Abstract
Background: HSG (hyperplasia suppressor gene, also named Mitofusion-2, Mfn-2) gene polymorphisms have been studied as a candidate gene in essential hypertension, but no clear consensus has been reached in the Chinese population. To systematically explore their possible association, a case-control study was conducted in a central Chinese population. Methods and Results: We recruited 402 EH patients and 267 normotensive (NT) control subjects. A total of 6 tag SNPs of HSG gene were genotyped successfully by TaqMan assay. The results showed that genotype distribution and the allelic frequency of rs873457, rs2236384, rs4846085, and rs1474868 in the EH and NT groups were significantly different (P < 0.05), although those of rs2295281 and rs17037564 were not. rs2336384, rs873457, rs4846085 and rs1474868 were also closely associated with EH under the dominant genetic model (P < 0.05). Gender-based subgroup analyses showed that significant associations between rs873457, rs2336384, rs4846085, and rs1474868 and EH could be found in males, but not in females. Haplotype analysis indicated that the C-G-T-T-T-G haplotype was positively correlated with EH. Conclusion: Our study suggested that HSG gene polymorphisms were significantly associated with EH in a central Han Chinese population, especially in male subjects.
Keywords: Essential hypertension, hyperplasia suppressor gene, polymorphism, haplotype, central Han Chinese population
Introduction
The high prevalence of hypertension is closely related to the morbidity and mortality of stroke, myocardial infarction, congestive heart failure, and end-stage renal disease [1] and is a major global public health problem.
Essential hypertension (EH) occurs due to the interaction of multiple factors, both genetic and environmental. It is estimated that genetics accounts for 20 to 60% of variations in blood pressure. A multitude of studies have attempted to focus on the factors of hypertension susceptibility. Genome-wide association study (GWAS) is a novel method now being used to detect genes which may raise one’s susceptibility to EH. Nevertheless, the outcomes reported from GWAS’s can only be used [2] to clarify relatively few genetic variations. As other factors such as ethnicity and environmental factors are vital to determining possible susceptibility genes, gene association remains an important area of research.
It is suggested that the hyperplasia suppressive gene (HSG), or Mitofusion-2 (Mfn-2), mapped to the chromosome 1p36.22, could be a hypertension-related susceptibility gene. This gene was isolated using differential display technology and is reported to have reduced expression in the vascular smooth muscle cells (VSMCs) of spontaneously hypertensive rats (SHRs) [3]. The HSG affects the proliferation of VSMCs [4,5], insulin resistance [6], and endoplasmic reticulum (ER) stress [7]. These studies suggest that HSG may participate in the occurrence and development of EH through these pathologic processes.
However, there has been little research investigating the relationship between the HSG gene and EH. While Wang et al. found that several polymorphisms, including rs2336384 in intron 2 of HSG, were associated with EH in a Chinese population, others reported only 1 SNP (rs2336384) in the HSG gene and found no significant association between the polymorphism and hypertension in Koreans [8]. Thus a study was needed to confirm the relationship between HSG polymorphisms and hypertension in Chinese Han populations.
A genome-wide association study (GWAS) [9] on the Chinese Han population demonstrated that the people in Shanghai City, Jiangsu, and Anhui provinces (which have high central Chinese Han populations) had unique genetic characteristics, which were remarkably different from the genetic background of the southern and the northern Han populations. This study aimed to investigate the associations between HSG gene polymorphisms and essential hypertension in a central Chinese Han population.
Materials and methods
Study population
All hypertensive patients and normotensive (NT) controls were collected from October 2015 to July 2017 at the health check-up centre of the Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University (Nanjing, China). A total of 402 unrelated hypertensive patients and 267 normotensive controls were identified and recruited. Blood pressure (BP) was accurately measured three times with a standardized mercury sphygmomanometer by experienced internists at their offices according to a common protocol recommended by European Society of Hypertension [10]. Exercise, alcohol, caffeine, and smoking were not generally allowed for a period of 30 min prior to blood pressure measurement. Measurements were taken on the right arm using the appropriate bladders after the participants had been seated in a chair with their feet on the floor for 10 min. All the participants were recruited into the study after they visited the clinical office several times.
This study defined hypertension as 1) an average systolic blood pressure (SBP) ≥ 140 mmHg, 2) and/or an average diastolic blood pressure (DBP) ≥ 90 mmHg, or 3) receiving antihypertensive treatment. Patients with a SBP/DBP < 140/90 mmHg and not on antihypertensive treatment were considered normotensive. Exclusion criteria included 1) secondary hypertension, 2) diabetes mellitus, 3) cancer, 4) and hepatic and renal dysfunction. For inclusion patients underwent a detailed clinical history assessment, physical exam, and laboratory panel. Participants who had smoked ≥ 100 cigarettes were identified as smokers, participants who had drunk ≥ 12 times during the past year were identified as drinkers [11]. This study followed the World Health Organization obesity guidelines on Asians by defining obesity as a body mass index ≥ 25 kg/m2 [12]. The study complied with the Declaration of Helsinki, and the local ethics committee of the Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University of Medical Sciences approved the research protocol. Written informed consent was obtained from each participant.
Genotyping
A 5 mL peripheral blood sample was collected after 12 h of overnight fasting and was drawn into ethylenediaminetetraacetic acid (EDTA)-containing receptacles. DNA was extracted according to standard phenol chloroform method and stored at -20°C for batch genotyping. The six single nucleotide polymorphisms (SNPs) of the HSG gene were genotyped using the TaqMan assay. The SNP genotyping kits for C__11461996_20 (rs873457); C__11461995_10 (rs2336384); C__16189654_10 (rs2295281); C__1267226_10 (rs4846085); C__1267235_20 (rs1474868); and C__32800152_10 (rs17037564) were acquired from Applied Biosystems. These kits contained two flanking primers and C- and T-specific (or G- and A-specific) probes labelled with VIC and FAM fluorescent dyes, respectively. Using the kit manufacturer’s instructions, the sample DNA was amplified by polymerase chain reaction (PCR) on a Gene Amp PCR System 9700 thermal cycler (Applied Biosystems). 380 samples of unknown genotype were placed into 384-well plates. For the negative control, two samples were prepared with no DNA but included reagents, and for the control group two duplicate samples were prepared. Plates were read on a ABI HT 7900 instrument using the end-point analysis mode of the SDS, version 2.0, software package (Applied Biosystems). Dye-component fluorescent emission data depicted on an X-Y scatter plot using SDS software were used to differentiate genotypes.
