Abstract
The pathogenesis of megaloblastic hemopathies (MH) is centered on the deficiency of vitamin B12 and folic acid with interruption of erythrocyte maturation. This study researched the participation of p53 and p21 in the pathophysiology of the disease. A retrospective study enrolled 95 patients with histopathologic diagnosis by biopsy or bone marrow clot (BMB/BMC), with clinical review and immunohistochemical study in tissue microarray (TMA) for p53 and p21, detailing their marking location. All patients had BMC and only 11 had BMB. The CMO was a differential of this study and it allowed an expanded sample. In the TMA, 63.7% (58/91) of the samples were immunopositive for p53; and 35.2% (31/88) were immunopositive for p21. Nuclear staining, divergent from the literature, was observed in 17.3% (10/58) among those p53+ and in 38.7% (12/31) among those p21+. The pattern of immunostaining showed non-significant differences (P=0.474) regarding morphologic and clinical aspects. The positivity for both may indicate an effective balance between apoptosis and anti-apoptotic action. Excessive inhibition of apoptosis would contribute to high global cellularity, but without functional maturation effectiveness. In conclusion, there is p21 and/or p53 immunoexpression in most cases of this study and there is no clear association between immunoexpression pattern and patient outcome. Unlike the literature, we also found a percentage of nuclear immunostaining, but the finding was not statistically significant. Combination of p21 and p53 results created different possibilities of pathologic interpretation for MH, reinforcing the importance of studies similar to this one.
Keywords: Anemia, megaloblastic, tumor suppressor protein p53, oncogene protein p21(ras), bone marrow, bone marrow cells, immunohistochemistry
Introduction
Schulz et al. (2000) [1] reported a case of a 41-year-old man with megaloblastic hemopathies (MH) and positivity for p21 and p53 in bone marrow erythroid cell cytoplasm, with no nuclear labeling and no alterations in the TP53, N-, K- and H-RAS. After treatment, the expression of such markers disappeared. According to the report, elevated p53 levels in the cytoplasm are associated with increased erythroblast apoptosis. Overexpression of p21 is related to elevated erythropoietin levels.
The report by Schulz et al. (2000) [1] was one of the motives for this study that evaluated the presence and pattern of p21 and p53 protein marking in bone marrow (BM) of patients with MH.
Methods
We reviewed the medical records and histopathology of bone marrow clots in 95 patients who underwent BM aspiration between March 1997 and March 2016 at the Hematology and Pathology services of Botucatu Medical School of São Paulo State University (FMB UNESP). The Research Ethics Committee of the institution approved the research (number: 57675416000005411), which obtained financial support from the São Paulo State Research Support Foundation (number 2016/19725-3). Immunohistochemistry (IHC) was performed using tissue microarray (TMA), the p21 antibodies (clone DCS-60.2, Ventana, ready to use), p53 antibodies (clone D0-7, Ventana/Dako, ready to use), and glycophorin-A antibodies (clone GA-R2, Ventana, ready for use). The statistics were carried out using SPSS 15.0, with descriptive tools and association tests (Chi-Square or Fisher’s Exact test), using a p-value of less than 5%.
Results
Of 95 patients, 45 (47.4%) were women, and the median age was 56 years (1-89 years). All patients had bone marrow clot (BMC), an influential factor in this study as this is not the reality in all services. This fact contributed to sampling and allowed better technical conditions for IHC since the materials did not need decalcification.
Megaloblasts were present in all samples and are exemplified in Figure 1B and 1C. Findings such as increased global cellularity (n=88; 92.6%) and inversion of granulocytic and erythroid series (n=66; 69.5%) were found in most cases of BMC. Cellular giantism (Figure 1D; n=59; 69.5%) can be demonstrated by the presence of metamyelocytes. Iron-deficiency concomitance was present in 56 (58.9%) patients, characterizing a possible multicarential condition.
Figure 1.
