Production of antibodies in response to viral infections constitutes an essential feature of adaptive immunity. Great efforts have been dedicated to characterize antibody responses in patients with coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. A recent article by Jiang et al., published in Trends in Immunology, discussed the state of research and development of neutralizing antibodies for the prevention and treatment of COVID-19, with a specific emphasis on cross-reactivity with two other highly pathogenic human coronaviruses, SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) [1]. However, the authors of this article largely disregarded the potential cross-reactive immunity triggered by the prevalence of low pathogenic human coronaviruses (LPH-CoV).
High and Low Pathogenic Human Coronaviruses That Naturally Infect Humans
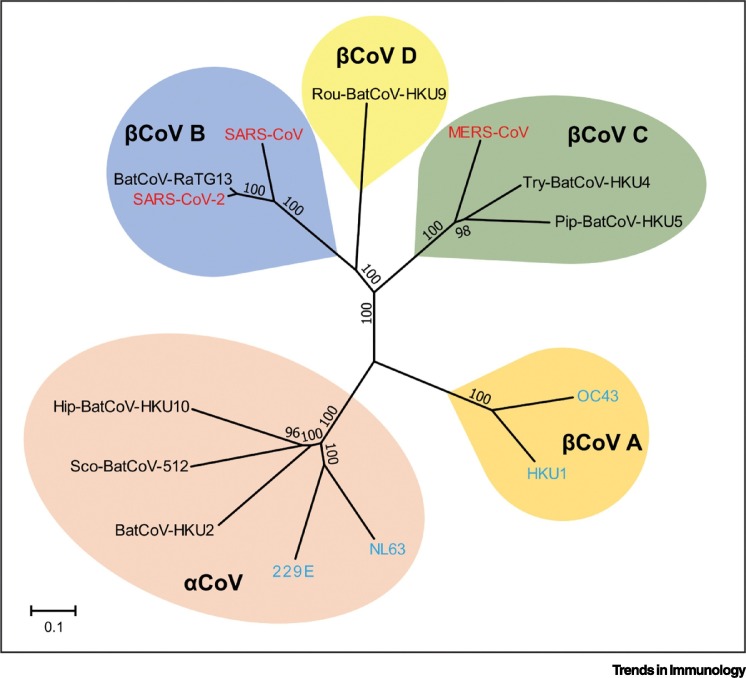
Coronaviruses are a large family of RNA viruses circulating among a wide range of animal species. Seven types of coronaviruses naturally infect humans, although all of them are thought to originate from animals [2]. The three highly pathogenic coronaviruses, including MERS-CoV, SARS-CoV, and SARS-CoV-2, can cause severe acute respiratory diseases in humans. By contrast, the four genotypes of LPH-CoV, including OC43, HKU1, 229E, and NL63, usually only cause mild and self-limiting respiratory tract infections [3]. Genetically, SARS-CoV-2, SARS-CoV, MERS-CoV, OC43, and HKU1 are betacoronaviruses, whereas 229E and NL63 are alphacoronaviruses. SARS-CoV-2 is most closely related to SARS-CoV, moderately to MERS-CoV, and is slightly distal to LPH-CoV (Figure 1 ) [4].
Figure 1.
Phylogenetic Tree and Genetic Relationships of Different Coronaviruses.
The high pathogenic human coronaviruses [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), SARS-CoV, and Middle East respiratory syndrome coronavirus (MERS-CoV)] are highlighted in red. The low pathogenic human coronaviruses (OC43, 229E, NL63, and HKU1) are highlighted in blue.
Potential Cross-neutralization among Highly Pathogenic Human Coronaviruses
Humans are proficient in antibody production in response to SARS-CoV-2 infection. Many monoclonal antibodies have been isolated and characterized from SARS-CoV-2 infected patients, in particular targeting the receptor-binding domain of the viral spike protein [5]. These antibodies can effectively neutralize SARS-CoV-2 infection in experimental models and this mechanistically supports the therapeutic application of neutralizing antibodies or convalescent plasma for treating COVID-19 patients [5]. As expected, cross-reactivity of these antibodies in binding to the counterpart’s spike protein has been commonly detected, but the ability of cross-neutralizing SARS-CoV or MERS-CoV has yet to be proven [6]. Conversely, as extensively highlighted by Jiang et al., several anti-SARS-CoV or anti-MERS-CoV antibodies have been reported to possess cross-reactive or cross-neutralizing activities against SARS-CoV-2 [1].
In the real world, SARS-CoV and MERS-CoV have only infected approximately 10 500 cases in total, according to the World Health Organization estimation [7]. Even if they are capable of triggering cross-neutralizing antibodies against SARS-CoV-2, their impact on the COVID-19 pandemic would be insignificant from a population perspective.