Statistical analysis
SPSS (Version 17.0; SPSS, Inc., Chicago, IL, USA) was used for database management and statistical analyses. A two-sample t-test was used to analyse all comparisons using continuous variables. The chi-square test was used to determine the allelic and genotypic frequencies of both the hypertensive cases and normotensive controls. Multivariate logistic regression adjusted for covariates was used to examine the frequency of genotypes among the participants using various genetic models (additive, dominant, recessive, and homozygote comparisons). Analyses used two tailed estimations of significance with a distinct cut-off at P < 0.05. The chi-square test for goodness-of-fit-based web program (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl) was used to test for the Hardy-Weinberg equilibrium.
The expectation maximization algorithm [13] was applied to estimate haplotype frequencies as well as to achieve the best haplotype configuration for each multi-locus genotype. Haplotypes with a frequency > 3% in the combined case and control samples were thoroughly investigated. To assess the haplotype distributions of the hypertensive and the normotensive groups a chi-square test was performed. To verify the associations of the haplotypes with the risk of disease, a global test statistic comparing the model with genetic data, to the model without genetic data was employed, making use of the online computer platform SHEsis (http://analysis.bio-x.cn/myAnalysis.php) [14,15].
Results
Characteristics of the participants in the case-control study
A total of 669 unrelated participants comprising 267 normotensive controls from the general public and 402 patients with EH were recruited for this study. The characteristics of our study population are presented in Table 1. There were 286 males and 116 females in the EH group, with an average age of 51.69±9.10. The mean age of the 174 males and 93 females in the normotensive control group was 50.53±7.49. Patients with EH and the normotensive controls had a similar age (P=0.086) and gender (P=0.102) distribution. In addition to BP changes, significant differences in body mass index, triglyceride level, glucose level, ratio of drinkers, and positive family history of EH were also observed between the EH and NT groups.
Table 1.
Study participant characteristics
| EH (n=402) | NT (n=267) | P | |
|---|---|---|---|
| Gender (M/F) | 286/116 | 174/93 | 0.102 |
| Age (years) | 51.69±9.10 | 50.53±7.49 | 0.086 |
| SBP (mmHg) | 159.34±17.25 | 115.84±12.38 | < 0.001 |
| DBP (mmHg) | 103.25±39.21 | 78.52±10.23 | < 0.001 |
| BMI (kg/m2) | 26.94±3.84 | 24.88±3.08 | < 0.001 |
| HR (bpm) | 69.10±9.94 | 66.22±10.04 | 0.660 |
| CRE (mmol/L) | 82.89±19.19 | 81.13±14.17 | 0.222 |
| TC (mmol/L) | 5.03±1.19 | 5.09±0.89 | 0.546 |
| TG (mmol/L) | 2.08±1.13 | 1.86±1.07 | 0.012 |
| LDL-C (mmol/L) | 3.38±0.89 | 3.42±0.74 | 0.509 |
| HDL-C (mmol/L) | 1.18±0.60 | 1.31±1.24 | 0.067 |
| GlU (mmol/L) | 5.60±1.01 | 5.24±0.66 | < 0.001 |
| ALT (mmol/L) | 26.98±23.18 | 24.92±15.38 | 0.202 |
| Smokers (n, %) | 119 (29.6) | 78 (29.2) | 0.914 |
| Drinkers (n, %) | 133 (33.1) | 39 (14.6) | < 0.001 |
| Positive family history of EH (n, %) | 249 (61.9) | 89 (33.3) | < 0.001 |
Abbreviations: BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BUN, blood urea nitrogen; CRE, creatinine; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; GLU, glucose; HR, heart rate.
Detection and distribution of the SNPs
Genotype and allele frequencies of the HSG gene variants (rs873457, rs2236384, rs2295281, rs4846085, rs1474868, and rs17037564) in the EH cases and NT controls are shown in Table 2. No deviation from the Hardy-Weinberg expectation was observed for rs873457, rs2236384, rs2295281, rs4846085, rs147486, and rs17037564 polymorphisms in either the case or the control group, or when broken down into male and female groups (P > 0.05).
Table 2.
Genotype distribution and allele frequency of HSG polymorphisms in the case and control groups
| SNP | Genotype (frequency, %) | P* | Allele (frequency, %) | P** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| rs873457 | CC | CG | GG | C | G | |||||
| Total | Case | 86 (21.4) | 219 (54.5) | 97 (24.1) | Case | 391 (48.6) | 413 (51.4) | |||
| Control | 53 (19.9) | 123 (46.1) | 91 (34.1) | 0.018 | Control | 229 (42.9) | 305 (57.1) | 0.039 | ||
| Male | Case | 65 (22.7) | 157 (54.9) | 64 (22.4) | Case | 287 (50.2) | 285 (49.8) | |||
| Control | 27 (15.5) | 87 (50.0) | 60 (34.5) | 0.010 | Control | 141 (40.5) | 207 (59.5) | 0.004 | ||
| Female | Case | 21 (18.1) | 62 (53.4) | 33 (28.4) | Case | 104 (44.8) | 128 (55.2) | |||
| Control | 26 (28.0) | 36 (38.7) | 31 (33.3) | 0.081 | Control | 88 (47.3) | 98 (52.7) | 0.613 | ||
