast administration, except for patients for whom pulmonary embolism (PE) was suspected as alternative diagnosis of COVID-19 pneumonia, after clinical probability assessment and D-dimer dosage. The contrast medium (Iomeron, iomeprol, 350 mg iodine/mL; Bracco, Milan, Italy) was injected using a power injector (Medrad® Stellant® Injector; Bayer Vital GmbH, Leverkusen, Germany) with a flow rate of 4 mL s−1 in all patients. CM injection was followed by a saline flush (40 mL, 3 mL s−1). The classical bolus trigger technique (CareBolus; Siemens Healthineer) (80 kVp) in the pulmonary trunk (PT) with a threshold of 230 HU was used to trigger the examinations. Breath-hold command was given after bolus triggering.
2.4. Image interpretation
All CT examinations were first read by a radiology resident and approved by a senior radiologist as part of the clinical routine. For the purpose of the study, they were secondarily independently reviewed by an experienced chest radiologist (Blinded for review) with 20 years of experience. All used a dedicated structured report for COVID-19 pneumonia findings on chest CT, on which they had to report the presence of ground glass opacities with or without crazy-paving pattern, isolated or admixed with perilobular or linear consolidation, and their peripheral or central distribution. They also had to report if CT features such as centrilobular nodules, mucoid impaction or other findings favoring another diagnosis were present. Radiologists had to conclude their report according to two options: either CT findings highly suggestive of COVID-19 pneumonia, or inconsistent with this diagnosis.
The severity of COVID-19 pneumonia was graded by the expert radiologist according to the extent of ground glass opacities and consolidation on lung window CT images, as follows: minimal (less than 10 % of lung parenchyma), moderate (10–25 %), intermediate (25–50 %), severe (50–75 %), critical (50–75 %).
2.5. Statistical analysis
Categorical variables are presented as numbers and percentages and continuous variables as median and interquartile range (IQR). Categorical variables were compared by using a Chi square or a Cochrane-Armitage test, whereas continuous variables were compared by using a Wilcoxon-Mann-Whitney test. Agreement between initial and expert reading was assessed by the Cohen’s kappa statistics. Binary logistic regression analysis was performed to evaluate factors independently associated with poor outcome (death or intubation).
P values below 0.05 were considered to be statistically significant. We used ‘R’ software (version 3.6.3, R Foundation, Vienna, Austria) for all analyses.
3. Results
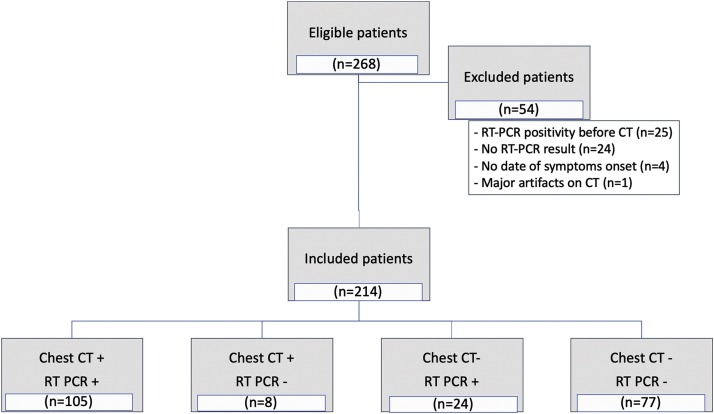
During the study period, a total of 268 individuals underwent chest CT scan for COVID-19 pneumonia suspicion at our institution. Twenty-five patients were excluded because radiologists were aware of RT-PCR positivity at time of CT examination, 24 other individuals were excluded because of unavailability of RT-PCR results. Clinical information regarding date of symptoms onset was lacking for 4 patients. A last patient was excluded because of major respiratory motion artifacts on CT images, making radiological interpretation impossible (Fig. 1 flow chart). Thus, 214 patients (119 men, median age 60 years) were finally included. Their characteristics and symptoms are presented in Table 1 . The median delay between CT examination and date of symptoms onset was 7 days. Eleven CT examinations had been performed with contrast administration allowing ruling out PE in all patients.
