Key Points
Question
Is the presence of metastases at diagnosis of sarcoma associated with socioeconomic status, race/ethnicity, or insurance status?
Findings
In this cross-sectional, population-based study of 47 337 sarcoma cases, metastases at diagnosis of sarcoma was not associated with socioeconomic status as measured by small-area Census characteristics. Among adults, those with Medicaid insurance or no insurance had a higher odds of metastases at diagnosis of most soft-tissue sarcomas, but not bone sarcomas, whereas the racial disparities in the prevalence of metastatic leiomyosarcoma and unclassified sarcomas were not associated with small-area socioeconomic status and insurance status.
Meaning
These findings suggest that Medicaid insurance or no insurance is associated with the presence of metastases at the time of diagnosis among adults with soft-tissue sarcomas, suggesting a diagnostic delay, but there is no such association for adults with bone sarcomas.
This cross-sectional study evaluates whether socioeconomic status, insurance status, or race/ethnicity are associated with the presence of metastases at the time of diagnosis of sarcoma.
Abstract
Importance
Approximately 10% to 30% of patients with sarcoma present with detectable metastases at diagnosis. However, the extent to which presentation with metastases is due to delayed diagnosis vs other factors remains unclear.
Objective
To evaluate whether socioeconomic status, insurance status, or race/ethnicity were associated with the presence of metastases at diagnosis of sarcoma.
Design, Setting, and Participants
This cross-sectional study used data from the population-based Surveillance, Epidemiology, and End Results program. Adult and pediatric patients with an initial diagnosis of soft-tissue and bone sarcoma between 2001 and 2015 were stratified by age group (pediatric, <20 years; adult, 20-65 years; older adult, >65 years) and sarcoma subtype. Statistical analyses were performed between August 2019 and January 2020.
Exposures
Surveillance, Epidemiology, and End Results Census tract–level socioeconomic status index, insurance status, and race/ethnicity.
Main Outcomes and Measures
The odds of presenting with metastases at diagnosis were calculated.
Results
A total of 47 337 patients with first primary malignant sarcoma were included (24 343 male patients [51.4%]), with 29 975 non-Hispanic White patients (63.3%), 5673 non-Hispanic Black patients (12.0%), 7504 Hispanic patients (15.8%), and 4185 American Indian–Alaskan Native and Asian Pacific Islander patients (8.8%). Liposarcoma in adults was the only subtype and age group combination that demonstrated a significant trend in incidence across socioeconomic status levels (odds ratio, 0.85; 99% CI, 0.76-0.96; P = .001). However, compared with having non-Medicaid insurance, having Medicaid or no insurance in adults was associated with an increased odds of metastases at diagnosis for 6 of the 8 sarcoma subtypes evaluated; osteosarcoma and Ewing sarcoma were the only 2 subtypes in adults for which metastases were not associated with insurance status. In addition, there was an increased risk of presenting with metastases among non-Hispanic Black adults diagnosed with leiomyosarcoma (odds ratio, 1.87; 99% CI, 1.41-2.48) and unclassified sarcomas (odds ratio, 1.65; 99% CI, 1.01-2.67) compared with non-Hispanic White adults that was independent of socioeconomic and insurance status.
Conclusions and Relevance
These findings suggest that delayed access to care is associated with advanced stage at diagnosis for several soft-tissue sarcoma subtypes in adults, whereas other factors may be associated with the metastatic progression of osteosarcoma and Ewing sarcoma, as well as the racial disparities observed with metastatic leiomyosarcoma and unclassified sarcomas.
Introduction
Soft-tissue sarcomas (STSs) and bone sarcomas comprise a group of more than 50 histologically distinct and exceedingly rare malignant tumors.1 Approximately 10% to 30% of patients with sarcoma present with detectable metastases at initial diagnosis, depending on subtype.2,3,4 Those with metastatic disease at diagnosis experience poorer outcomes than those with localized disease, with overall 5-year survival rates ranging from 10% to 30% for those with metastases and 65% to 80% for those without metastases.5,6,7,8,9 Sarcomas cause few early recognizable signs and symptoms that can often lead to an extended delay between symptom onset and a definitive diagnosis.10,11 However, the extent to which a delayed diagnosis increases the likelihood of metastases and a higher risk of death from sarcoma remains unclear.10,11,12,13
A lower socioeconomic status (SES) may be representative of factors at the individual or community level that prevent patients from receiving timely access to medical care14,15 and has been associated with advanced stage at diagnosis of more common cancers, including those of the breast, prostate, colorectal, lung, and cervix.16 Prior analyses2,3 of the effect of SES on the diagnosis of sarcoma in the US are few and largely based on county-level analyses of SES, which may have introduced bias, considering that counties may comprise economically heterogeneous populations. Further insight can be obtained from analyses of race/ethnicity because any observed associations that are independent of SES may point toward variation in genetic risk for presentation with metastases. Results from analyses of race/ethnicity are limited by the extreme rarity of the disease, often constraining investigators to analyze histologically distinct sarcoma subtypes as a single group (eg, sarcoma) and few adjusted for likely confounding by SES.17,18 Of note, a genome-wide association study19 of osteosarcoma identified 2 germline genetic variants that were associated with an increased risk of metastases. This indicates that the metastatic potential for at least 1 sarcoma subtype is present at the start of tumorigenesis and not solely through sequential accumulation of mutations during disease progression.
The purpose of this cross-sectional study was to use a large, contemporary cohort of patients with sarcoma within the Surveillance, Epidemiology, and End Results (SEER) registry to describe associations of small-area SES, race/ethnicity, and insurance status with metastases present at the diagnosis of sarcomas across the age spectrum. The results of this study provide a comprehensive overview regarding these associations among individually rare subtypes of sarcoma.
Methods
Study Population
We used the specialized SEER Census Tract–level SES Database,20 which includes cancer cases diagnosed between 2000 and 2015 in the catchment area of 16 SEER registries; the Alaska Native and Louisiana Tumor registries are excluded.21 Microscopically confirmed, first primary malignant sarcoma cases were identified using International Classification of Disease for Oncology, 3rd Edition (ICD-O-3) histology codes recognized by the 2013 World Health Organization’s Classification of Tumours of Soft Tissue and Bone.22 We then categorized sarcoma cases into subtypes of interest according to the World Health Organization classifications and the recommendations of an expert sarcoma oncologist (B.J.W.) and pathologist (P.M.) (eTable 1 in the Supplement). Kaposi sarcoma was excluded from analyses because it primarily arises in the setting of HIV infection.23 We also excluded cases diagnosed in the year 2000 because they were not directly coded under the ICD-O-3 histology coding guidelines introduced in 2001.24
This study is exempt from institutional review board approval and the need for informed consent because it involves the use of publicly available, deidentified data in accordance with 45 CFR §46. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies.25
Metastatic Disease at Diagnosis, SES, Race/Ethnicity, and Other Covariate Categories
Metastatic sarcoma was characterized on the basis of the SEER summary staging variable. Cases with a staging of distant were classified as having metastases at diagnosis, whereas those with a staging of localized or regional were not. Cases with an unknown stage at diagnosis were excluded from further analyses. Race and ethnicity were evaluated using mutually exclusive groups—non-Hispanic White, non-Hispanic Black, Asian Pacific Islander (API) or American Indian and Alaska Native (AIAN), and Hispanic—that were assigned to an individual according to the reported race/ethnicity in a patient’s medical records. The identification of individuals of Hispanic ethnicity was further enhanced by use of the North American Association for Central Cancer Registries Hispanic-Latino identification algorithm, which is based principally on surname and birth place.26 We combined the API and AIAN race categories because of sample size constraints. Five-year age groups were categorized into 9 broader age group categories to account for small sample sizes: 0 to 9 years, 10 to 19 years, 20 to 29 years, 30 to 39 years, 40 to 49 years, 50 to 64 years, 65 to 74 years, 75 to 84 years, and 85 years and older.