| rs2236384 | GG | GT | TT | G | T | |||||
| Total | Case | 87 (21.6) | 219 (54.5) | 96 (23.9) | Case | 393 (48.9) | 411 (51.1) | |||
| Control | 53 (19.9) | 123 (46.1) | 91 (34.1) | 0.015 | Control | 229 (42.9) | 305 (57.1) | 0.031 | ||
| Male | Case | 66 (23.1) | 156 (54.5) | 64 (22.4) | Case | 288 (50.3) | 284 (49.7) | |||
| Control | 27 (15.5) | 87 (50.0) | 60 (34.5) | 0.009 | Control | 141 (40.5) | 207 (59.5) | 0.004 | ||
| Female | Case | 21 (18.1) | 63 (54.3) | 32 (27.6) | Case | 105 (45.3) | 127 (54.7) | |||
| Control | 26 (28.0) | 36 (38.7) | 31 (33.3) | 0.065 | Control | 88 (47.3) | 98 (52.7) | 0.675 | ||
| rs2295281 | CC | CT | TT | C | T | |||||
| Total | Case | 48 (11.9) | 192 (47.8) | 162 (40.3) | Case | 288 (35.8) | 516 (64.2) | |||
| Control | 48 (18.0) | 115 (43.1) | 104 (39.0) | 0.085 | Control | 211 (39.5) | 323 (60.5) | 0.171 | ||
| Male | Case | 32 (11.2) | 133 (46.5) | 121 (42.3) | Case | 197 (34.4) | 375 (65.6) | |||
| Control | 33 (19.0) | 80 (46.0) | 61 (35.1) | 0.047 | Control | 146 (42.0) | 202 (58.0) | 0.022 | ||
| Female | Case | 16 (13.8) | 59 (50.9) | 41 (35.3) | Case | 91 (39.2) | 141 (60.8) | |||
| Control | 15 (16.1) | 35 (37.6) | 43 (46.2) | 0.156 | Control | 65 (34.9) | 121 (65.1) | 0.368 | ||
| rs4846085 | CC | CT | TT | C | T | |||||
| Total | Case | 98 (24.4) | 217 (54.0) | 87 (21.6) | Case | 413 (51.4) | 391 (48.6) | |||
| Control | 91 (34.1) | 122 (45.7) | 54 (20.2) | 0.021 | Control | 304 (56.9) | 230 (43.1) | 0.046 | ||
| Male | Case | 65 (22.7) | 155 (54.2) | 66 (23.1) | Case | 285 (49.8) | 287 (50.2) | |||
| Control | 60 (34.5) | 86 (49.4) | 28 (16.1) | 0.014 | Control | 206 (59.2) | 142 (40.8) | 0.005 | ||
| Female | Case | 33 (28.4) | 62 (53.4) | 21 (18.1) | Case | 128 (55.2) | 104 (44.8) | |||
| Control | 31 (33.3) | 36 (38.7) | 26 (28.0) | 0.081 | Control | 98 (52.7) | 88 (47.3) | 0.613 | ||
| rs1474868 | CC | CT | TT | C | T | |||||
| Total | Case | 118 (29.4) | 207 (51.5) | 77 (19.2) | Case | 433 (55.1) | 361 (44.9) | |||
| Control | 104 (39.0) | 117 (43.8) | 46 (17.2) | 0.035 | Control | 325 (60.9) | 209 (39.1) | 0.037 | ||
| Male | Case | 77 (26.9) | 150 (52.4) | 59 (20.6) | Case | 304 (53.1) | 268 (46.9) | |||
| Control | 67 (38.5) | 82 (47.1) | 25 (14.4) | 0.023 | Control | 216 (62.1) | 132 (37.9) | 0.008 | ||
| Female | Case | 41 (35.3) | 57 (49.1) | 18 (15.5) | Case | 139 (59.9) | 93 (40.1) | |||
| Control | 37 (39.8) | 35 (37.6) | 21 (22.6) | 0.201 | Control | 109 (58.6) | 77 (41.4) | 0.786 | ||
| rs17037564 | AA | AG | GG | A | G | |||||
| Total | Case | 8 (2.0) | 80 (19.9) | 314 (78.1) | Case | 96 (11.9) | 708 (88.1) | |||
| Control | 5 (1.3) | 52 (19.5) | 254 (78.7) | 0.984 | Control | 62 (11.6) | 472 (88.4) | 0.855 | ||
| Male | Case | 4 (1.4) | 55 (19.2) | 227 (79.4) | Case | 63 (11.0) | 509 (89.0) | |||
| Control | 3 (1.7) | 32 (18.4) | 139 (79.9) | 0.942 | Control | 38 (10.9) | 310 (89.1) | 0.964 | ||
| Female | Case | 4 (3.4) | 25 (21.6) | 87 (75.0) | Case | 33 (14.2) | 199 (85.8) | |||
| Control | 2 (2.2) | 20 (21.5) | 71 (76.3) | 0.854 | Control | 24 (12.9) | 162 (87.1) | 0.695 | ||
Abbreviations: SNPs, single-nucleotide-polymorphisms; *P and **P values representing the comparison of genotype frequencies and allelic frequencies, respectively.
The genotype distributions and allele frequency of the SNPs (rs873457, rs2236384, rs4846085, and rs1474868) in the hypertensive cases and normotensive controls were statistically different (P < 0.05). The C allele of rs873457 and the G allele of rs2236384 were significantly more prevalent in the EH cases, whereas the C allele frequency of rs4846085 and rs1474868 were significantly higher in the NT control subjects. No significant difference in the genotype and allele frequency distribution of SNP rs17037564 was observed between the EH cases and the NT controls. When the subjects were subdivided by gender, similar findings for rs873457, rs2336384, rs4846085, and rs1474868 polymorphisms were observed in males, but not in females. For rs2295281, there were significant differences in the genotype and allele frequencies of the male EH cases and the NT controls, whereas for rs17037564 polymorphisms, there were no significant differences in the genotype and allele frequency of the EH cases and the NT groups whether in females or males.
Association analyses
Logistic regression analyses were performed under different genetic models (additive, dominant, and recessive) after adjusting for potential confounding factors, such as gender, age, BMI, total cholesterol, triglyceride, glucose, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and smoking and drinking habits. The logistic regression analyses results are presented in Table 3.
Table 3.