Fig. 1.
Flow chart of the study population.
Table 1.
Patient characteristics.
| All (n = 214) |
PCR + (n = 129) |
PCR- (n = 85) |
p value | |
|---|---|---|---|---|
| AGE | 60 [46–73] | 62 [51–75] | 56 [36–71] | 0.003 |
| Male gender | 119 (56) | 82 (64) | 37 (44) | 0.006 |
| Delay from symptom onset | 7 [3–9] | 7 [4–8] | 5 [2–10] | 0.100 |
| Symptoms | ||||
|
179 (84) | 118 (91) | 61 (72) | <0.001 |
|
173 (81) | 109 (84) | 64 (75) | 0.135 |
|
158 (74) | 93 (72) | 65 (76) | 0.58 |
|
57 (27) | 32 (25) | 25 (29) | 0.557 |
|
45 (21) | 32 (25) | 13 (15) | 0.134 |
|
78 (36) | 57 (44) | 21 (25) | 0.006 |
|
77 (36) | 48 (37) | 29 (34) | 0.752 |
| Blood tests | ||||
|
54 [14–115] | 69 [31–120] | 24 [4–94] | 0001 |
|
1.1 [0.8–1.6] | 0.9 [0.7–1.3] | 1.6 [1.0–2.2] | <0.001 |
|
5.0 [3.4–7.7] | 4.0 [3.2–6.2] | 6.2 [4.5–10.2] | <0.001 |
| Medical History | ||||
|
49 (23) | 21 (16) | 28 (33) | 0.008 |
|
36 (17) | 25 (19) | 11 (13) | 0.296 |
|
||||
| COVID-19 pneumonia on CT | ||||
|
116 (54) | 102 (79) | 14 (16) | <0.001 |
|
113 (53) | 105 (81) | 8 (9) | <0.001 |
Continuous variables are presented as median and interquartile range in brackets and were compared between PCR + and PCR - groups using Mann Whitney U test Categorical variables are presented as numbers and percentages and were compared between the two groups using chi-squared test.
The first RT-PCR result was positive for 120 (56 %) of the 214 patients. Of the 94 individuals with a first negative test, nine had positive result on subsequent testing, but only 25 of the 94 initially negative individuals had further testing. The second RT-PCR result was positive in 6 patients and remained negative in 19 patients. Four of the latter patients had a third RT-PCR test which turned out to be positive in 3 of them. Thus, 129 of all included patients (129/214, 60 %) had at least one positive RT-PCR result. Sensitivity and negative predictive value of the first RT-PCR were 93 (95 % confidence interval (95 % CI): 87–97 %) and 90 % (83–96 %), respectively.
Considering RT-PCR as the standard of reference, the sensitivities, specificities, negative and positive predictive values of CT were 79 % (71–86 %) and 81 % (74–88 %), 84 % (74–91 %) and 91 % (82–96 %), 72 % (63–81 %) and 76 % (67–84 %), and 88 % (81–93 %) and 93 % (87–97 %), for initial and expert reading, respectively. There was strong inter-reader agreement between initial and expert reading with a Cohen’s kappa value of 0.89.
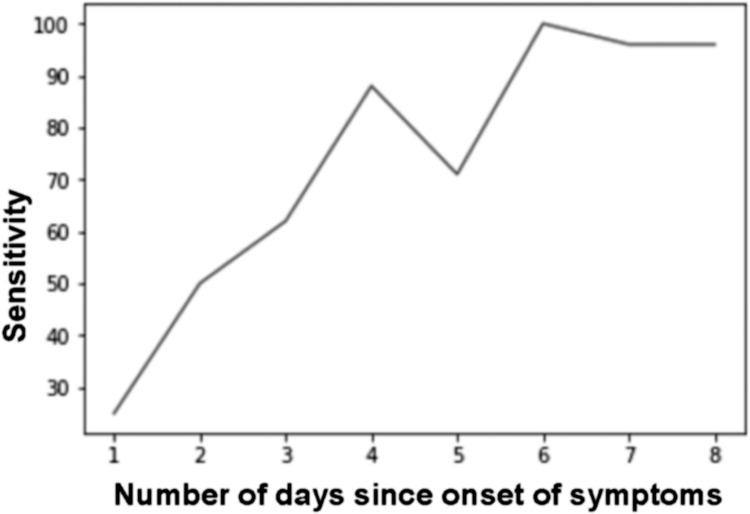
CT sensitivity was higher for the 123 patients having symptoms for more than 5 days, with sensitivity and specificity of 93 (85–97 %) and 88 % (74–96 %), respectively for the expert reading. The increase of sensitivity according to delay from symptoms onset is illustrated in Fig. 2 .