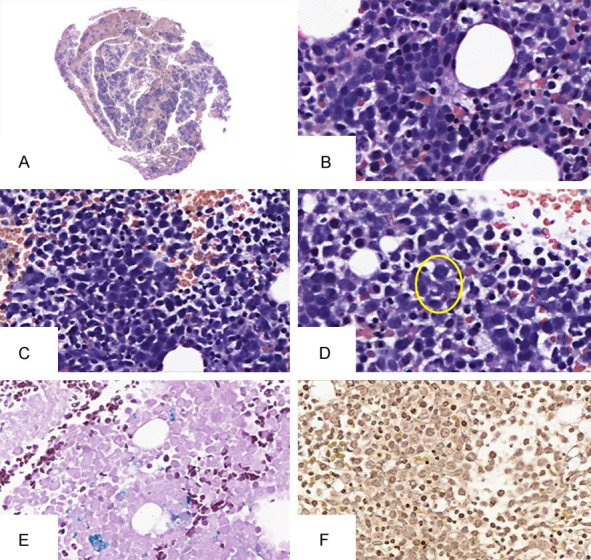
Histopathologic evaluation in bone marrow clot. A. (scanning, H&E) Bone marrow clot. At least five spikes are required to ensure sample representativeness. Note the increased global cellularity. B and C. (400×, H&E) Megaloblasts in the context of granulocytic hypercellularity. D. (400×, H&E) Cellular gigantism of the granulocytic series demonstrated by a metamyelocyte. The cell under analysis is circled in yellow. E. (200×, Perls) Exemplification of a patient without iron deficiency. The hemosiderin deposits are stained blue. F. (Reticulin, 400×) Case example with grade 2 reticulin.
Of the 95 patients, 81 had medical records, and the analyzes showed initial anemia in 84% of cases (n=68) and pancytopenia in 50.6% of cases (n=41). Hepatomegaly was found in 11 (13.6%) patients and splenomegaly in 14 (17.3%) patients. Two (2.5%) patients used anticonvulsants, while 26 (32.1%) patients took antihypertensives, 9 (9.5%) reported use of antidepressants, and 4 (4.9%) used corticosteroids. Among the comorbidities, the most frequent were systemic arterial hypertension (n=32; 39.5%), atrophic gastritis (n=22, 27.2%), diabetes mellitus (n=18; 22.2%), and hypothyroidism (n=10, 12.3%). Ethylism was documented in 4 patients (4.9%), equivalent to the percentage who had a gastrectomy. Regarding clinical outcomes, remission with disease control was achieved in 31 patients (38.3%). However, unfavorable outcomes were also detected and characterized as maintenance of the disease (n=12; 14.8%), remission with relapse (n=5; 6.2%), appearance of segment hematologic neoplasia (n=3; 3.7%). Thirty patients did not have a full recorded clinical follow-up. There are several reasons for this phenomenon, including the fact that these patients often come to the service for neoplastic investigation and, once the hypothesis is ruled out, they return to their original health services.
Regarding IHC (Figure 2), the p53 and p21 evaluations included the pattern (cytoplasmic or nuclear) and the intensity (1+ to 4+) of staining. The glycophorin-A marker stained erythroid series cells and served to discriminate the cells of interest for p53 and p21 IHC interpretation.
Figure 2.
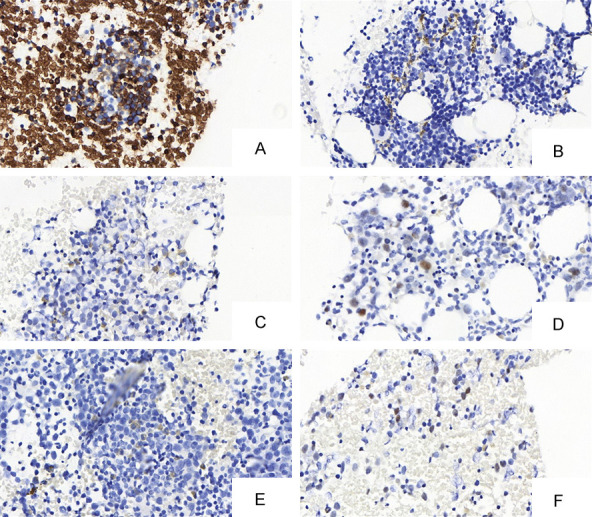
IHC in the bone marrow of patients with MH. A. (400× glycophorin A) Labeling is positive on erythroid series precursor cells and megaloblasts. The internal control of the slide is represented by red blood cell positivity. B. (400×) This image demonstrates false positivity of the IHC. It has a granular and coarse pattern, probably corresponding to hemosiderin deposition. This coloring is negative. C. (400×, p53) Cytoplasmic marking. D. (400×, p53) Nuclearm. E. (400×, p21) Cytoplasmic marking. F. (400×, p21) Nuclear marking.
