Abstract
Members of the family Nudiviridae are large dsDNA viruses with distinctive rod-shaped nucleocapsids and circular genomes of 96–232 kbp. Nudiviruses have been identified from a diverse range of insects and crustaceans and are closely related to baculoviruses. This is a summary of the International Committee on Taxonomy of Viruses Report on the taxonomy of the family Nudiviridae, which is available at ictv.global/report/nudiviridae.
Keywords: Nudiviridae, ICTV report, taxonomy
Virion
Nudivirus virions consist of cylindrical nucleocapsids enveloped to produce ellipsoidal or rod-shaped virions of variable length and width (Fig. 1, Table 1). In some cases, virions are assembled into occlusion bodies, with a matrix consisting of a protein similar to the baculovirus polyhedrin protein [1] or of a completely unrelated protein [2].
Fig. 1.
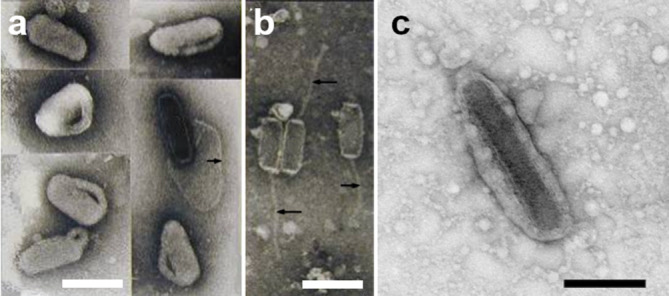
Negatively-stained virions (a) and nucleocapsids (b) of Oryctes rhinoceros nudivirus (genus Alphanudivirus). Arrows indicate (a) the membrane from a disrupted virion, (b) tail-like appendages. (c) A virion of Helicoverpa zea nudivirus 2. Bars, 0.2 µm. Micrographs (a) and (b) modified and reprinted from [5] with permission from Elsevier, (c) produced by Jean Adams (USDA-ARS).
Table 1.
Characteristics of members of the family Nudiviridae
|
Typical member: |
Oryctes rhinoceros nudivirus Ma07 (EU747721), species Oryctes rhinoceros nudivirus, genus Alphanudivirus |
|---|---|
|
Virion |
Enveloped, rod-shaped or ellipsoidal, compact (approximately 100×200 nm) or elongated (approximately 81×415 nm) |
|
Genome |
A single covalently closed circular dsDNA molecule of 96–232 kbp encoding 89–155 proteins |
|
Replication |
Nuclear, including nucleocapsid assembly and envelopment |
|
Translation |
From mRNAs transcribed from viral DNA |
|
Host range |
Immature and adult stages of insects and crustaceans |
|
Taxonomy |
Multiple genera and species |
Genome
The nudivirus genome is a single, covalently closed circular molecule of dsDNA, with approximately 89–155 potential protein-encoding ORFs (Fig. 2). These ORFs are distributed throughout the genome in either orientation. ORF content and order can vary significantly in members of different species. Most genomes also contain regions of short repeated sequences. The 32 ORFs present in all nudivirus genomes make up the set of core genes [1], many of which are homologues of baculovirus core genes [3]. These core genes include ORFs encoding DNA polymerase B and baculovirus RNA polymerase subunit homologues that probably participate in viral genome replication and gene expression, respectively.
Fig. 2.
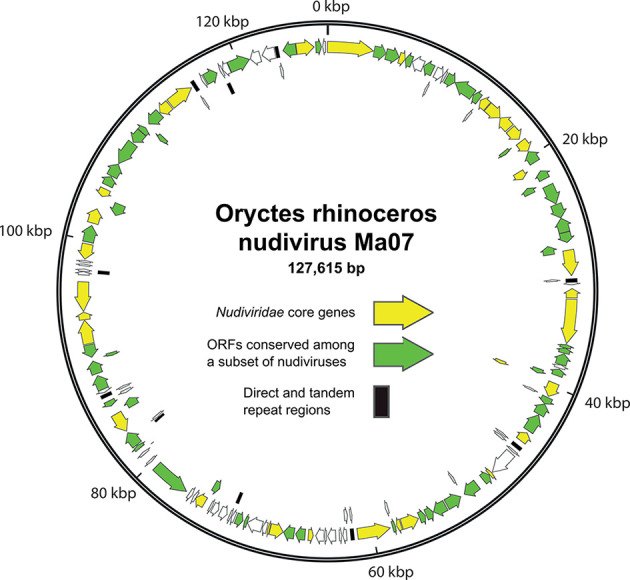
Genome map of Oryctes rhinoceros nudivirus isolate Ma07. Positions and orientations of annotated ORFs are represented as arrows, with filled arrows corresponding to core genes (yellow) and ORFs conserved among other nudiviruses (green). Locations of direct (dr) and tandem (tr) repeat sequence regions are also shown.