Fig. 2.
Evolution of CT sensitivity according to its delay from symptoms ‘onset.
Sensitivity (%) is represented along the Y axis and the number of days since onset of symptoms is represented along the X axis.
Regarding symptoms, fever and myalgia were significantly more frequent in patients with RT-PCR positivity, whereas other symptoms were equally distributed among the positive and negative groups. All biological variables evaluated (CRP, neutrophil and lymphocyte counts) were significantly different in the 2 groups.
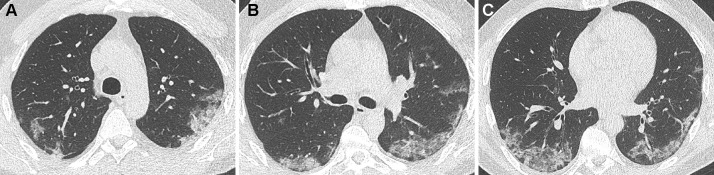
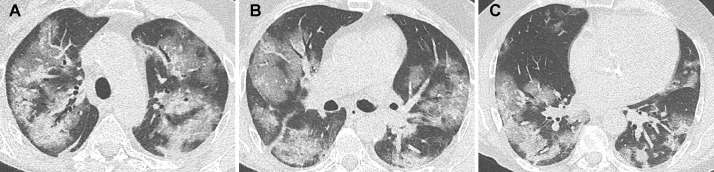
Only 8 patients with negative RT-PCR were considered as positive for COVID-19 pneumonia on expert CT reading. Six of them had a second negative RT-PCR result and the other 2 had only one RT-PCR test performed. Of the 8 patients with positive CT result, disease extent was minimal in 2 patients, moderate in 4 patients (Fig. 3 ) and severe in 2 patients (Fig. 4 ).
Fig. 3.
Unenhanced CT examination highly suggestive of COVID-19 pneumonia in a 47 year-old-man with 2 negative RT-PCR results.
Subpleural, bilateral ground glass opacities are seen posteriorly, in association with linear consolidation in the left lung base. The patient presented a respiratory deterioration 9 days after admission, with an increase of oxygen needs and was finally discharged from hospital 7 days later.
Fig. 4.
Unenhanced CT showing severe disease extent in a 63 year-old-man with positive RT-PCR results.
Peripheral and central ground glass opacities are demonstrated bilaterally. The patient was intubated 5 days later. He was discharged from hospital after one month.
Five of these patients were hospitalized and considered as having a false negative RT-PCR result, which would increase CT specificity from to 91 to 96% (89–99 %) for the expert reading.
Of the 129 patients with confirmed SARS-Cov-2 infection, 34 (26 %) had a severe outcome, defined as intubation or death in the 3 weeks following CT examination (Table 2 ). Regarding clinical characteristics, diabetes was the only significantly different clinical variable between severe and non-severe patients. Consolidation and disease extent on CT were also associated with clinical severity. Sixty-eight percent of patients with severe outcome had more than 25 % of lung parenchyma involvement, as compared to only 26 % patients of the non-severe category (p < 0.001).
Table 2.