P53 was positive in 58 of 91 samples (63.7%) in TMA. Intensity was generally low (1+/4+) in most patients (n=44; 75.8%). The remaining 58 positive patients had intensity of 2+ in 10 (17.3%) samples, 3+ in 3 (5.6%) samples and 4+ in 1 (1.3%) sample. Regarding the pattern, 48 (82.7%) had a cytoplasmic pattern and 10 (17.3%) had nuclear pattern.
Regarding p21, there was immunopositivity in 31 (35.2%) patients from 88 samples evaluable in TMA. Staining intensities in the samples were: 1+ (n=25; 80.6%) and 2+ (n=6; 19.4%). The cytoplasmic pattern was predominant and observed in 19 (61.3%) positive cases, and the remaining (n=12; 38.7%) exhibited nuclear pattern.
Discussion
The P53 gene has tumor suppressor functions, including disruption of the cell cycle in phase G1, before phase S, ensuring the possibility of repair in damaged DNA; apoptosis induction; and regulationbetween phase S and G2 of the cell cycle [2]. Immunohistochemistry allows the detection of p53 protein, which has a short half-life and is usually detected in cell nuclei. The short half-life makes it difficult to detect in healthy tissues. However, the existence of mutation prolongs its exposure and, consequently, there is an increase in detection capacity. In the case of the relationship between p53 and apoptosis, it is important to remember that p53 is initially maintained in the cytoplasm by binding to the murine double minute (MDM-2), a caspase substrate. The enzymatic action results in the release of p53, migrating to the nucleus, and activating the transcription of pro-apoptotic genes such as BAX25 [3-11].
Regarding protein p21, called cyclin-dependent kinase inhibitor, its functions are related to the control of cell growth and may prevent or even regulate it by inhibiting apoptosis. The anti-apoptotic activity of p21 is induced by different pathways such as growth factor deprivation or p53 overexpression. Under these conditions, cytoplasmic p21 binds and inhibits the activity of proteins directly involved in apoptosis induction, such as caspases. In addition, p21 may mediate the up-regulation of genes encoding the secretion of anti-apoptotic factors [12]. The p53 acts on the P21 gene promoter with consequent cell cycle arrest still in the G1 phase, allowing repairs to damaged DNA [2].
According to Schulz et al. (2000) [1], the cytoplasmic localization of the label is not related to the possible inactivation of the functions of this protein, including apoptosis. High levels of cytoplasmic p53 are related to high rates of erythroblast apoptosis. With respect to p21, its labeling is a result of protein activation by erythropoietin elevation or hematopoietic growth factors. Koury et al. (1997 [13], 2000 [14]), in experimental models, reported that, due to damage to the genetic material, there is increased expression of p53 and p21 [13,14].
In the context of MH, the genetic damage represented by folate and cobalamin deficiency would induce p53 and p21 expression, favoring apoptosis. Yadav et al. (2016) [15], in a comparative study between cobalamin, folic acid, and homocysteine levels with p53 expression, concluded that increased expression in MH-bearing megaloblasts is associated with low levels of B12 and folic acid. However, the control group cells did not show the same with another anemia subtype [15].
During the study, we noticed a predominant cytoplasmic immunoexpression of p53 and p21, confirming, in a large number of cases, the hypothesis of Schulz et al. (2000) [1]. An interesting point is the concomitance of expression of p21 and p53. In the case of MH, we consider that there are patients with both immunoexpressions of p21 and p53, which would be in equilibrium. There are patients with isolated p53 or p21 immunoexpression, representing states of high apoptotic activity and states of apoptotic inhibition. Finally, there are immunonegative patients for both p53 and p21, with no stimulation to these proteins. Pathophysiologically, the positivity for both indicates an effective balance between apoptosis and anti-apoptotic action. Excessive inhibition of apoptosis would contribute to high global cellularity, but without functional maturation effectiveness.
On the other hand, excess apoptosis leads the body to metabolic stress and could gradually contribute to hematopoietic failure. The double negative cases represent a worrisome group in which there is no cellular response to the harmful or isolated event through which the bone marrow passes. Five patients (19.6%) had an unfavorable outcome and TMA evaluation score were double negative for p53 and p21 (P=0.474).