Small-area SES was analyzed from a composite index. The index is calculated by SEER using a principal component analysis of several Census tract–level SES indicator variables as specified by Yost et al27: median household income, median house value, median rent, percentage of the population below 150% of the poverty line, an education index, percentage of the population with working class occupations, and percentage of the population older than 16 years in the workforce without a job. Cases were geocoded to a Census tract according to their address at diagnosis. The SES indices were linked to a Census tract using either the 2000 Decennial Census long-form survey or a series of American Community Survey 5-year estimates, depending on year of diagnosis. The SES index is provided to SEER users as a 5-level categorical variable, with the first category representing the lowest SES quintile. Cases with a missing SES index or an address that could not be geocoded to a Census tract were excluded from analysis.
Statistical Analyses
Multivariable logistic regression models were used to evaluate the odds of presenting with metastases at diagnosis across quintiles of small-area SES (hereafter referred to as SES) and race/ethnicity, adjusted for age at diagnosis, sex, and year of diagnosis. Covariates were selected a priori for inclusion on the basis of previous associations with sarcoma and/or our exposures of interest.9,28,29 All models were stratified by broad age group categories (pediatric, <20 years; adult, 20-65 years; and older adult, >65 years). Sixty-five years was chosen as the cutoff point for older adults because it is the age of Medicare eligibility. Within each age group strata, associations were reported for any sarcoma subtype with more than 100 metastatic cases, a cutoff that was chosen a priori to maintain adequate power in strata specific analyses. For all sarcomas, SES was assessed as either a categorical variable (with the lowest quintile serving as the reference) or an ordinal variable, with the P value from the ordinal variable serving as a test for trend. We focus our reporting here on results from SES evaluated as an ordinal variable (Figure 1); results from SES evaluated as a categorical variable are presented in eTable 2 in the Supplement.
Figure 1. Multivariable Adjusted Odds Ratios (ORs) and 99% CIs for Metastatic Sarcoma by Socioeconomic Status, Stratified by Age Group and Sarcoma Subtype: Surveillance, Epidemiology, and End Results 16 Registries, 2001-2015.
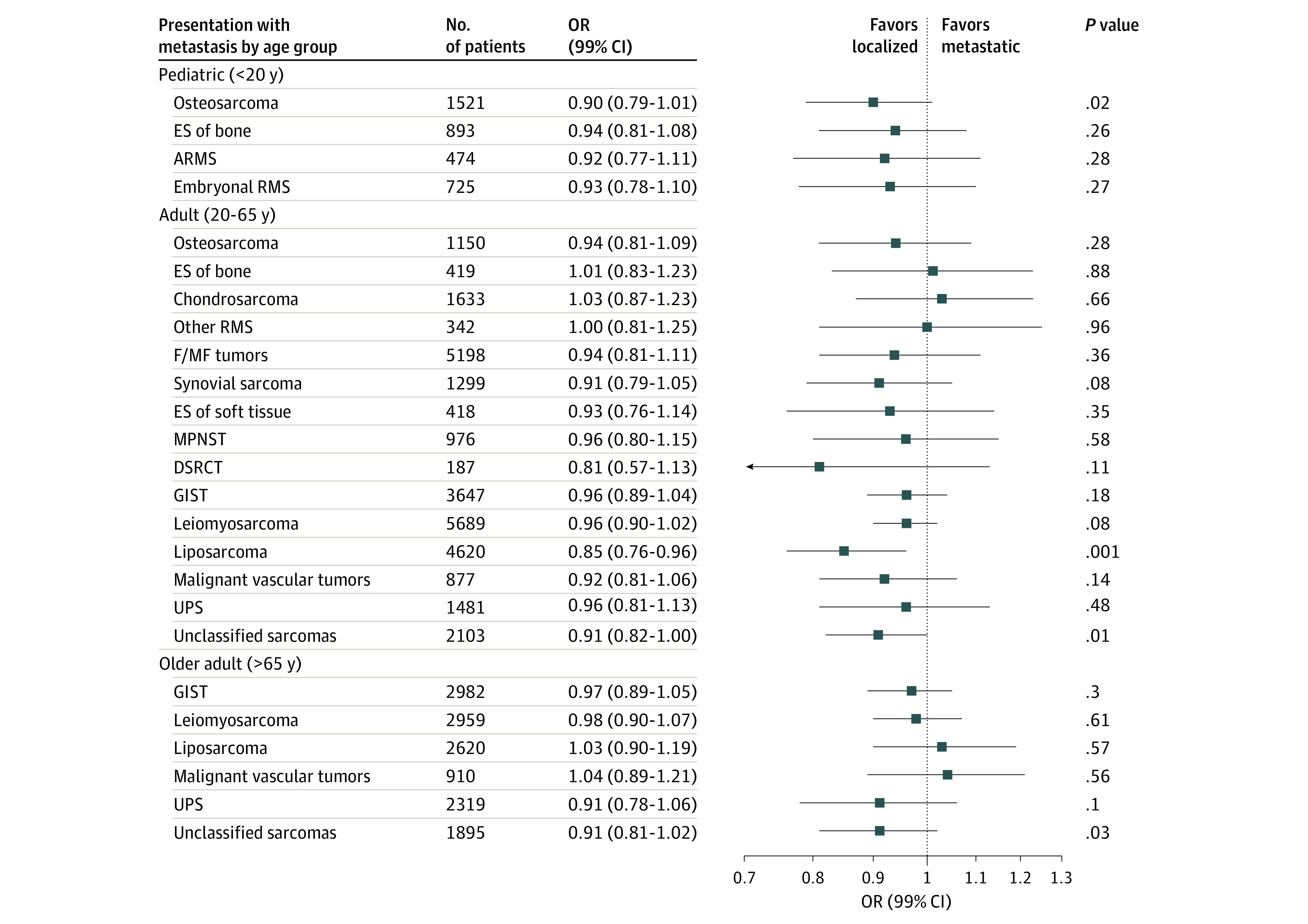
All data are adjusted for race, sex, age at diagnosis, and year of diagnosis. ARMS indicates alveolar rhabdomyosarcoma; DSRCT, desmoplastic small round cell tumor; ES, Ewing sarcoma; F/MF, fibroblastic or myofibroblastic; GIST, gastrointestinal stromal tumor; MPNST, malignant peripheral nerve sheath tumor; RMS, rhabdomyosarcoma; and UPS, undifferentiated pleomorphic sarcoma.
