Abstract
Objective: This project investigated the inhibitory effect of Fraxetin on endometrial cancer cell proliferation, and explored the possibility of applying Fraxetin in the treatment of endometrial cancer. Methods: Human endometrial cancer RL95-2 cell line was cultured in vitro, and the cells were administered different concentrations of Fraxetin. MTS was used to detect the inhibitory effect of Fraxetin on proliferation. Flow cytometry was applied to detect the effect of Fraxetin on RL95-2 cell cycle. Western blot was employed to determine the expression of apoptosis-related proteins, such as caspase-3, caspase-9, p-AMPK, AMPK, p-mTOR, and mTOR. JC-1 staining was used to measure the mitochondrial membrane potential changes in the cells before and after the administration. The glucose oxidase method and the lactate oxidase method were used to detect changes in glucose consumption and lactic acid production in endometrial cancer cells before and after drug intervention, respectively. Results: Fraxetin inhibited cell proliferation and promoted apoptosis. The expressions of caspase-3 and caspase-9 increased significantly, p-AMPK gradually increased, and mitochondrial membrane potential weakened. Glucose consumption and lactic acid production increased significantly. Conclusion: Fraxetin can inhibit the proliferation of RL95-2 cells, promote apoptosis, inhibit mitochondrial oxidation of endometrial cancer cells, promote anaerobic metabolism of cells, and exert an inhibitory effect on endometrial cancer cells by inhibiting mitochondria.
Keywords: Fraxetin, RL95-2 cells, apoptosis, mitochondria
Introduction
Endometrial cancer is one of the most common malignant tumors in the female reproductive system. The incidence rate is the fourth highest, and rising. The prognosis of patients with early EC is relatively good, but the survival rate of patients with advanced or relapsed EC is significantly reduced. The prognosis of EC is affected by various factors including tumor grade, stage, muscle layer infiltration and lymph node metastasis. There is no effective treatment for advanced and recurrent endometrial cancer. Therefore, the study of EC metastasis, recurrence, and other related issues is of great significance for improving the treatment of EC patients [1-3].
The adenosine phosphate-activated protein kinase family belongs to a serine/threonine protein kinase, and is one of the regulators of cell energy metabolism. When the cell is deficient in energy, the intracellular AMP/ATP ratio rises, which leads to the upregulation of AMPK expression, and finally restores the energy metabolism balance through a series of complex and delicate mechanisms [4]. AMPK can promote blood glucose absorption, reduce blood sugar and blood lipid levels, increase insulin sensitivity, and improve insulin resistance. In addition, AMPK increases fatty acid oxidation, inhibits fat and protein synthesis, and exerts a significant effect on insulin. AMPK family members are closely related to metabolic diseases such as diabetes and obesity. At the molecular level, there is a complex relationship between AMPK and insulin signaling pathways. For example, AMPK can up- or down-regulate the activities of PI3K and PKB combined with insulin receptor substrate-1, while insulin and PKB can down-regulate AMPK activity. In recent years, the relationship between AMPK and tumors has received attention [5,6]. However, the inhibitory effect of Fraxetin on endometrial cancer cells through the AMPK signaling pathway is unclear. In this paper, the mechanism is studied, and the possibility of applying Fraxetin to the treatment of endometrial cancer is explored.
Methods
Cell culture
Endometrial cancer RL95-2 cells were routinely cultured in DMEM medium containing 10% fetal bovine serum, temperature at 37°C, with 5% CO2, under saturated humidity conditions. The cells were digested and passaged with 0.25% trypsin and 0.02% EDTA. Cells in exponential growth phase were obtained for the experiment.
MTT to detect the inhibitory effect of drugs on proliferation of RL95-2 cells
A cell suspension was prepared, and 100 μl of the cell suspension was inoculated into a 96-well culture plate for 48 h. Fraxetin was diluted in gradient at 160 μM, 80 μM, 40 μM, 20 μM, 10 μM, 5 μM, and 2.5 μM. 150 μl of cells were processed in each well. After 48 h culture, 10 μl (5 mg/ml) of MTT solution was added to each well, and the culture was continued in the incubator for 1-2 h. The absorbance was measured at 490 nm using a microplate reader.
Annexin V staining to detect apoptosis
The cells were washed once with PBS and then digested with trypsin. After being washed with ice-cold PBS, the cells were resuspended in staining buffer. An appropriate amount of FITC-Annexin V staining solution was added according to the kit instructions. The cells were incubated in darkness, and then stained with propidium iodide solution.