Association of HSG gene polymorphisms with essential hypertension under different genetic models
| SNPS | Models | Genotypes | Overall | Male | Female | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| OR (95% CI) | P* | OR (95% CI) | P** | OR (95% CI) | P*** | |||
| rs873457 | dominant | (CC+CG) vs. GG | 0.738 (0.597-0.913) | 0.005 | 0.724 (0.566-0.926) | 0.010 | 0.768 (0.502-1.175) | 0.224 |
| recessive | CC vs. (CG+GG) | 0.925 (0.575-1.488) | 0.748 | 0.726 (0.398-1.324) | 0.296 | 2.229 (0.866-5.734) | 0.096 | |
| additive | CC vs. CG vs. GG | 0.781 (0.603-1.012) | 0.061 | 0.637 (0.444-0.913) | 0.014 | 1.056 (0.624-1.787) | 0.839 | |
| rs2236384 | dominant | (GG+GT) vs. TT | 0.730 (0.590-0.903) | 0.004 | 0.709 (0.545--0.921) | 0.010 | 0.837 (0.539-1.300) | 0.429 |
| recessive | GG vs. (GT+CT) | 0.921 (0.572-1.481) | 0.734 | 0.661 (0.354-1.237) | 0.196 | 2.229 (0.866-5.734) | 0.096 | |
| additive | GG vs. GT vs. TT | 0.744 (0.564-0.983) | 0.038 | 0.839 (0.493-1.427) | 0.517 | 1.042 (0.614-1.769) | 0.878 | |
| rs2295281 | dominant | (TT+CT) vs. CC | 1.017 (0.836-1.237) | 0.870 | 1.713 (0.894-3.284) | 0.105 | 0.834 (0.605-1.151) | 0.270 |
| recessive | TT vs. (CT+CC) | 1.710 (0.983-2.974) | 0.058 | 1.050 (0.829-1.331) | 0.685 | 0.750 (0.303-1.861) | 0.535 | |
| additive | TT vs. CT vs. CC | 1.170 (0.882-1.551) | 0.275 | 1.291 (0.767-2.176) | 0.336 | 1.008 (0.568-1.792) | 0.977 | |
| rs4846085 | dominant | (TT+CT) vs. CC | 0.740 (0.599-0.915) | 0.005 | 0.710 (0.546-0.922) | 0.010 | 0.768 (0.502-1.175) | 0.224 |
| recessive | TT vs. (CT+CC) | 0.929 (0.578-1.492) | 0.759 | 0.670 (0.359-1.250) | 0.208 | 2.229 (0.866-5.734) | 0.096 | |
| additive | TT vs. CT vs. CC | 0.785 (0.606-1.015) | 0.065 | 0.849 (0.501-1.438) | 0.542 | 1.056 (0.624-1.787) | 0.839 | |
| rs1474868 | dominant | (CC+CT) vs. TT | 0.813 (0.669-0.989) | 0.038 | 0.726 (0.566-0.932) | 0.012 | 1.062 (0.532-1.076) | 0.821 |
| recessive | CC vs. (CT+TT) | 0.862 (0.524-1.417) | 0.557 | 0.691 (0.363-1.319) | 0.263 | 1.554 (0.577-4.188) | 0.383 | |
| additive | CC vs. CT vs. TT | 0.767 (0.583-1.009) | 0.058 | 0.763 (0.451-1.289) | 0.311 | 1.038 (0.630-1.793) | 0.887 | |
| rs17037564 | dominant | (AA+AG) vs. GG | 0.907 (0.721-1.142) | 0.408 | 0.988 (0.743-1.314) | 0.935 | 0.803 (0.510-1.262) | 0.341 |
| recessive | AA vs. (AG+GG) | 0.899 (0.465-1.737) | 0.752 | 1.484 (0.281-7.828) | 0.642 | 0.308 (0.035-2.730) | 0.290 | |
| additive | AA vs. AG vs. GG | 0.846 (0.566-1.264) | 0.414 | 1.252 (0.575-2.724) | 0.571 | 0.642 (0.304-1.356) | 0.245 | |
Abbreviations: SNPs, single-nucleotide-polymorphisms; *P, **P and ***P representing the results adjusted for age, BMI, total cholesterol, triglyceride, glucose, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and smoking and drinking habits in the overall participants, male and female subjects, respectively.
The analyses showed that rs2336384 was significantly associated with EH risk under both the additive genetic model (GG vs. GT vs. TT: P=0.038, OR=0.744, 95% CI=0.564-0.983) and dominant genetic model [(GG+GT) vs. TT: P=0.004, OR=0.730, 95% CI=0.590-0.903], which indicated that G allele carriers of rs2336384 have a higher risk of EH. Furthermore, the rs873457, rs4846085, and rs1474868 SNPs were significantly associated with EH under the dominant genetic model [rs873457 (CC+CG) vs. GG: P=0.005, OR=0.738, 95% CI=0.597-0.913; rs4846085 (TT+CT) vs. CC: P=0.005, OR=0.740, 95% CI=0.599-0.915; rs1474868 (CC+CT) vs. TT: P=0.038, OR=0.813, 95% CI=0.669-0.989]. No significant associations were found between rs2295281 or rs17037564 polymorphisms and EH risk. Gender-based subgroup analyses found significant associations between rs873457, rs2336384, rs4846085, and rs1474868, and EH in males, but not in females. For rs2295281 or rs17037564 polymorphisms, no significant associations with EH were found in either males or females.
Haplotype analyses
As seen in Table 4, linkage disequilibrium (LD) analysis showed that rs873457 and rs2236384 were almost in complete LD (D’=1.00, r2=0.994). LD could also be found in rs2336384 and rs4846085 (D’=0.997, r2=0.991), as well as in rs873457 and rs4846085 (D’=0.997, r2=0.991), and rs2295281 and rs17037564 (D’=0.999, r2=0.080), respectively (Figure 1). The haplotype analyses identified five haplotypes (C-G-T-T-C-A, C-G-T-T-T-G, G-T-C-C-C-G, G-T-T-C-C-A, and G-T-T-C-C-G) defined by the composition of alleles at each SNP in the following order: rs873457-rs2236384-rs2295281-rs4846085-rs1474868-rs17037564 with a frequency greater than 3%. Multiple logistic regression analyses showed that the frequency of the haplotype C-G-T-T-T-G was higher in the EH cases [OR=1.259, 95% CI (1.007-1.575), P=0.043] than in the NT controls, and that the G-T-C-C-C-G haplotype might be a protective haplotype [OR=0.851 95% CI (0.678~1.068), P=0.164]. No significant associations were observed between the other three haplotypes (C-G-T-T-C-A, G-T-T-C-C-A, and G-T-T-C-C-G) and EH risk.
Table 4.
Linkage disequilibrium (LD) analysis of HSG polymorphisms
| D’/r2 | rs2236384 | rs2295281 | rs4846085 | rs1474868 | rs17037564 |
|---|---|---|---|---|---|
| rs873457 | 1.000/0.994 | 0.995/0.508 | 0.997/0.991 | 0.969/0.807 | 0.242/0.007 |
| rs2236384 | 0.995/0.510 | 0.997/0.991 | 0.969/0.802 | 0.218/0.006 | |
| rs2295281 | 0.995/0.510 | 0.982/0.401 | 0.999/0.080 | ||
| rs4846085 | 0.969/0.805 | 0.533/0.006 | |||
| rs1474868 | 0.950/0.090 |
Values in upper diagonal and lower diagonal represent the D’ and r2 of the SNP combinations, respectively.
Figure 1.