In addition, we conducted multivariable logistic regression analyses to evaluate the odds of metastases at diagnosis by insurance status, while controlling for small-area SES (as an ordinal variable), race/ethnicity, age at diagnosis, sex, and year of diagnosis. Insurance status was defined according to the SEER variable as non-Medicaid insurance (insured or insured and no specifics), Medicaid coverage (any Medicaid), or uninsured. Analyses were confined to sarcoma subtypes with more than 100 metastatic cases diagnosed after 2007, when insurance status data began to be collected by SEER. We further restricted analyses to cases diagnosed in the adult age group strata because few pediatric or older adult cases were uninsured. Cases with unknown insurance status were excluded from analysis.
A P < .01 was considered statistically significant. This significance threshold was used because it is approximately equal to 0.05 divided by 4, the number of hypothesis tests conducted within each sarcoma subtype and age group (ie, the ordinal SES and 3 race/ethnicity comparisons). All reported P values are 2-sided and are calculated from logistic regression models that used the Wald χ2 statistic. All data sets were generated using SEER*stat statistical software version 8.3.6 (National Cancer Institute),30 and statistical analyses were performed in R statistical software version 3.4.4 (R Project for Statistical Computing).31 Statistical analyses were performed between August 2019 and January 2020.
Results
eTable 3 in the Supplement presents the clinical and demographic distribution of included cases. After excluding 3613 cases with unknown stage of diagnosis, 836 cases with a missing SES index, and 705 cases with missing race/ethnicity, we analyzed 90.7% of sarcoma cases with a first primary, malignant sarcoma subtype of interest diagnosed between 2001 and 2015 in the SEER 16 registries (47 337 of 52 183 cases). There was a total of 24 343 male patients (51.4%) and 22 994 female patients (48.6%). With regard to race/ethnicity, 29 975 patients (63.3%) were non-Hispanic White, 5673 (12.0%) were non-Hispanic Black, 7504 (15.8%) were Hispanic, and 4185 (8.8%) were AIAN or API. Most patients with sarcoma (30 039 patients [63.5%]) were aged 20 to 65 years at diagnosis. Overall, the proportion of patients presenting with metastatic disease at diagnosis was 17.3% for STS and 20.5% for bone sarcomas. Among the subtypes evaluated, the prevalence of metastases at diagnosis was highest for alveolar rhabdomyosarcoma (223 patients [47.0%]) among pediatric patients, desmoplastic round cell tumor (134 patients [71.7%]) among adult patients, and gastrointestinal stromal tumors (698 patients [23.4%]) among older adult patients.
Socioeconomic Status and Metastasis at Diagnosis
Figure 1 shows the odds ratios (ORs) and 99% CIs for presenting with metastatic disease at diagnosis by SES, stratified by sarcoma category and age group strata (crude results are presented in eTable 4 in the Supplement). Liposarcoma in adults was the only subtype and age group combination evaluated to show a significant association, with 15% lower odds of metastatic liposarcoma observed for every increase in SES quintile (OR, 0.85; 99% CI, 0.76-0.96; P = .001).
Race/Ethnicity and Metastasis at Diagnosis
Figure 2, Figure 3, and Figure 4 show the adjusted ORs and 99% CIs for presenting with metastatic disease at diagnosis by race and ethnicity, stratified by sarcoma category and age group strata (crude results are given in eTable 5 in the Supplement). In the pediatric age group, we did not observe significant racial disparities in the odds of presenting with metastases at diagnosis for any of the subtypes evaluated. Conversely, in adults, we observed non-Hispanic Black patients to have an elevated odds of metastatic Ewing sarcoma of bone (OR, 5.00; 99% CI, 1.16-30.54), leiomyosarcoma (OR, 1.79; 99% CI, 1.42-2.24), and unclassified sarcomas (OR, 1.58; 99% CI, 1.04-2.37) compared with non-Hispanic White patients. The odds of metastatic leiomyosarcoma was also elevated in Hispanic (OR, 1.31; 99% CI, 1.04-1.65) and AIAN or API (OR, 1.45; 99% CI, 1.09-1.91) adults compared with non-Hispanic White adults. In the older age group strata, non-Hispanic Black patients had higher odds of metastatic leiomyosarcoma (OR, 1.59; 99% CI, 1.11-2.27) than non-Hispanic White patients, similar to the results observed in adults.
Figure 2. Multivariable Adjusted Odds Ratios (ORs) and 99% CIs for Metastatic Sarcoma in non-Hispanic Black vs non-Hispanic White Patients, Stratified by Age Group and Sarcoma Subtype: Surveillance, Epidemiology, and End Results 16 Registries, 2001-2015.
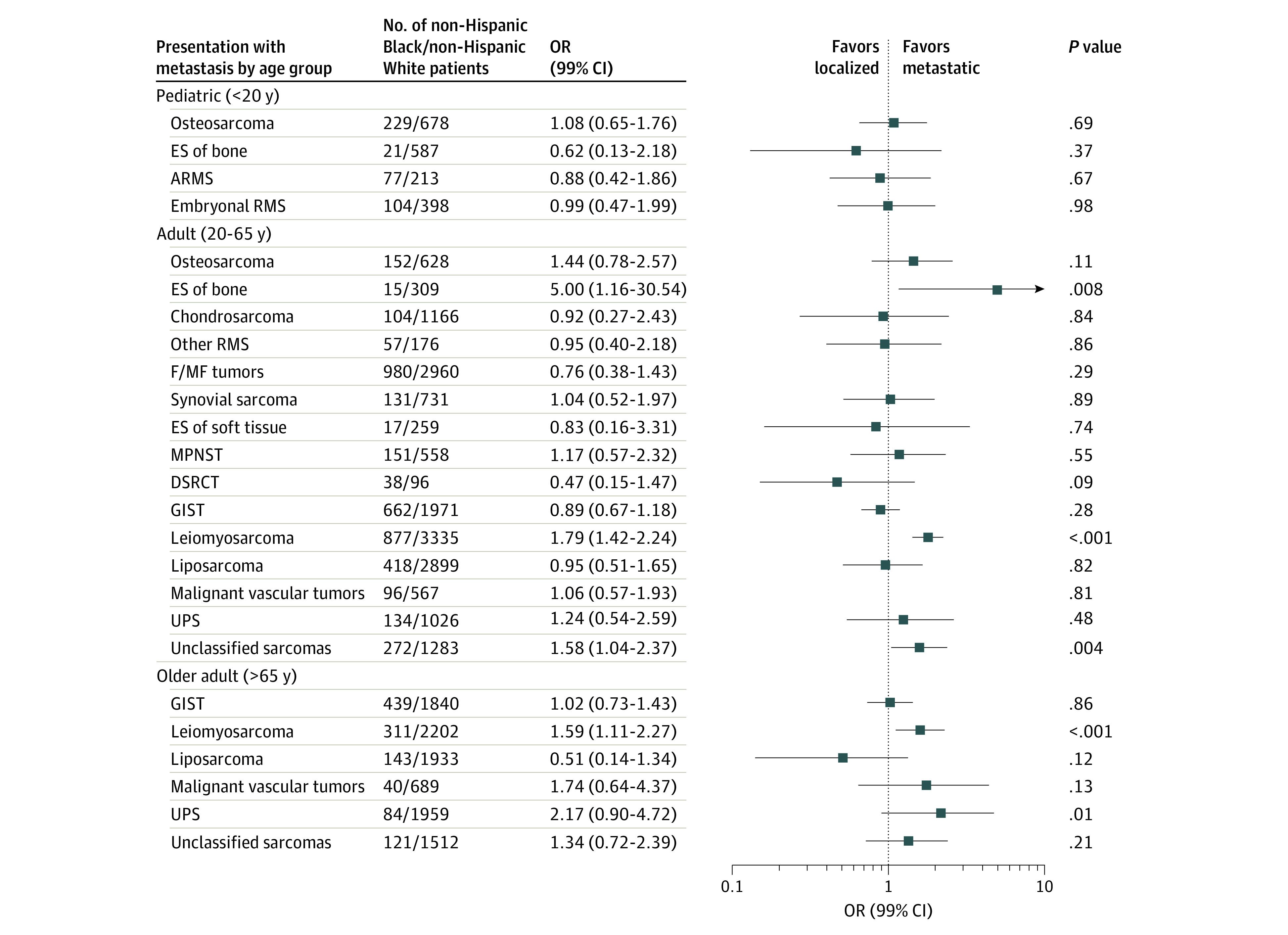
All data are adjusted for small-area socioeconomic status (ordinal variable), sex, age at diagnosis, and year of diagnosis. ARMS indicates alveolar rhabdomyosarcoma; DSRCT, desmoplastic small round cell tumor; ES, Ewing sarcoma; F/MF, fibroblastic or myofibroblastic; GIST, gastrointestinal stromal tumor; MPNST, malignant peripheral nerve sheath tumor; RMS, rhabdomyosarcoma; and UPS, undifferentiated pleomorphic sarcoma.