JC-1 staining to study the effect of BBR on mitochondrial function of endometrial cancer cells
A cell suspension was prepared. 0.6 ml of the cell suspension was inoculated into a 24-well plate to culture for 24 h. The cells were administered with 40 μM of Fraxetin for 24 h. 0.5 ml of JC-1 staining solution was added. The cells were agitated gently, and placed in 37°C incubator for 20 minutes. The JC-1 staining buffer was prepared as 1:4 to distilled water, and then placed in an ice bath. After 37°C incubation, the supernatant was discarded. The cells were washed twice with JC-1 staining buffer. 1 ml of JC-1 staining buffer was added again, and the cells were observed under a fluorescent microscope.
Glucose oxidase method to detect changes in glucose consumption in endometrial cancer cells before and after drug intervention
A cell suspension was prepared. 0.6 ml of cell suspension was inoculated into a 24-well plate to culture for 24 hours. The cells were administered Fraxetin at 0 μM, 5 μM, 10 μM, 20 μM, and 40 μM for 24 h, respectively. 500 µl culture medium was obtained, and centrifuged at 1500 rpm at room temperature. 10 µl of the supernatant was prepared and added to 1 ml glucose detection reagent. The cells were incubated for 20 minutes at room temperature. 200 µl of the product was inoculated into a 96-well plate. The absorbance of the sample at 500 nm within 1 h was measured, and was compared with the absorbance of the standard value. The glucose concentration in the sample was calculated.
Lactate oxidase method to detect changes in lactic acid in cells after drug intervention
A cell suspension was prepared. 0.6 ml of cell suspension was inoculated into a 24-well plate to culture for 24 hours. The cells were treated with Fraxetin of 0 μM, 5 μM, 10 μM, 20 μM, and 40 μM for 24 h. 500 µl of culture medium was prepared and centrifuged at 1500 rpm under room temperature. 10 µl of the supernatant was obtained and added with 1 ml of lactic acid detection reagent. The cells were incubated for 10 minutes at room temperature. 200 µl of the reactant was inoculated into a 96-well plate. The absorbance of the sample was detected at 500 nm within 30 min, and was compared with absorbance of the standard value to calculate the lactic acid concentration.
Western blotting to detect changes in related proteins during drug-induced apoptosis
The cells were processed to prepare a cell suspension. 3 ml cell suspension was inoculated into a 6-well plate for 24 hours’ culture. Rl95-2 cells were treated with 0 μM, 5 μM, 10 μM, 20 μM, and 40 μM Fraxetin for 48 h respectively. A protein sample was prepared. BCA method was applied to detect protein concentration. SDS-polyacrylamide gel electrophoresis, transferring, blocking, hybridization, and ECL reaction were conducted. Imaging was performed on the ECL detector and the test results were saved. The image processing software ImagePro was used to determine the average density value, and the ratio of target protein to internal reference protein gray was calculated.
Statistical analysis
The data was processed using SPSS 13.0 statistical software. Differences between groups were compared using t test and one-way analysis of variance. P<0.05 indicated a significant difference. All data are expressed as mean ± SD, with values from at least three samples.
Results
Effect of Fraxetin on RL95 proliferation
MTT showed that Fraxetin can effectively inhibit cell proliferation, and the greater the concentration, the higher the inhibition rate, see Figure 1.
Figure 1.
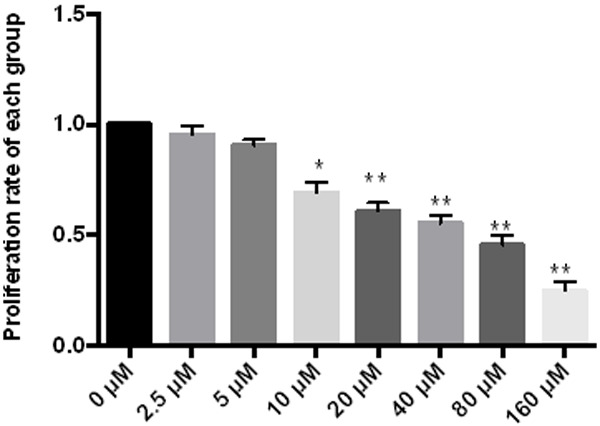
Effect of Fraxetin on RL95 proliferation. **P < 0.01 vs 0 μM, *P < 0.05 vs 0 μM.
Effect of Fraxetin on RL95 apoptosis
Annexin V/PI double staining showed that there was significant apoptosis in the drug groups, but almost no such effect in the control group. The results showed that Fraxetin can regulate cell proliferation and survival, see Figure 2.
Figure 2.
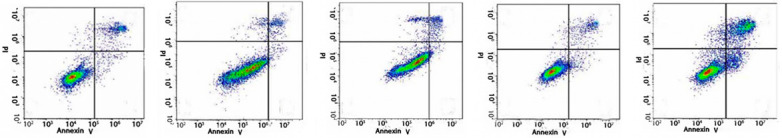
Effect of Fraxetin on RL95 apoptosis.