Replication
After virus entry, replication occurs in the nucleus and is accompanied by nuclear hypertrophy. Progeny nucleocapsids are assembled and enveloped within the nucleus. Mature virions either bud from the cytoplasmic membrane of infected cells or are released upon cell lysis. A persistent, asymptomatic infection may be established.
Taxonomy
Alphanudivirus. Members of two species in this genus include Oryctes rhinoceros nudivirus from the coconut rhinoceros beetle, Oryctes rhinoceros, and Gryllus bimaculatus nudivirus from a field cricket, Gryllus bimaculatus. Virions of these viruses are relatively compact, although a tail-like appendage extends from Oryctes rhinoceros nudivirus nucleocapsids (Fig. 1a, b).
While Oryctes rhinoceros nudivirus exclusively infects adults and larvae of O. rhinoceros, Gryllus bimaculatus nudivirus can infect different species of field crickets [4]. Infections can result in death of the host. Oryctes rhinoceros nudivirus has been developed and applied successfully to control infestations of O. rhinoceros on coconut palm trees [5].
Betanudivirus. The species Heliothis zea nudivirus is represented by two isolates, Heliothis zea nudivirus 1 and Helicoverpa zea nudivirus 2, whose genome sequences are 93.5 % identical. The virions of these viruses are longer and thinner than those of the alphanudiviruses (Fig. 1c and Table 1).
Heliothis zea nudivirus 1 initially causes a lytic infection, but surviving cells harbour the virus in a persistent state facilitated by virally encoded micro-RNAs [6]. Although Heliothis zea nudivirus 1 can infect cell lines from the noctuid Helicoverpa (formerly Heliothis) zea and other host species, attempts to infect H. zea insects have been unsuccessful. Helicoverpa zea nudivirus 2 can infect H. zea larvae, but replication is constrained to reproductive tissue and may result in gonadal atrophy and sterility. Helicoverpa zea nudivirus 2 is transmitted both vertically to progeny and between adult moths during mating [7].
Resources
Current ICTV Report on the family Nudiviridae: ictv.global/report/nudiviridae.
Funding information
Production of this summary, the online chapter and associated resources was funded by a grant from the Wellcome Trust (WT108418AIA).
Acknowledgements
Members of the ICTV Report Consortium are Stuart G. Siddell, Andrew J. Davison, Elliot J. Lefkowitz, Sead Sabanadzovic, Peter Simmonds, Donald B. Smith, Richard J. Orton and Balázs Harrach.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Bézier A, Thézé J, Gavory F, Gaillard J, Poulain J, et al. The genome of the nucleopolyhedrosis-causing virus from Tipula oleracea sheds new light on the Nudiviridae family. J Virol. 2015;89:3008–3025. doi: 10.1128/JVI.02884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y-T, Lee D-Y, Wang Y, Hu J-M, Li W-H, et al. The genome and occlusion bodies of marine Penaeus monodon nudivirus (PmNV, also known as MBV and PemoNPV) suggest that it should be assigned to a new nudivirus genus that is distinct from the terrestrial nudiviruses. BMC Genomics. 2014;15:628. doi: 10.1186/1471-2164-15-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, Bininda-Emonds ORP, van Oers MM, Vlak JM, Jehle JA. The genome of Oryctes rhinoceros nudivirus provides novel insight into the evolution of nuclear arthropod-specific large circular double-stranded DNA viruses. Virus Genes. 2011;42:444–456. doi: 10.1007/s11262-011-0589-5. [DOI] [PubMed] [Google Scholar]
- 4.Huger AM. A new virus disease of crickets (Orthoptera: Gryllidae) causing macronucleosis of fatbody. J Invertebr Pathol. 1985;45:108–111. doi: 10.1016/0022-2011(85)90055-2. [DOI] [Google Scholar]
- 5.Huger AM. The Oryctes virus: its detection, identification, and implementation in biological control of the coconut palm rhinoceros beetle, Oryctes rhinoceros (Coleoptera: Scarabaeidae) J Invertebr Pathol. 2005;89:78–84. doi: 10.1016/j.jip.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Wu Y-L, Wu CP, Liu CYY, Hsu PW-C, Wu EC, et al. A non-coding RNA of insect HzNV-1 virus establishes latent viral infection through microRNA. Sci Rep. 2011;1:60. doi: 10.1038/srep00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burand JP, Rallis CP, Tan W. Horizontal transmission of Hz-2V by virus infected Helicoverpa zea moths. J Invertebr Pathol. 2004;85:128–131. doi: 10.1016/j.jip.2004.01.004. [DOI] [PubMed] [Google Scholar]