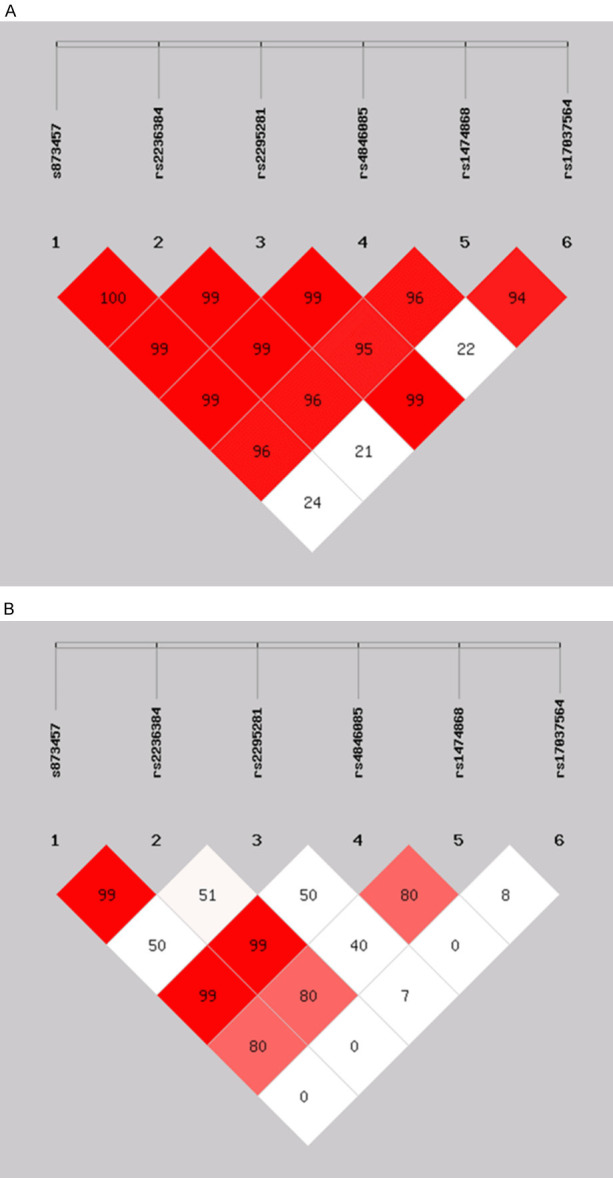
Linkage disequilibrium (LD) plots illustrating the degree of linkage disequilibrium of HSG polymorphisms in this study. A. Values in the LD blocks are the D’ in percentage. B. Values in the LD blocks are the r2 in percentage.
Discussion
Essential hypertension (EH) is a polygenic disease and elucidating its molecular and genetic mechanism will help us have a deeper understanding of its pathophysiologic mechanism. Single nucleotide polymorphisms (SNPs) are ever more vital in exposing the mechanisms of polygenic diseases. In this study, we examined six tagged HSG SNPs (rs873457, rs2236384, rs2295281, rs4846085, rs1474868, and rs17037564) and their contributions to the development of essential hypertension in the central Han Chinese population.
The results from this study confirmed that among the enrolled EH patients and NT subjects, the genotype distribution and allele frequency of four SNPs (rs873457, rs2236384, rs4846085, and rs1474868) were significantly different (P < 0.05). Logistic regression analyses indicated that rs2336384 was significantly associated with EH under the additive (OR=0.744, 95% CI=0.564-0.983, P=0.038) and dominant (OR=0.730, 95% CI=0.590-0.903, P=0.004) genetic models even after adjustment for multiple covariates. Under the dominant genetic model, rs873457 (OR=0.738, 95% CI=0.597-0.913, P=0.005), rs4846085 (OR=0.740, 95% CI=0.599-0.915, P=0.005), and rs1474868 (OR=0.813, 95% CI=0.669-0.989, P=0.038) SNPs were closely associated with EH. Our results confirm the findings of Li et al. [16] who reported the significant association of HSG/Mfn2 gene polymorphisms rs2336384 and rs2236057 to an increased risk of EH. They also reported the significant association of rs2236058 and rs3766741 polymorphisms with a decreased risk of EH in a northern Chinese Han population. Wang et al. [17] examined the association of seven SNPs (rs873457, rs2336384, rs1474868, rs4846085, rs2236055, and rs873458) on the HSG gene intron 2 and the risk of EH and reported that rs873457, rs2336384, rs1474868, rs4846085, and rs2236055 were significantly associated with a risk of EH. In addition, in 2013, Wang et al. [18] investigated the role of the -1248 A > G polymorphism in the 5’-UTR region of HSG in the development of EH. They found that the A allele frequency of the 1248 A > G locus in the NT group was significantly lower in the EH group, which indicated that people who carried the A allele had a lower risk of developing EH. We found that the rs873457, rs2336384, rs4846085, and rs1474868 polymorphisms were correlated with the risk of EH, which was also consistent with the results of Wang et al. [17]. However, Jin et al. [8] found no correlation between rs2336384 of HSG/Mfn2 and the risk of EH in a Korean population. In contrast, in this study, we observed a significant association between rs2336384 and EH risk. We inferred that this inconsistency might be due to the following reasons. First, there are differences in the genetic backgrounds of Chinese and Korean populations [9,19]. Second, the sample sizes of the studied populations were different. Although the SNP sites selected in these studies were different, the results suggested that HSG gene SNPs were associated with the risk of EH. We speculate that HSG single nucleotide polymorphisms play an important role in the pathogenesis of EH.
When grouped by gender, our study found that the association between HSG polymorphisms and EH were male-specific. The genotype distribution and allele frequency of the rs873457, rs2336384, rs4846085, and rs1474868 polymorphisms were significantly different in male EH patients and NT subjects (P < 0.05) but not in females (P > 0.05). Logistic regression showed that the rs873457, rs2336384, rs4846085, and rs1474868 polymorphisms were closely associated with EH after adjusting for covariates. These findings were consistent with a study reported by Wang et al. [17].
We inferred that there were two main reasons for this phenomenon. Firstly, there are differences in the genetic backgrounds of men and women [20]. Some previous studies indicated that there were significant gender differences in the distribution of some EH susceptibility gene SNPs [21,22], which supported our findings. Secondly, HSG gene expression might also be regulated by various external environmental factors, including cold stimulation exposure and inflammatory cytokines [23-26]. Especially in China, men have more exposure to risk factors, such as smoking, drinking, and mental stress, than women. These factors might influence blood pressure through the interaction between genes and the environment. The rs873457, rs2336384, rs4846085, and rs1474868 mutations may make men more sensitive to these risk factors, and oxidative stress injury may further promote a higher incidence of EH in men [27]. The difference may also be due to sex hormones, though this requires further study.