Figure 3. Multivariable Adjusted Odds Ratios (ORs) and 99% CIs for Metastatic Sarcoma in Hispanic vs non-Hispanic White Patients, Stratified by Age Group and Sarcoma Subtype: Surveillance, Epidemiology, and End Results 16 Registries, 2001-2015 .
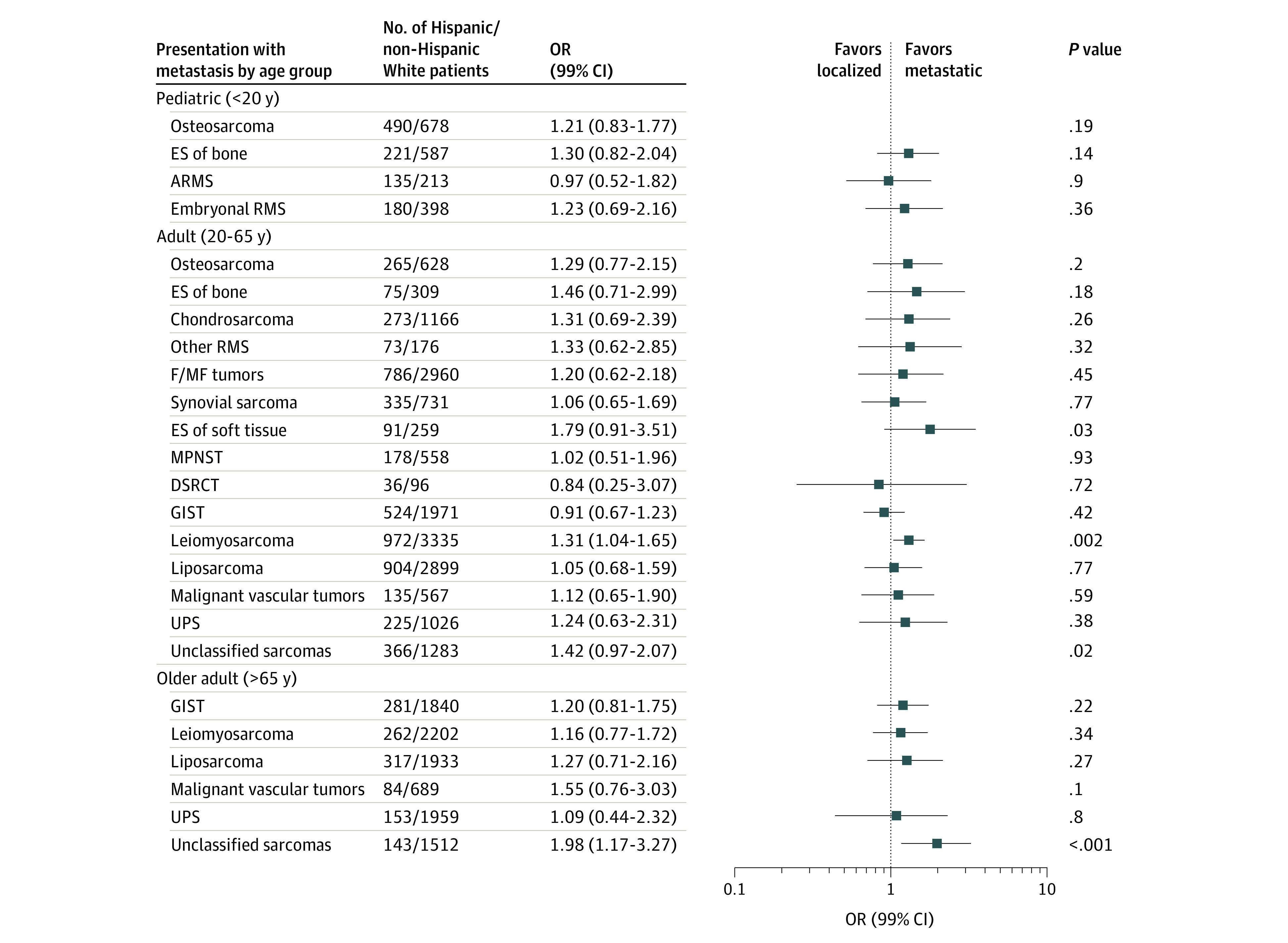
ARMS indicates alveolar rhabdomyosarcoma; DSRCT, desmoplastic small round cell tumor; ES, Ewing sarcoma; F/MF, fibroblastic or myofibroblastic; GIST, gastrointestinal stromal tumor; MPNST, malignant peripheral nerve sheath tumor; RMS, rhabdomyosarcoma; and UPS, undifferentiated pleomorphic sarcoma.
Figure 4. Multivariable Adjusted Odds Ratios (ORs) for Metastatic Sarcoma in American Indian and Alaska Native (AIAN) or Asian Pacific Islander (API) Patients vs non-Hispanic White Patients, Stratified by Age Group and Sarcoma Subtype: Surveillance, Epidemiology, and End Results 16 Registries, 2001-2015 .