Patient’s characteristics according to severity profile (death or intubation at 3 weeks follow-up).
| Severe (n = 34) |
Non severe (n = 95) |
p value | |
|---|---|---|---|
| Male | 25 (74) | 57 (60) | 0.23 |
| Age | 66 [55–80] | 59 [51–72] | 0.059 |
| Comorbidities | |||
|
10 (29) | 15 (16) | 0.141 |
|
13 (38) | 17 (18) | 0.03 |
|
6 (18) | 15 (16) | 0.985 |
|
9 (26) | 16 (17) | 0.334 |
|
5 (15) | 7 (7) | 0.358 |
| CT findings : | |||
|
30 (88) | 73 (77) | 0.241 |
|
23 (68) | 40 (42) | 0.018 |
| Disease extent* : | <0.001 | ||
|
5 (15) | 30 (31) | – |
|
6 (18) | 40 (42) | – |
|
16 (47) | 22 (23) | – |
|
5 (15) | 3 (3) | – |
|
2 (6) | 0 (0) | – |
Cochran-Armitage trend test.
Continuous variables are presented as median and interquartile range in brackets and were compared between PCR + and PCR - groups using Mann Whitney U test Categorical variables are presented as numbers and percentages and were compared between the two groups using chi-squared test.
Following binary logistic regression analysis, only comorbidities (p = 0.003) and CT disease extent (p = 0.008) were independently associated to death/intubation.
4. Discussion
In the present study, we found strong inter reader agreement between initial and expert reading for the diagnosis of COVID-19 pneumonia and a sensitivity reaching 93 % for expert reading when CT was performed after 5 days of symptoms, considering RT-PCR positivity only as standard of reference.
Other studies have used a different reference standard to confirm SARS-Cov-2 infection, not exclusively relying on RT-PCR results but also on a clinical validation based on the combination of symptoms, exposure, and presence of lung imaging features consistent with coronavirus pneumonia [11].
When the standard of reference is not RT-PCR, the risk is to falsely consider as COVID-19 pneumonia viral pneumonia of other origin. Indeed, a large study in which radiologists were blinded to RT-PCR results demonstrated that radiologists are capable of distinguishing COVID-19 from viral pneumonia on chest CT with only moderate sensitivity [12].
AI et al. in a large series of 1014 patients reported a higher sensitivity for CT scan than in our study, (97 % versus 93 % at best in our series) but with lower specificity [13]. In this study, two radiologists decided on positive or negative CT findings by consensus, but the authors do not specify on which criteria the diagnosis of COVID-19 was based. In a meta-analysis including 63 studies mainly from China, the pooled specificity of CT was only 37 % [14]. An Italian study performed on 156 patients reported 56 % specificity for CT [15].
The use of a structured report suggesting another diagnosis when CT findings such as centrilobular nodules or mucoid impaction were present probably accounts for the higher specificity of both initial and expert reading in our study.
Regarding clinical symptoms, the vast majority of our patients had respiratory symptoms which is in phase with the guidelines from the European Society of Radiology and the European Society of Thoracic Imaging which we followed, not recommending CT as a screening test in patients with mild or no symptoms. The advice paper from the two societies recommends CT after the clinical evaluation of patients with respiratory symptoms such as dyspnea and desaturation [16]. This is also a recommendation from the Fleischner society to not use imaging as a screening test in asymptomatic individuals [17].
An important result of our study was the increase of CT sensitivity after 5 days of evolution, not after 2 days, as reported by Bernheim et al. [18]. In their series, sensitivity increased from 56 % to 99 % between the initial and the intermediate phase which correspond to 3–5 days after symptoms onset whereas CT sensitivity in our study was only 62 % in the first 5 days and increased to 93 % only after 5 days of symptoms.
As already demonstrated, disease extent on CT is associated with prognosis, and allows predicting poor outcome [[19], [20], [21], [22]]. We found that 68 % of patients with disease extent exceeding 25 % of the lung parenchyma were intubated or deceased in the 3 weeks following CT. In the series reported by Zhao et al. [23], 79 % of patients of the severe and fatal group had diffuse disease extent on CT. Based on their CT score, Yuan et al. were able to predict mortality with a sensitivity of 85.6 % and a specificity of 84.5 %. A visual assessment of less than 73 % of well aerated lung was predictive of ICU admission or death in the series by Colombi et al. based on 236 patients [21].