Haplotype analysis is considered to be more powerful than single locus analysis for identifying genetic variants of complex diseases, such as EH [28]. In this study, haplotype analyses identified 5 haplotypes (C-G-T-T-C-A, C-G-T-T-T-G, G-T-C-C-C-G, G-T-T-C-C-A, and G-T-T-C-C-G) with a frequency greater than 3%. After adjustment for multiple covariates, logistic regression showed that haplotype C-G-T-T-T-G could increase the risk of EH [OR=1.259, 95% CI [1.007-1.575], P=0.043]. In addition, we found that the distribution frequency of haplotype G-T-C-C-C-G was lower in the EH group than the NT Group, but it did not reach statistical significance (P=0.164), which suggested that people with haplotype G-T-C-C-C-G wereless likely to develop EH. Therefore, we believe that the C-G-T-T-T-G and G-T-C-C-C-G haplotypes might be a risk factor and a protective factor for EH, respectively. Wang et al. [17] reported that the C-G-A-A-A-C-C haplotype, established by rs873457, rs2336384, rs1474868, rs4846065, rs4240897, rsrs2236055, and rs873458, was significantly higher in EH patients than in control individuals (P < 0.01 for all). Logistic regression showed that this haplotype was an independent risk factor for EH. Although the SNP sites involved in that study differed from our study and led to different haplotype results, the implications of our study are consistent with Wang’s. To some extent, the analysis of the HSG haplotype in this study provides additional potentially valuable information for the exploration of hypertension susceptibility factors.
Previous studies have demonstrated that vascular proliferation is an important factor in established genetic and experimental hypertension models [29,30]. In spontaneously hypertensive rats (SHRs), HSG gene expression was decreased in hyper-proliferative VSMCs from SHR arteries, as well as in white blood cells, explanted vessels, and cultured VSMCs from hypertensive patients [31]. Therefore, we concluded that HSG may be involved in the development of EH by regulating the proliferation of vascular smooth muscle cells. In addition, in vivo studies have shown that HSG/Mfn2 deficiency may lead to insulin resistance by increasing the concentration of H2O2 in the liver and muscles and impairing insulin signalling pathways [6]. Given that insulin resistance and compensatory hyperinsulinemia due to insulin resistance play a role in the development of hypertension [32], we speculate that HSG/Mfn2 may also participate in the development of EH by influencing insulin resistance.
The two positive SNP sites in this study are located on HSG introns. The first possible mechanism is that HSG/Mfn2 gene expression may be affected by transcriptional regulation, thereby affecting its function. The second mechanism is a linkage imbalance between these polymorphism sites and other functional polymorphism sites. Further functional studies are needed to demonstrate how these polymorphisms affect the pathogenesis of hypertension.
This study has several limitations. First, selection bias may exist due to the study design, reducing the power of our study. Second, this study did not include a functional study of the positive SNP sites, and the molecular mechanism by which these positive SNP sites affect the risk of essential hypertension is still unclear. Lastly, this is a single case-control study and relatively small sample size is too weak to fully explore the relationship between HSG SNPs and susceptibility to EH.
Conclusions
Our study showed that the HSG gene variants (rs873457, rs2236384, rs4846085, and rs1474868) and the related haplotype C-G-T-T-T-G were significantly associated with EH in a central Han Chinese population. Interestingly, our stratified analysis suggested that the rs873457, rs2236384, rs4846085, and rs1474868 HSG variants were found to be significantly associated with EH in males, but not in females. Studies with a larger sample size and further functional studies of HSG in essential hypertension are needed to confirm this discovery.
Acknowledgements
We are very grateful and thank all the participants in this study. LS W conceived and designed the study, LS W made critical revision of the draft manuscript. XQ L, SB W interpreted the results, finished the data analysis, and wrote the draft manuscript. XQ L, SB W, H W participated in the laboratory tests and data collection. All authors read and approved the final manuscript. This work was supported by grants from the National Natural Science Foundation of China (No. 81770361) and the “333 high level talents Project” of Jiangsu Province (No. BRA2017408).
Disclosure of conflict of interest
None.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of Cardiology/American heart association task force on clinical practice guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Lu X, Wang L, Lin X, Huang J, Charles Gu C, He M, Shen H, He J, Zhu J, Li H, Hixson JE, Wu T, Dai J, Lu L, Shen C, Chen S, He L, Mo Z, Hao Y, Mo X, Yang X, Li J, Cao J, Chen J, Fan Z, Li Y, Zhao L, Li H, Lu F, Yao C, Yu L, Xu L, Mu J, Wu X, Deng Y, Hu D, Zhang W, Ji X, Guo D, Guo Z, Zhou Z, Yang Z, Wang R, Yang J, Zhou X, Yan W, Sun N, Gao P, Gu D. Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet. 2015;24:865–874. doi: 10.1093/hmg/ddu478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen KH, Guo X, Ma D, Guo Y, Li Q, Yang D, Li P, Qiu Y, Wen S, Xiao R, Tang J. Dysregulation of HSG triggers vascular proliferative disorders. Nat Cell Biol. 2004;6:872–883. doi: 10.1038/ncb1161. [DOI] [PubMed] [Google Scholar]
- 4.Xia Y, Wu Y, He X, Gong J, Qiu F. Effects of mitofusin-2 gene on cell proliferation and chemotherapy sensitivity of MCF-7. J Huazhong Univ Sci Technolog Med Sci. 2008;28:185–189. doi: 10.1007/s11596-008-0218-2. [DOI] [PubMed] [Google Scholar]