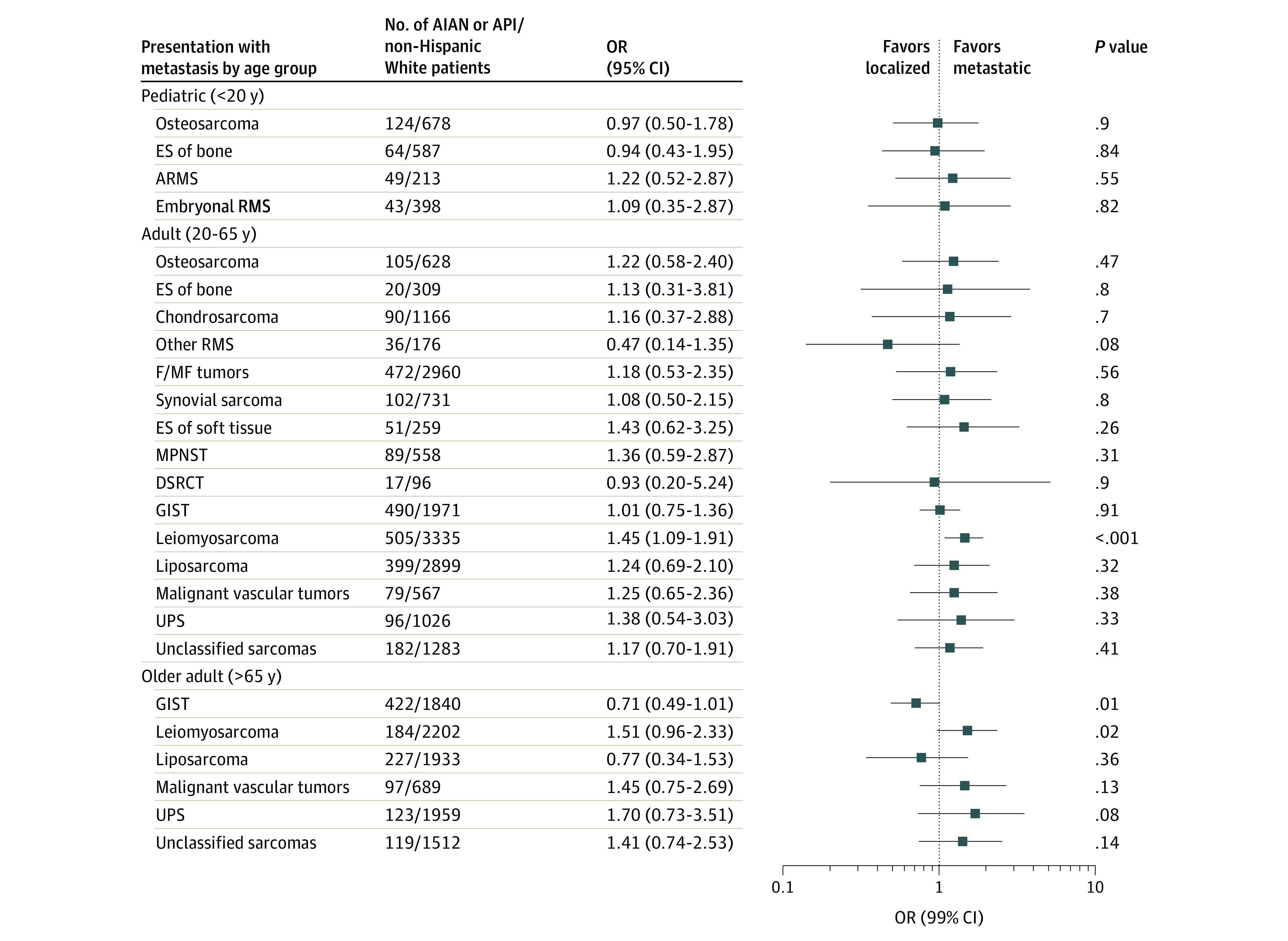
ARMS indicates alveolar rhabdomyosarcoma; DSRCT, desmoplastic small round cell tumor; ES, Ewing sarcoma; F/MF, fibroblastic or myofibroblastic; GIST, gastrointestinal stromal tumor; MPNST, malignant peripheral nerve sheath tumor; RMS, rhabdomyosarcoma; and UPS, undifferentiated pleomorphic sarcoma.
Insurance Status and Metastasis at Diagnosis
The Table presents results from the analysis of insurance status on metastatic disease at diagnosis in the adult age group strata (crude results are presented in eTable 6 in the Supplement). In total, 12 890 adults with sarcoma diagnosed after 2007 were identified, and 322 (2.5%) were excluded from analysis for having missing insurance data. Across most sarcoma subtypes evaluated (6 of 8 subtypes), non-Medicaid insured patients had a lower odds of having metastases at diagnosis compared with Medicaid insured or uninsured patients; Ewing sarcoma of bone and osteosarcoma were the only subtypes evaluated to show null associations with both Medicaid insured and uninsured. In addition, insurance status appeared to attenuate the association observed with small-area SES among adult patients with liposarcoma (OR, 0.94; 99% CI, 0.80-1.10). However, non-Hispanic Black patients still had elevated odds of metastatic leiomyosarcoma (OR, 1.87; 99% CI, 1.41-2.48) and unclassified sarcomas (OR, 1.65; 99% CI, 1.01-2.67) compared with non-Hispanic White patients. The odds of non-Hispanic Black patients having metastatic Ewing sarcoma of bone also remained elevated but was extremely imprecise (OR, 9.15; 99% CI, 1.32-146.12). After adjustment for insurance status, there was no association of metastatic leiomyosarcoma with AIAN or API or Hispanic ethnicity.
Table. Multivariable Adjusted Odds Ratios for Metastases at Diagnosis in Adults Aged 20-65 y at Diagnosis, by Insurance Status, Stratified by Sarcoma Subtype: Surveillance, Epidemiology, and End Results 16 Registries, 2007-2015.
| Subtype | Patients, No. | Adjusted OR (99% CI)a | |||||
|---|---|---|---|---|---|---|---|
| Insurance statusb | Ordinal socioeconomic status | Race/ethnicityc | |||||
| Medicaid | Uninsured | Non-Hispanic Black | Hispanic | AIAN or API | |||
| Osteosarcoma | 677 | 1.68 (0.9-3.09) | 1.04 (0.33-2.85) | 0.94 (0.77-1.15) | 1.3 (0.58-2.79) | 1.11 (0.55-2.18) | 0.85 (0.29-2.13) |
| Ewing sarcoma of bone | 255 | 1.6 (0.63-4.06) | 0.99 (0.14-5.79) | 1.17 (0.89-1.56) | 9.15 (1.32-146.12) | 1.48 (0.57-3.81) | 0.67 (0.08-3.85) |
| Synovial sarcoma | 777 | 2.15 (1.11-4.09) | 2.04 (0.86-4.61) | 0.98 (0.81-1.18) | 0.91 (0.36-2.1) | 0.91 (0.49-1.68) | 1.37 (0.58-3.03) |
| Gastrointestinal stromal tumor | 2350 | 1.71 (1.18-2.44) | 1.72 (1.08-2.71) | 0.97 (0.89-1.07) | 0.84 (0.58-1.21) | 0.82 (0.56-1.2) | 0.86 (0.58-1.26) |
| Leiomyosarcoma | 3403 | 1.46 (1.1-1.95) | 0.94 (0.59-1.45) | 0.96 (0.89-1.04) | 1.87 (1.41-2.48) | 1.23 (0.92-1.63) | 1.31 (0.92-1.85) |
| Liposarcoma | 2957 | 2.27 (1.33-3.78) | 2.44 (1.06-5.04) | 0.94 (0.8-1.1) | 0.79 (0.35-1.58) | 0.78 (0.44-1.33) | 1.31 (0.67-2.41) |
| Vascular tumors | 564 | 1.9 (1.06-3.44) | 1.13 (0.34-3.56) | 0.95 (0.8-1.14) | 0.79 (0.36-1.69) | 1.08 (0.56-2.08) | 0.91 (0.4-1.98) |
| Unclassified sarcomas | 1585 | 1.71 (1.1-2.63) | 1.32 (0.69-2.4) | 0.92 (0.81-1.04) | 1.65 (1.01-2.67) | 1.34 (0.85-2.09) | 1.22 (0.67-2.13) |
Abbreviations: AIAN, American Indian and Alaska Native; API, Asian Pacific Islander; OR, odds ratio.