The Potential Impact of Low Pathogenic Human Coronaviruses
LPH-CoV, including OC43, HKU1, 229E, and NL63, are endemic and have been widely circulating among the global population for decades. Of relevance, SARS-CoV-2-reactive T cell immune responses have been observed in healthy individuals without exposure to SARS-CoV-2, but these individuals have harbored antibodies against LPH-CoV, including OC43 and NL63 [8]. As characterized, this cross-immune reactivity specifically targets viral proteins [8]. Unfortunately, this raises great concerns regarding the specificity of antibody-based serologic assays in estimating SARS-CoV-2 prevalence. This may be relevant, especially in regions where the number of SARS-CoV-2 cases are low (e.g., outside of an epicenter), and where false-positivity can be caused by cross-reactivity to anti-LPH-CoV antibodies, likely drastically overestimating the real prevalence rates [9].
It remains unproven whether such cross-reactivity could functionally neutralize SARS-CoV-2 infection. If it does provide cross-protective effects against SARS-CoV-2 to some extent, either by preventing infection or by mitigating pathogenesis, this would have a fundamental impact on the COVID-19 pandemic because LPH-CoV infection is extremely common in humans. The global incidence of upper respiratory infections is several billion episodes per year and, among these, over 5% are LPH-CoV infections [3]. In addition, viral antigen-triggered antibodies are usually long-lived and infection with OC43 or 229E effectively triggers IgG antibody production, which peaks 2 weeks post-infection [10]. And, although the antibody titers appear to wane gradually, they persist for at least 1 year [10]. Therefore, a large proportion of the global population should be seropositive to LPH-CoV.
LPH-CoV have a clear seasonal feature, in that they are more prevalent in the winter time, based on studies from countries in the northern hemisphere [3]. It would be interesting to monitor whether this seasonal frequency might have an impact on the possible seasonality of SARS-CoV-2. It is also intriguing that LPH-CoV appear to be highly prevalent in young children. Thus, it will be relevant to investigate whether this prevalence can influence in any way the clinical features of COVID-19, whereby most children appear to be hardly affected by SARS-CoV-2 infection [3,11]. In the clinic, intravenous immunoglobulin (IVIG) has been explored to treat COVID-19 patients, showing some evidence of clinical benefits [12]. Because IVIG is a pool of immunoglobulins from many healthy donors that might contain anti-LPH-CoV antibodies, this might in theory mechanistically support some of the protective effects against SARS-CoV-2 infection due to cross-reactivity. However, this hypothesis remains entirely conjectural at this point.
We posit that pre-exposure of high or low pathogenic human coronaviruses conceivably generates cross-reactive antibodies towards SARS-CoV-2, but whether they are functionally capable of neutralizing SARS-CoV-2 remains to be rigorously examined. As Jiang et al. have highlighted [1], previous efforts to generate anti-SARS-CoV or anti-MERS-CoV neutralizing antibodies might potentially serve for drug repurposing, or to provide guidelines for developing similar strategies to target SARS-CoV-2. In the real world, the cross-immunity triggered by circulating LPH-CoV might have an unexpected relevance to the global COVID-19 pandemic, which deserves close monitoring and investigation.
Acknowledgments
The work was supported by the Ministry of Education of China for an Innovative Research Team in University grant (No. IRT_17R88; to Z.M.).
References
- 1.Jiang S. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Z.W. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16:1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li P. Estimating global epidemiology of low-pathogenic human coronaviruses in relation to the COVID-19 context. J. Infect. Dis. 2020;222:695–696. doi: 10.1093/infdis/jiaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forni D. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y. Potent neutralizing antibodies against SARS-CoV-2 identified by high-throughput single-cell sequencing of convalescent patients' B cells. Cell. 2020;182:73–84. doi: 10.1016/j.cell.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv H. Cross-reactive antibody response between SARS-CoV-2 and SARS-CoV infections. Cell Rep. 2020;31:107725. doi: 10.1016/j.celrep.2020.107725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert G.L. SARS, MERS and COVID-19-new threats; old lessons. Int. J. Epidemiol. 2020;49:726–728. doi: 10.1093/ije/dyaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grifoni A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood N. Seroprevalence of SARS-CoV-2-specific antibodies among adults in Los Angeles County, California, on April 10-11, 2020. JAMA. 2020;323:2425–2427. doi: 10.1001/jama.2020.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Callow K.A. The time course of the immune response to experimental coronavirus infection of man. Epidemiol. Infect. 1990;105:435–446. doi: 10.1017/s0950268800048019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji Y. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob. Health. 2020;8:e480. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Y. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]