- 5.de Brito OM, Scorrano L. Mitofusin 2: a mitochondria-shaping protein with signaling roles beyond fusion. Antioxid Redox Signal. 2008;10:621–633. doi: 10.1089/ars.2007.1934. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian D, Hernandez-Alvarez MI, Segales J, Sorianello E, Munoz JP, Sala D, Waget A, Liesa M, Paz JC, Gopalacharyulu P, Oresic M, Pich S, Burcelin R, Palacin M, Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl Acad Sci U S A. 2012;109:5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ngoh GA, Papanicolaou KN, Walsh K. Loss of mitofusin 2 promotes endoplasmic reticulum stress. J Biol Chem. 2012;287:20321–20332. doi: 10.1074/jbc.M112.359174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin HS, Sober S, Hong KW, Org E, Kim BY, Laan M, Oh B, Jeong SY. Age-dependent association of the polymorphisms in the mitochondria-shaping gene, OPA1, with blood pressure and hypertension in Korean population. Am J Hypertens. 2011;24:1127–1135. doi: 10.1038/ajh.2011.131. [DOI] [PubMed] [Google Scholar]
- 9.Xu S, Yin X, Li S, Jin W, Lou H, Yang L, Gong X, Wang H, Shen Y, Pan X, He Y, Yang Y, Wang Y, Fu W, An Y, Wang J, Tan J, Qian J, Chen X, Zhang X, Sun Y, Zhang X, Wu B, Jin L. Genomic dissection of population substructure of Han Chinese and its implication in association studies. Am J Hum Genet. 2009;85:762–774. doi: 10.1016/j.ajhg.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Task Force for the management of arterial hypertension of the European Society of Hypertension; Task Force for the management of arterial hypertension of the European Society of Cardiology. 2013 ESH/ESC guidelines for the management of arterial hypertension. Blood Press. 2013;22:193–278. doi: 10.3109/08037051.2013.812549. [DOI] [PubMed] [Google Scholar]
- 11.Gu D, Su S, Ge D, Chen S, Huang J, Li B, Chen R, Qiang B. Association study with 33 single-nucleotide polymorphisms in 11 candidate genes for hypertension in Chinese. Hypertension. 2006;47:1147–1154. doi: 10.1161/01.HYP.0000219041.66702.45. [DOI] [PubMed] [Google Scholar]
- 12.Krahe R, Lou Y, Liu J, Li Y, Liu Y, Wang Z, Liu K, Wu H, Niu Q, Gu W, Guo Y, Li Z, Wen S. Association Study of the β2-adrenergic receptor gene polymorphisms and hypertension in the northern Han Chinese. PLoS One. 2011;6:e18590. doi: 10.1371/journal.pone.0018590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–3557. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–98. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 15.Li Z, Zhang Z, He Z, Tang W, Li T, Zeng Z, He L, Shi Y. A partition-ligation-combination-subdivision EM algorithm for haplotype inference with multiallelic markers: update of the SHEsis (http://analysis.bio-x.cn) Cell Res. 2009;19:519–523. doi: 10.1038/cr.2009.33. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Zhang B, Li C, Liu J, Liu Y, Sun D, Ma H, Wen S. The association of mitofusion-2 gene polymorphisms with susceptibility of essential hypertension in northern Han Chinese population. Int J Med Sci. 2016;13:39–47. doi: 10.7150/ijms.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z, Liu Y, Liu J, Liu K, Wen J, Wen S, Wu Z. HSG/Mfn2 gene polymorphism and essential hypertension: a case-control association study in Chinese. J Atheroscler Thromb. 2011;18:24–31. doi: 10.5551/jat.5611. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Liu Y, Liu J, Niu Q, Wen J, Wen S, Wu Z. A Novel 5’-uncoding region -1248 A>G variation of mitofusin-2 gene is associated with hypertension in Chinese. Yonsei Med J. 2013;54:603–8. doi: 10.3349/ymj.2013.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen B, Li H, Lu D, Song X, Zhang F, He Y, Li F, Gao Y, Mao X, Zhang L, Qian J, Tan J, Jin J, Huang W, Deka R, Su B, Chakraborty R, Jin L. Genetic evidence supports demic diffusion of Han culture. Nature. 2004;431:302–305. doi: 10.1038/nature02878. [DOI] [PubMed] [Google Scholar]
- 20.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat Rev Genet. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu D, Yin R, Aung L, Li Q, Yan T, Zeng X, Huang K, Huang P, Wu J, Pan S. Sex-specific association of ACAT-1 rs1044925 SNP and serum lipid levels in the hypercholesterolemic subjects. Lipids Health Dis. 2012;11:9. doi: 10.1186/1476-511X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.International Consortium for Blood Pressure Genome-Wide Association Studies; Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, Smith AV, Tobin MD, Verwoert GC, Hwang SJ, Pihur V, Vollenweider P, O’Reilly PF, Amin N, Bragg-Gresham JL, Teumer A, Glazer NL, Launer L, Zhao JH, Aulchenko Y, Heath S, Sõber S, Parsa A, Luan J, Arora P, Dehghan A, Zhang F, Lucas G, Hicks AA, Jackson AU, Peden JF, Tanaka T, Wild SH, Rudan I, Igl W, Milaneschi Y, Parker AN, Fava C, Chambers JC, Fox ER, Kumari M, Go MJ, van der Harst P, Kao WH, Sjögren M, Vinay DG, Alexander M, Tabara Y, Shaw-Hawkins S, Whincup PH, Liu Y, Shi G, Kuusisto J, Tayo B, Seielstad M, Sim X, Nguyen KD, Lehtimäki T, Matullo G, Wu Y, Gaunt TR, Onland-Moret NC, Cooper MN, Platou CG, Org E, Hardy R, Dahgam S, Palmen J, Vitart V, Braund PS, Kuznetsova T, Uiterwaal CS, Adeyemo A, Palmas W, Campbell H, Ludwig B, Tomaszewski M, Tzoulaki I, Palmer ND CARDIoGRAM consortium; CKDGen Consortium; KidneyGen Consortium; EchoGen consortium; CHARGE-HF consortium. Aspelund T, Garcia M, Chang YP, O’Connell JR, Steinle NI, Grobbee DE, Arking DE, Kardia SL, Morrison AC, Hernandez D, Najjar S, McArdle WL, Hadley D, Brown MJ, Connell JM, Hingorani AD, Day IN, Lawlor DA, Beilby JP, Lawrence RW, Clarke R, Hopewell JC, Ongen H, Dreisbach AW, Li Y, Young JH, Bis JC, Kähönen M, Viikari J, Adair LS, Lee NR, Chen MH, Olden M, Pattaro C, Bolton JA, Köttgen A, Bergmann S, Mooser V, Chaturvedi N, Frayling TM, Islam