JC-1 staining to detect the effect of Fraxetin on mitochondrial membrane potential
JC-1 staining showed that, after treating RL95-2 cells with Fraxetin, the green fluorescence intensity of JC-1 increased while the red fluorescence intensity decreased, indicating that the mitochondrial membrane potential was reduced as the drug inhibited mitochondrial function. See Figure 3.
Figure 3.
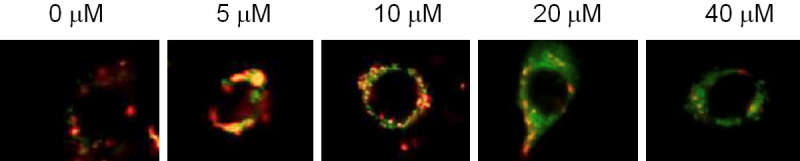
Effect of Fraxetin on mitochondrial membrane potential.
Effect of Fraxetin on glucose consumption and lactic acid production in cells
Glucose oxidase method was used to detect the changes of glucose consumption in cells after the administration of Fraxetin. As the dose increased, glucose consumption changed. At 40 μM, the intracellular glucose consumption is reduced, which may be related to the inhibition of more cells, as shown in Figure 4.
Figure 4.
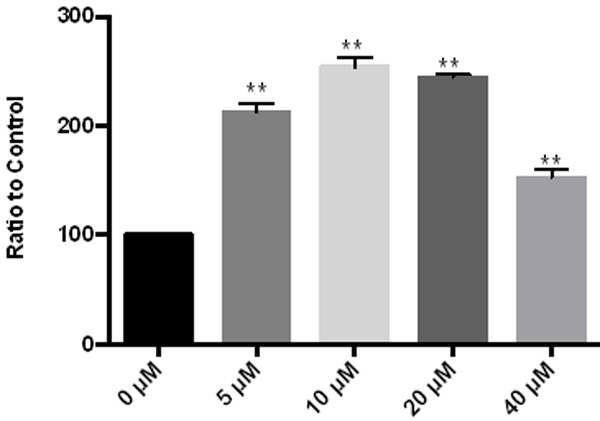
Effect of Fraxetin on glucose consumption in cells. **P < 0.01 vs 0 μM, *P < 0.05 vs 0 μM.
The lactate oxidase method was used to detect changes in intracellular lactic acid production after different concentrations of Fraxetin were intervened on endometrial cancer cells. After drug administration, the intracellular lactic acid production significantly increased. As the concentration increased, the lactic acid production changed. See Figure 5.
Figure 5.
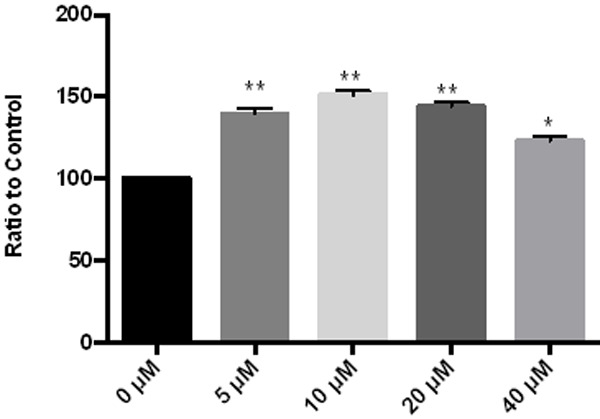
Effect of Fraxetin on lactic acid consumption of cells. **P < 0.01 vs 0 μM, *P < 0.05 vs 0 μM.
The effect of Fraxetin on various proteins
The results showed that the expressions of Caspase-3 and Caspase-9 increased with the increase of drug concentration, but there was no significance. The expression of p-AMPK increased significantly, the expression of AMPK decreased greatly, while the expression of mTOR exhibited just the opposite of AMPK, see Figure 6.
Figure 6.
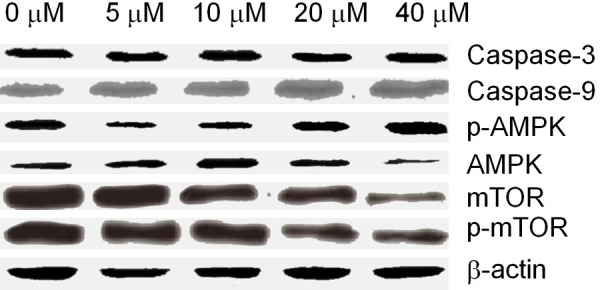
Effect of Fraxetin on various proteins.