Adjusted for insurance status, area-level socioeconomic status (ordinal), race, sex, age at diagnosis, and year of diagnosis.
Reference category = insured.
Reference category = non-Hispanic white.
Discussion
To our knowledge, this is the largest analysis aimed at ascertaining whether SES and race/ethnicity were independently associated with metastatic presentation of sarcoma. Overall, we found no association with small-area SES across most of the 25 sarcoma subtype–age group combinations evaluated. However, compared with having non-Medicaid insurance, having Medicaid or no insurance in adults was associated with an increased odds of metastases at diagnosis in 6 of the 8 subtypes evaluated; osteosarcoma and Ewing sarcoma were the only 2 subtypes not associated with insurance status. In addition, we observed an increased odds of presenting with metastases in non-Hispanic Black adults diagnosed with leiomyosarcoma and unclassified sarcomas that was independent of SES and insurance status. Our results suggest that those without insurance or who are underinsured are more likely to present with metastatic disease at diagnosis for several STS subtypes. Also, the fact that SES-related factors were not associated with the odds of metastatic presentation in patients with osteosarcoma or Ewing sarcoma, or with the racial disparities observed with metastatic leiomyosarcoma or unclassified sarcomas, indicates that factors other than diagnostic delay may contribute to the metastatic progression of these subtypes.
A limitation of our analysis is that we used area-level SES measured at the Census tract level as an indicator of individual-level SES. Although Census tracts comprise homogeneous populations,32 the variation in SES measures among individuals within a Census tract33,34 and the independent effects of individual and area level SES on health-related conditions35,36,37 indicate that area-based measures of SES should not be interpreted as if they were collected from individuals.38 Rather, area-based measures capture information on community and neighborhood factors that influence health, which are generally conceptualized to be independent of individual SES characteristics.38 We, therefore, sought to further explore the influence of SES on metastatic disease at presentation by evaluating insurance status, one of the few measures of SES captured by SEER at the individual level.
Across most STS subtypes evaluated in the adult age group strata, having Medicaid or no insurance was associated with an increased risk of presenting with metastases compared with having private insurance. Both Medicaid insured and uninsured patients can experience numerous barriers to receiving medical care, including reduced access to regular sources of health care services, prolonged referral times to specialists, an inability to navigate the health care system, transportation issues, or poor psychosocial support.39,40,41,42,43,44 Our study concurs with a prior analysis45 that reported an elevated risk of metastatic presentation in Medicaid insured patients diagnosed with STS of the extremities. Moreover, it builds on prior work in part because our analyses were stratified by sarcoma subtype, thereby providing a more detailed overview regarding the extent to which delayed access to medical care is associated with sarcoma stage at presentation.
Notably, we observed that insurance status and small-area SES were not associated with metastases at diagnosis of osteosarcoma or Ewing sarcoma, the 2 bone sarcoma subtypes evaluated in adults. These results both contrast with and confirm prior analyses. For instance, the finding that insurance status is not associated with metastatic osteosarcoma concurs with reports of a genetic predisposition to metastatic disease46,47,48 and null associations with SES measured at the county level,2 but is in contrast with our prior observation of a higher prevalence of metastases in countries with lower human development indices,49 which may harbor greater barriers in providing timely access to medical care. One possible explanation for these discrepant findings is that the population of patients with metastatic osteosarcoma is composed of both individuals with genetic susceptibility and those who experienced diagnostic delays, although the attributable proportion of each remains unexamined. Similarly, Ewing sarcoma tumors were not associated with either small-area SES or insurance status in adults, a finding consistent with prior reports.3 Future genetic analyses should be pursued to identify whether there exist risk variants for metastatic Ewing sarcoma.
We observed non-Hispanic Black adults to have a higher odds of metastatic leiomyosarcoma and unclassified sarcomas at diagnosis than non-Hispanic White adults after controlling for SES and insurance status. It is unclear what factors may underlie these observations. That they were independent of SES-related factors in our analysis highlights the possibility that genetic variants associated with ancestry may contribute to an inherently aggressive tumor phenotype, as hypothesized with other cancer types.50,51,52 However, we note that a single measure of SES cannot fully characterize the complexities and multifactorial nature of racial disparities in health. Other social, cultural, or lifestyle factors that are related to access to medical care, but that are unaccounted for by use of small-area SES or insurance status, may have confounded our observations. Further research is needed to determine whether there are associations between genetic ancestry and stage at diagnosis of these subtypes.
Limitations
In addition to the use of an area-level SES measure, there are several additional limitations with our analyses that should be considered. First, the Medicaid insured category likely includes uninsured patients who were found to qualify for public health insurance after their cancer diagnosis, at which point they were retroactively coded as having Medicaid.53,54 We speculate that this misclassification likely inflated associations with Medicaid insurance and may explain the seemingly counterintuitive finding that Medicaid insured patients had a similar or higher risk of metastasis than uninsured patients. We also note that small sample sizes may have resulted in null associations with the uninsured category in instances when Medicaid insured patients had an increased risk. Second, the SES measure used in our analysis is a composite of several SES characteristics, each of which may have an effect on health that differs in direction or magnitude. However, the individual SES characteristics were not available for analysis. Third, the quality of sarcoma pathology55,56 and the possible inaccuracy of ICD-O-3 coding of sarcomas57,58 is a limitation of our study and sarcoma research in general. Fourth, we note that SEER race/ethnicity categories are reported to be in high agreement with self-report race, except for patients identified as AIAN,59 but are imperfect measures of genomic ancestry.60
Conclusions
In the current study, we provide evidence that factors related to diagnostic delay, including having Medicaid insurance or no insurance, were associated with an increased risk of presenting with more advanced staged STS in adults, but other factors were more likely associated with metastases at diagnosis for osteosarcoma and Ewing sarcoma, as well as leiomyosarcoma and unclassified sarcomas in non-Hispanic Black adults. These data may be used to guide efforts to detect metastatic sarcoma earlier to improve patient outcomes.