M, Jafar TH, Erdmann J, Kulkarni SR, Bornstein SR, Grässler J, Groop L, Voight BF, Kettunen J, Howard P, Taylor A, Guarrera S, Ricceri F, Emilsson V, Plump A, Barroso I, Khaw KT, Weder AB, Hunt SC, Sun YV, Bergman RN, Collins FS, Bonnycastle LL, Scott LJ, Stringham HM, Peltonen L, Perola M, Vartiainen E, Brand SM, Staessen JA, Wang TJ, Burton PR, Soler Artigas M, Dong Y, Snieder H, Wang X, Zhu H, Lohman KK, Rudock ME, Heckbert SR, Smith NL, Wiggins KL, Doumatey A, Shriner D, Veldre G, Viigimaa M, Kinra S, Prabhakaran D, Tripathy V, Langefeld CD, Rosengren A, Thelle DS, Corsi AM, Singleton A, Forrester T, Hilton G, McKenzie CA, Salako T, Iwai N, Kita Y, Ogihara T, Ohkubo T, Okamura T, Ueshima H, Umemura S, Eyheramendy S, Meitinger T, Wichmann HE, Cho YS, Kim HL, Lee JY, Scott J, Sehmi JS, Zhang W, Hedblad B, Nilsson P, Smith GD, Wong A, Narisu N, Stančáková A, Raffel LJ, Yao J, Kathiresan S, O’Donnell CJ, Schwartz SM, Ikram MA, Longstreth WT Jr, Mosley TH, Seshadri S, Shrine NR, Wain LV, Morken MA, Swift AJ, Laitinen J, Prokopenko I, Zitting P, Cooper JA, Humphries SE, Danesh J, Rasheed A, Goel A, Hamsten A, Watkins H, Bakker SJ, van Gilst WH, Janipalli CS, Mani KR, Yajnik CS, Hofman A, Mattace-Raso FU, Oostra BA, Demirkan A, Isaacs A, Rivadeneira F, Lakatta EG, Orru M, Scuteri A, Ala-Korpela M, Kangas AJ, Lyytikäinen LP, Soininen P, Tukiainen T, Würtz P, Ong RT, Dörr M, Kroemer HK, Völker U, Völzke H, Galan P, Hercberg S, Lathrop M, Zelenika D, Deloukas P, Mangino M, Spector TD, Zhai G, Meschia JF, Nalls MA, Sharma P, Terzic J, Kumar MV, Denniff M, Zukowska-Szczechowska E, Wagenknecht LE, Fowkes FG, Charchar FJ, Schwarz PE, Hayward C, Guo X, Rotimi C, Bots ML, Brand E, Samani NJ, Polasek O, Talmud PJ, Nyberg F, Kuh D, Laan M, Hveem K, Palmer LJ, van der Schouw YT, Casas JP, Mohlke KL, Vineis P, Raitakari O, Ganesh SK, Wong TY, Tai ES, Cooper RS, Laakso M, Rao DC, Harris TB, Morris RW, Dominiczak AF, Kivimaki M, Marmot MG, Miki T, Saleheen D, Chandak GR, Coresh J, Navis G, Salomaa V, Han BG, Zhu X, Kooner JS, Melander O, Ridker PM, Bandinelli S, Gyllensten UB, Wright AF, Wilson JF, Ferrucci L, Farrall M, Tuomilehto J, Pramstaller PP, Elosua R, Soranzo N, Sijbrands EJ, Altshuler D, Loos RJ, Shuldiner AR, Gieger C, Meneton P, Uitterlinden AG, Wareham NJ, Gudnason V, Rotter JI, Rettig R, Uda M, Strachan DP, Witteman JC, Hartikainen AL, Beckmann JS, Boerwinkle E, Vasan RS, Boehnke M, Larson MG, Järvelin MR, Psaty BM, Abecasis GR, Chakravarti A, Elliott P, van Duijn CM, Newton-Cheh C, Levy D, Caulfield MJ, Johnson T. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soriano FX, Liesa M, Bach D, Chan DC, Palacin M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-coactivator-1, estrogen-related receptor-, and mitofusin 2. Diabetes. 2006;55:1783–1791. doi: 10.2337/db05-0509. [DOI] [PubMed] [Google Scholar]
- 24.Fealy CE, Mulya A, Lai N, Kirwan JP. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. J Appl Physiol. 2014;117:239–245. doi: 10.1152/japplphysiol.01064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvi E, Wang Z, Rizzi F, Gong Y, McDonough CW, Padmanabhan S, Hiltunen TP, Lanzani C, Zaninello R, Chittani M, Bailey KR, Sarin AP, Barcella M, Melander O, Chapman AB, Manunta P, Kontula KK, Glorioso N, Cusi D, Dominiczak AF, Johnson JA, Barlassina C, Boerwinkle E, Cooper-DeHoff RM, Turner ST. Genome-wide and gene-based meta-analyses identify novel loci influencing blood pressure response to hydrochlorothiazide. Hypertension. 2017;69:51–59. doi: 10.1161/HYPERTENSIONAHA.116.08267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bach D, Naon D, Pich S, Soriano FX, Vega N, Rieusset J, Laville M, Guillet C, Boirie Y, Wallberg-Henriksson H, Manco M, Calvani M, Castagneto M, Palacín M, Mingrone G, Zierath J, Vidal H, Zorzano A. Expression of Mfn2, the charcot-marie-tooth neuropathy type 2A gene, in human skeletal muscle: effects of type 2 diabetes, obesity, weight loss, and the regulatory role of tumor necrosis factor alpha and interleukin-6. Diabetes. 2005;54:2685–2693. doi: 10.2337/diabetes.54.9.2685. [DOI] [PubMed] [Google Scholar]
- 27.Jezek P, Plecita-Hlavata L. Mitochondrial reticulum network dynamics in relation to oxidative stress, redox regulation, and hypoxia. Int J Biochem Cell Biol. 2009;41:1790–1804. doi: 10.1016/j.biocel.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Morris RW, Kaplan NL. On the advantage of haplotype analysis in the presence of multiple disease susceptibility alleles. Genet Epidemiol. 2002;23:221–233. doi: 10.1002/gepi.10200. [DOI] [PubMed] [Google Scholar]
- 29.Belo VA, Guimaraes DA, Castro MM. Matrix metalloproteinase 2 as a potential mediator of vascular smooth muscle cell migration and chronic vascular remodeling in hypertension. J Vasc Res. 2015;52:221–231. doi: 10.1159/000441621. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Chen W, Li J, Qi S, Hong S, Wang Y, Gao L, Shi Z, Liu Y, Liu W, Chi Y, Liu C, Fu Y, Yin X. Involvement of mitochondrial fission in calcium sensing receptor-mediated vascular smooth muscle cells proliferation during hypertension. Biochem Biophys Res Commun. 2018;495:454–460. doi: 10.1016/j.bbrc.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 31.Liu YP, Wen SJ, Liu Y, Zhao LM, Guo YH, Chen XJ, Wang ZG, Liu JL, Wen J, Wang SQ, Tang J. [Hyperplasia suppressor gene expression in vascular smooth muscle cells derived from normotensive and hypertensive patients underwent bypass surgery] . Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:914–918. [PubMed] [Google Scholar]
- 32.Wang F, Han L, Hu D. Fasting insulin, insulin resistance and risk of hypertension in the general population: a meta-analysis. Clin Chim Acta. 2017;464:57–63. doi: 10.1016/j.cca.2016.11.009. [DOI] [PubMed] [Google Scholar]