Our study has several limitations. The number of RT-PCR assays was uneven, with only 26 % of individuals with an initial negative RT-PCR having further tests. This might have negatively affected our evaluation of the CT specificity, since 5 patients with negative RT-PCR with positive CT findings were hospitalized and considered as having COVID-19 pneumonia. However, this limitation does not change our main study results, reporting higher specificity for Ct than that reported in Chinese studies. The prognostic role of disease extent on CT was only evaluated based on visual assessment performed by the expert reader and we did not evaluate the agreement for disease extent between initial and expert reading, but CT visual quantitative evaluation has been reported to have high consistency [22]. Automated quantification of COVID-19 pneumonia extent might also be used and has been shown to predict severe outcome [21].
In conclusion, we found that CT had higher sensitivity for the diagnosis of COVID-19 pneumonia in patients reporting symptoms for at least 6 days. CT specificity in our study was higher than that previously reported, which we believe is due to use of exclusion criteria within a dedicated structure report. We confirm the prognostic role of CT with the limit that it was only based on expert reading in our study.
Funding
Mp Revel reports personal fees for lectures from MSD France, travel support from Guerbet, outside the submitted work.
CRediT authorship contribution statement
Enora Guillo: Investigation, Data curation, Writing - original draft. Ines Bedmar Gomez: Investigation, Data curation. Severine Dangeard: Methodology, Formal analysis. Souhail Bennani: Investigation, Data curation. Ines Saab: Investigation, Data curation. Mickael Tordjman: Writing - review & editing. Lea Jilet: Formal analysis. Guillaume Chassagnon: Writing - review & editing. Marie-Pierre Revel: Conceptualization, Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
References
- 1.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding X., Xu J., Zhou J., Long Q. Chest CT findings of COVID-19 pneumonia by duration of symptoms. Eur. J. Radiol. 2020;127 doi: 10.1016/j.ejrad.2020.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan F., Ye T., Sun P., et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H., Han X., Jiang N., et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song J., Tian J., Zhang L., et al. Development and validation of a prognostic index for efficacy evaluation and prognosis of first-line chemotherapy in stage III-IV lung squamous cell carcinoma. Eur. Radiol. 2019;29:2388–2398. doi: 10.1007/s00330-018-5912-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Z., Zhang Y., Wang Y., et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur. Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z., Guo D., Li C., et al. Coronavirus disease 2019: initial chest CT findings. Eur. Radiol. 2020;30:4398–4406. doi: 10.1007/s00330-020-06816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hani C., Trieu N.H., Saab I., et al. COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn. Interv. Imaging. 2020;101:263–268. doi: 10.1016/j.diii.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am. J. Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 10.Liang T., Liu Z., Wu C.C., et al. Evolution of CT findings in patients with mild COVID-19 pneumonia. Eur. Radiol. 2020 doi: 10.1007/s00330-020-06823-8. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai H.X., Hsieh B., Xiong Z., et al. Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology. 2020 doi: 10.1148/radiol.2020200823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ai T., Yang Z., Hou H., et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020 doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H., Hong H., Yoon S.H. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology. 2020 doi: 10.1148/radiol.2020201343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caruso D., Zerunian M., Polici M., et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020 doi: 10.1148/radiol.2020201237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revel M.-P., Parkar A.P., Prosch H., et al. COVID-19 patients and the radiology department – advice from the European Society of Radiology (ESR) and the European Society of Thoracic Imaging (ESTI) Eur. Radiol. 2020 doi: 10.1007/s00330-020-06865-y. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin Gd, Ryerson Cj, Haramati Lb, et al. The role of chest imaging in patient management during the COVID-19 pandemic: a multinational consensus statement from the fleischner society. Chest. 2020;158:106–116. doi: 10.1016/j.chest.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernheim A., Mei X., Huang M., et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyu P., Liu X., Zhang R., et al. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Invest. Radiol. 2020;55:412–421. doi: 10.1097/RLI.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan M., Yin W., Tao Z., et al. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colombi D., Bodini F.C., Petrini M., et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li K., Fang Y., Li W., et al. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur. Radiol. 2020;30:4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao W., Zhong Z., Xie X., et al. Relation between chest CT findings and clinical conditions of coronavirus disease (COVID-19) pneumonia: a multicenter study. Am. J. Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]