eTable 1. Soft Tissue and Bone Sarcoma Classifications and ICD-O-3 Histology Codes
eTable 2. Multivariable Adjusted OR for Metastatic Sarcoma by SES, Stratified by Age Group and Sarcoma Subtype: SEER 16 Registries, 2001–2015
eTable 3. Distribution of Sarcoma Cases by Demographic and Clinical Characteristics: SEER 16 Registries, 2001–2015
eTable 4. Unadjusted OR for Metastatic Sarcoma by SES, Stratified by Age Group and Sarcoma Subtype SEER 16 Registries, 2001–2015
eTable 5. Unadjusted OR for Metastatic Sarcoma by Race/Ethnicity, Stratified by Age Group and Sarcoma Subtype: SEER 16 Registries, 2001–2015
eTable 6. Unadjusted OR for Metastases at Diagnosis in Adults (20–65 Years at Diagnosis) by Insurance Status, Stratified by Sarcoma Subtype: SEER 16 Registries (2007–2015)
References
- 1.Helman LJ, Meltzer P. Mechanisms of sarcoma development. Nat Rev Cancer. 2003;3(9):685-694. doi: 10.1038/nrc1168 [DOI] [PubMed] [Google Scholar]
- 2.Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am. 2013;95(13):e89. doi: 10.2106/JBJS.L.01189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramkumar DB, Ramkumar N, Miller BJ, Henderson ER. Risk factors for detectable metastatic disease at presentation in Ewing sarcoma: an analysis of the SEER registry. Cancer Epidemiol. 2018;57:134-139. doi: 10.1016/j.canep.2018.10.013 [DOI] [PubMed] [Google Scholar]
- 4.Italiano A, Mathoulin-Pelissier S, Cesne AL, et al. Trends in survival for patients with metastatic soft-tissue sarcoma. Cancer. 2011;117(5):1049-1054. doi: 10.1002/cncr.25538 [DOI] [PubMed] [Google Scholar]
- 5.Cotterill SJ, Ahrens S, Paulussen M, et al. Prognostic factors in Ewing’s tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing’s Sarcoma Study Group. J Clin Oncol. 2000;18(17):3108-3114. doi: 10.1200/JCO.2000.18.17.3108 [DOI] [PubMed] [Google Scholar]
- 6.Mialou V, Philip T, Kalifa C, et al. Metastatic osteosarcoma at diagnosis: prognostic factors and long-term outcome—the French pediatric experience. Cancer. 2005;104(5):1100-1109. doi: 10.1002/cncr.21263 [DOI] [PubMed] [Google Scholar]
- 7.Savina M, Le Cesne A, Blay JY, et al. Patterns of care and outcomes of patients with metastatic soft tissue sarcoma in a real-life setting: the METASARC Observational Study. BMC Med. 2017;15(1):78. doi: 10.1186/s12916-017-0831-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng VY, Scharschmidt TJ, Mayerson JL, Fisher JL. Incidence and survival in sarcoma in the United States: a focus on musculoskeletal lesions. Anticancer Res. 2013;33(6):2597-2604. [PubMed] [Google Scholar]
- 9.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531-1543. doi: 10.1002/cncr.24121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. The symptom-to-diagnosis delay in soft tissue sarcoma influence the overall survival and the development of distant metastasis. J Surg Oncol. 2011;104(7):771-775. doi: 10.1002/jso.22006 [DOI] [PubMed] [Google Scholar]
- 11.Rougraff BT, Davis K, Lawrence J. Does length of symptoms before diagnosis of sarcoma affect patient survival? Clin Orthop Relat Res. 2007;462(462):181-189. doi: 10.1097/BLO.0b013e3180f62608 [DOI] [PubMed] [Google Scholar]
- 12.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790. doi: 10.1200/JCO.2002.20.3.776 [DOI] [PubMed] [Google Scholar]
- 13.Bacci G, Ferrari S, Longhi A, et al. High-grade osteosarcoma of the extremity: differences between localized and metastatic tumors at presentation. J Pediatr Hematol Oncol. 2002;24(1):27-30. doi: 10.1097/00043426-200201000-00008 [DOI] [PubMed] [Google Scholar]
- 14.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54(2):78-93. doi: 10.3322/canjclin.54.2.78 [DOI] [PubMed] [Google Scholar]
- 15.Miller BJ, Gao Y, Duchman KR. Socioeconomic measures influence survival in osteosarcoma: an analysis of the National Cancer Data Base. Cancer Epidemiol. 2017;49:112-117. doi: 10.1016/j.canep.2017.05.017 [DOI] [PubMed] [Google Scholar]
- 16.Schwartz KL, Crossley-May H, Vigneau FD, Brown K, Banerjee M. Race, socioeconomic status and stage at diagnosis for five common malignancies. Cancer Causes Control. 2003;14(8):761-766. doi: 10.1023/A:1026321923883 [DOI] [PubMed] [Google Scholar]
- 17.Jacobs AJ, Lindholm EB, Levy CF, Fish JD, Glick RD. Racial and ethnic disparities in treatment and survival of pediatric sarcoma. J Surg Res. 2017;219:43-49. doi: 10.1016/j.jss.2017.05.031 [DOI] [PubMed] [Google Scholar]
- 18.Joseph M, Hamilton EC, Hayes-Jordan A, Huh WW, Austin MT. The impact of racial/ethnic disparities on survival for children and young adults with chest wall sarcoma: a population-based study. J Pediatr Surg. 2018;53(8):1621-1626. doi: 10.1016/j.jpedsurg.2018.04.011 [DOI] [PubMed] [Google Scholar]
- 19.Savage SA, Mirabello L, Wang Z, et al. Genome-wide association study identifies two susceptibility loci for osteosarcoma. Nat Genet. 2013;45(7):799-803. doi: 10.1038/ng.2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Cancer Institute Census tract-level SES and rurality database (2000-2015). Accessed March 27, 2019. https://seer.cancer.gov/seerstat/databases/census-tract/index.html
- 21.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81-92. doi: 10.1007/s10552-013-0310-1 [DOI] [PubMed] [Google Scholar]
- 22.Fletcher CDM, Bridge JA, Hogendoorn PCW, eds. WHO Classification of Tumours of Soft Tissue and Bone. 4th ed IARC Publications; 2013. [Google Scholar]
- 23.Renwick N, Halaby T, Weverling GJ, et al. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi’s sarcoma. AIDS. 1998;12(18):2481-2488. doi: 10.1097/00002030-199818000-00018 [DOI] [PubMed] [Google Scholar]
- 24.North American Association of Central Cancer Registries Guidelines for ICD-O-3 histology code and behavior update implementation. Published January 1, 2018. Accessed July 7, 2020. https://www.naaccr.org/wp-content/uploads/2018/01/Updated-Jan-10-2018-ICD-O-3-Guidelines-v2.pdf
- 25.Equator Network The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observation studies. Accessed April 17, 2020. https://www.equator-network.org/reporting-guidelines/strobe/
- 26.North American Association of Central Cancer Registries NAACCR guideline for enhancing Hispanic-Latino identification: revised NAACCR Hispanic/Latino identification algorithm [NHIA v2.2.1]. Published September 12, 2011. Accessed July 7, 2020. https://www.naaccr.org/wp-content/uploads/2016/11/NHIA-v2.2.1.pdf
- 27.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703-711. doi: 10.1023/A:1011240019516 [DOI] [PubMed] [Google Scholar]
- 28.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2(1):14. doi: 10.1186/2045-3329-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? a population-based epidemiologic study and literature review. Ann Oncol. 2010;21(5):1106-1111. doi: 10.1093/annonc/mdp415 [DOI] [PubMed] [Google Scholar]
- 30.National Cancer Institute SEER*Stat software. Accessed October 15, 2019. https://seer.cancer.gov/seerstat/
- 31.R core team R: a language and environment for statistical computing. Published 2016. Accessed July 7, 2020. https://www.R-project.org
- 32.Boscoe FP, Johnson CJ, Sherman RL, Stinchcomb DG, Lin G, Henry KA. The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer. 2014;120(14):2191-2198. doi: 10.1002/cncr.28632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geronimus AT, Bound J. Use of census-based aggregate variables to proxy for socioeconomic group: evidence from national samples. Am J Epidemiol. 1998;148(5):475-486. doi: 10.1093/oxfordjournals.aje.a009673 [DOI] [PubMed] [Google Scholar]