Conclusion
Therefore, there is p21 and/or p53 immunoexpression in most cases of MH studied based on MBC. There is no clear association between immunoexpression pattern and patient outcome, but this may represent a way to understand the pathophysiology of this disease in patients with negative evolution regardless of the treatments employed. Unlike the case report that was the focus of the study, we also found a percentage of nuclear immunostaining, but the finding was not statistically significant.
Acknowledgements
The group received financial support of São Paulo Research Foundation (FAPESP). Project number: 2016/19725-3.
Disclosure of conflict of interest
None.
References
- 1.Schulz E, Arruda V, Saad S. Cytoplasmic overexpression of p53 and p21rasin megaloblastic anemia. Haematologica. 2000;85:874–875. [PubMed] [Google Scholar]
- 2.Fett-Conte AC, Salles ABCF. A importância do gene p53 na carcinogênese humana. Rev Bras Hematol Hemoter. 2002;24:85–89. [Google Scholar]
- 3.Tornosello ML, Annunziata C, Tornosello AL, Buonaguro L, Buonaguro FM. Human oncoviruses and p53 tumor suppressor pathway deregulation at the origin of human cancers. Cancers. 2018;10:213–227. doi: 10.3390/cancers10070213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grivicich I, Regner A, Rocha AB. Apoptosis: programmed cell death. Revista Brasileira de Cancerologia. 2007;53:335–343. [Google Scholar]
- 5.Klumb C, Cavalcanti GB Jr. Avaliação dos métodos de detecção das alterações do gene e proteína P53 nas neoplasias linfoides. Revista Brasileira de Hematologia e Hemoterapia. 2002;24:111–125. [Google Scholar]
- 6.Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer. 2009;9:400–414. doi: 10.1038/nrc2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parveen A, Akash MS, Rehman K, Kyunn WW. Dual role of p21 in the progression of cancer and its treatment. Crit Rev Eukaryot Gene Expr. 2016;26:49–62. doi: 10.1615/CritRevEukaryotGeneExpr.v26.i1.60. [DOI] [PubMed] [Google Scholar]
- 8.Georgakilas AG, Martin OA, Bonner WM. p21: a two-faced genome guardian. Trends Mol Med. 2017;23:310–319. doi: 10.1016/j.molmed.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Pérez-Yépez EA, Sáldivar-Céron I, Cruz OV, Plasencia CP, Romero LEA. P21 actived kinase1: nuclear activity and its role during DNA damage repair. DNA Repair. 2018;65:42–46. doi: 10.1016/j.dnarep.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Koury MJ, Horne DW, Brown ZA, Pietenpol JA, Blount BC, Ames BN, Hard R, Koury ST. Apoptosis of late stage erythroblasts in megaloblastic anemia: association with DNA damage and macrocyte production. Blood. 1997;89:4617–4623. [PubMed] [Google Scholar]
- 11.Koury MJ. Apoptosis in megaloblastic anemia occurs during DNA synthesis by a p53-independent, nucleoside-reversible mechanism. Blood. 2000;96:3249–3250. [PubMed] [Google Scholar]
- 12.Hall JA, Mason J, Choi J, Holguin M. B12 deficiency leading to marked poikilocytosis versus true schistocytosis, a pernicious problem. Transfus Apher Sci. 2017;56:576–577. doi: 10.1016/j.transci.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Yadav MK, Manoli NM, Madhunapantula SV. Comparative assessment of vitamin-B12, folic acid and homocysteine levels in relation to p53 expression in megaloblastic anemia. PLoS One. 2016;11:e0164559. doi: 10.1371/journal.pone.0164559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav MK, Manoli NM, Madhunapantula SV. Unmethylated promoter DNA correlates with p53 expression apoptotic levels only in Vitamin B9 and B12 deficient megaloblastic anemia but not in non-megaloblastic anemia controls. Int J Biol Macromol. 2018;109:76–84. doi: 10.1016/j.ijbiomac.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira NFP, Planello AC, Andia DC, Pardo APS. Metilação de DNA e câncer. Revista Brasileira De Cancerologia. 2010;56:493–499. [Google Scholar]