- 34.Soobader M, LeClere FB, Hadden W, Maury B. Using aggregate geographic data to proxy individual socioeconomic status: does size matter? Am J Public Health. 2001;91(4):632-636. doi: 10.2105/AJPH.91.4.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pardo-Crespo MR, Narla NP, Williams AR, et al. Comparison of individual-level versus area-level socioeconomic measures in assessing health outcomes of children in Olmsted County, Minnesota. J Epidemiol Community Health. 2013;67(4):305-310. doi: 10.1136/jech-2012-201742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juhn YJ, Sauver JS, Katusic S, Vargas D, Weaver A, Yunginger J. The influence of neighborhood environment on the incidence of childhood asthma: a multilevel approach. Soc Sci Med. 2005;60(11):2453-2464. doi: 10.1016/j.socscimed.2004.11.034 [DOI] [PubMed] [Google Scholar]
- 37.O’Campo P, Xue X, Wang MC, Caughy M. Neighborhood risk factors for low birthweight in Baltimore: a multilevel analysis. Am J Public Health. 1997;87(7):1113-1118. doi: 10.2105/AJPH.87.7.1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krieger N, Williams DR, Moss NE. Measuring social class in US public health research: concepts, methodologies, and guidelines. Annu Rev Public Health. 1997;18(1):341-378. doi: 10.1146/annurev.publhealth.18.1.341 [DOI] [PubMed] [Google Scholar]
- 39.Greenwald HP, O’Keefe S, Dicamillo M. The importance of public sector health care in an underserved population. J Health Hum Serv Adm. 2004;27(2):142-157. [PubMed] [Google Scholar]
- 40.Berk ML, Schur CL. Access to care: how much difference does Medicaid make? Health Aff (Millwood). 1998;17(3):169-180. doi: 10.1377/hlthaff.17.3.169 [DOI] [PubMed] [Google Scholar]
- 41.Kim CY, Wiznia DH, Hsiang WR, Pelker RR. The effect of insurance type on patient access to knee arthroplasty and revision under the Affordable Care Act. J Arthroplasty. 2015;30(9):1498-1501. doi: 10.1016/j.arth.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 42.Wiznia DH, Nwachuku E, Roth A, et al. The influence of medical insurance on patient access to orthopaedic surgery sports medicine appointments under the affordable care act. Orthop J Sports Med. 2017;5(7):2325967117714140. doi: 10.1177/2325967117714140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niu X, Roche LM, Pawlish KS, Henry KA. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411. doi: 10.1002/cam4.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ward E, Halpern M, Schrag N, et al. Association of insurance with cancer care utilization and outcomes. CA Cancer J Clin. 2008;58(1):9-31. doi: 10.3322/CA.2007.0011 [DOI] [PubMed] [Google Scholar]
- 45.Smartt AA, Jang ES, Tyler WK. Is there an association between insurance status and survival and treatment of primary bone and extremity soft-tissue sarcomas? a SEER Database study. Clin Orthop Relat Res. 2020;478(3):527-536. doi: 10.1097/CORR.0000000000000889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirabello L, Koster R, Moriarity BS, et al. A genome-wide scan identifies variants in NFIB associated with metastasis in patients with osteosarcoma. Cancer Discov. 2015;5(9):920-931. doi: 10.1158/2159-8290.CD-15-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott MC, Sarver AL, Tomiyasu H, et al. Aberrant retinoblastoma (RB)-E2F transcriptional regulation defines molecular phenotypes of osteosarcoma. J Biol Chem. 2015;290(47):28070-28083. doi: 10.1074/jbc.M115.679696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirabello L, Zhu B, Koster R, et al. Frequency of pathogenic germline variants in cancer-susceptibility genes in patients with osteosarcoma. JAMA Oncol. 2020;6(5):724-734. doi: 10.1001/jamaoncol.2020.0197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marko TA, Diessner BJ, Spector LG. Prevalence of metastasis at diagnosis of osteosarcoma: an international comparison. Pediatr Blood Cancer. 2016;63(6):1006-1011. doi: 10.1002/pbc.25963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Libes JM, Seeley EH, Li M, et al. ; Kenyan Wilms Tumor Consortium . Race disparities in peptide profiles of North American and Kenyan Wilms tumor specimens. J Am Coll Surg. 2014;218(4):707-720. doi: 10.1016/j.jamcollsurg.2013.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodman MT, Correa CN, Tung K-H, et al. Stage at diagnosis of ovarian cancer in the United States, 1992-1997. Cancer. 2003;97(10)(suppl):2648-2659. doi: 10.1002/cncr.11347 [DOI] [PubMed] [Google Scholar]
- 52.Lantz PM, Mujahid M, Schwartz K, et al. The influence of race, ethnicity, and individual socioeconomic factors on breast cancer stage at diagnosis. Am J Public Health. 2006;96(12):2173-2178. doi: 10.2105/AJPH.2005.072132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradley CJ, Gardiner J, Given CW, Roberts C. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005;103(8):1712-1718. doi: 10.1002/cncr.20954 [DOI] [PubMed] [Google Scholar]
- 54.Koroukian SM, Bakaki PM, Raghavan D. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer. 2012;118(17):4271-4279. doi: 10.1002/cncr.27380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray-Coquard I, Montesco MC, Coindre JM, et al. ; Conticanet group . Sarcoma: concordance between initial diagnosis and centralized expert review in a population-based study within three European regions. Ann Oncol. 2012;23(9):2442-2449. doi: 10.1093/annonc/mdr610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thway K, Fisher C. Histopathological diagnostic discrepancies in soft tissue tumours referred to a specialist centre. Sarcoma. 2009;2009(17):741975. doi: 10.1155/2009/741975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyu HG, Haider AH, Landman AB, Raut CP. The opportunities and shortcomings of using big data and national databases for sarcoma research. Cancer. 2019;125(17):2926-2934. doi: 10.1002/cncr.32118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lyu HG, Stein LA, Saadat LV, Phicil SN, Haider A, Raut CP; Dana-Farber/Brigham and Women’s Cancer Center Sarcoma Surgery Group . Assessment of the accuracy of disease coding among patients diagnosed with sarcoma. JAMA Oncol. 2018;4(9):1293-1295. doi: 10.1001/jamaoncol.2018.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177-187. doi: 10.1007/s10552-006-0089-4 [DOI] [PubMed] [Google Scholar]
- 60.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. 2015;9(1):1-15. doi: 10.1186/s40246-014-0023-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Soft Tissue and Bone Sarcoma Classifications and ICD-O-3 Histology Codes
eTable 2. Multivariable Adjusted OR for Metastatic Sarcoma by SES, Stratified by Age Group and Sarcoma Subtype: SEER 16 Registries, 2001–2015
eTable 3. Distribution of Sarcoma Cases by Demographic and Clinical Characteristics: SEER 16 Registries, 2001–2015
eTable 4. Unadjusted OR for Metastatic Sarcoma by SES, Stratified by Age Group and Sarcoma Subtype SEER 16 Registries, 2001–2015
eTable 5. Unadjusted OR for Metastatic Sarcoma by Race/Ethnicity, Stratified by Age Group and Sarcoma Subtype: SEER 16 Registries, 2001–2015
eTable 6. Unadjusted OR for Metastases at Diagnosis in Adults (20–65 Years at Diagnosis) by Insurance Status, Stratified by Sarcoma Subtype: SEER 16 Registries (2007–2015)