Discussion
Apoptosis occurs through the mitochondrial pathway or the death receptor pathway. Mitochondrial transmembrane potential changes when cells undergo apoptosis, and the change is related to multiple mechanisms. The pore that causes the mitochondrial membrane permeability to change is a complex composition of proteins, located between the inner and outer membranes of the mitochondria. When apoptosis occurs, the PT pore is opened. Substances that promote PT pore opening can induce apoptosis, while substances that inhibit PT pore opening can prevent apoptosis. Changes in mitochondrial transmembrane potential are also closely related to the Caspase family. Caspase is mainly responsible for selectively cutting certain proteins to activate or deactivate them. Caspase-3 is mainly responsible for cutting structural and regulatory proteins in the nucleus and cytoplasm. Studies have shown that Caspase-3 is correlated with changes in mitochondrial transmembrane potential [7].
Caspase plays an important role in the apoptosis of a variety of mammalian species. At least 11 caspases have been identified. Among them, caspase-2, caspase-8, caspase-9, and caspase-10 are involved in the initiation of apoptosis. Caspase-3, caspase-6 and caspase-7 participate in the execution of apoptosis, of which caspase-3 and caspase-7 have similar substrate and inhibitor specificity. Caspase-3 and caspase-7 can degrade PARP and DFF-45, leading to the inhibition of DNA repairment and the initiation of DNA degradation. The caspase family proteins are probably the most important effector molecules that can start various biochemical processes and lead to the initiation of apoptosis. Therefore, the analysis of the caspase family protein activation is more effective than any other method to detect the early occurrence of apoptosis. Caspase-3 activation plays an important role in the initiation of cellular events during the apoptotic process, and there is evidence that Caspase-3 is the core during protease cascade cleavage. Caspase-3 isinvolved in many pathways of apoptosis. Caspase-3 normally exists in the form of zymogen in the cytoplasm, and is activated in the early stage of apoptosis [8]. Therefore, in the experiment, the occurrence of apoptosis was examined through the analysis of Caspase-3 activity.
Our study explored the inhibitory effect of Fraxetin on endometrial cancer cells. The results showed that Fraxetin activated caspase-3 in RL95-2 cells, confirming that Fraxetin can promote the apoptosis of endometrial cancer cells. The increase of caspase-9 activation indicated that Fraxetin induced apoptosis through mitochondria. JC-1 staining results demonstrated that Fraxetin lowered mitochondrial membrane potential and inhibited mitochondrial function in RL95-2 cells. Fraxetin promoted lactic acid production and glucose consumption. The results confirmed that the inhibition of endometrial cancer cell proliferation is through inhibiting mitochondrial function.
AMPK is widely present in eukaryotic cells and is a member of the serine/threonine protein kinase family. When the intracellular AMP/ATP ratio increases due to metabolic stress in vitro, AMPK undergoes phosphorylation and further activates its downstream target molecules, reducing the consumption of ATP, and increasing the generation of ATP, or catabolism. When the AMP/ATP ratio decreases, AMPK promotes anabolic metabolism. When the body is under sugar starvation, AMPK is activated. The activated AMPK can further activate the nodular sclerosis complex mutant gene and inhibit its target mammalian rapamycin target protein by TSC [9].
mTOR is an important regulator of insulin regulatory protein synthesis with multiple effects. The important function of mTOR is to sense changes in nutritional components in cells and to participate in the regulation of mRNA transcription initiation and protein translation. In an amino acid-rich environment, activated mTOR can regulate protein synthesis. Conversely, when cells sense amino acid deficiency or other stressors, mTOR activity is inhibited, resulting in slower protein synthesis to provide more ATP to combat stress. It can be seen that AMPK and mTOR react in the opposite direction for the same change of cell energy state. In fact, many studies have confirmed that the activation of AMPK can lead to the inhibition of mTOR [10].
Due to the correlation between metabolic ab0normalities and the occurrence of endometrial cancer, as well as the regulatory effects of Fraxetin on various aspects of metabolic abnormalities, we speculated that Fraxetin can treat endometrial cancer through regulating the metabolism of endometrial cancer cells [11]. Our results confirmed that, after Fraxetin intervention on RL95-2 cells, intracellular AMPK phosphorylation is increased, accompanied by mTOR function inhibition, indicating p-mTOR expression is reduced. This suggested that the mechanism of mitochondrial apoptosis induced by Fraxetin in tumor cells may be related to the specific energy metabolism of tumor cells.
In summary, Fraxetin can inhibit the proliferation of RL95-2 cells, promote apoptosis, inhibit the mitochondrial oxidation function of endometrial cancer cells, promote anaerobic metabolism, and exerts its effect on endometrial cancer cells by inhibiting the mitochondria.
Acknowledgements
This study was supported by the Capacity Improvement and Continuing Education Center of National Health Commission, No: GWJJ2019 100307.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Moor K, Brewer MA. Endometrial cancer: is this a new disease. Am Soc Clin Oncol Educ Book. 2017;37:435–442. doi: 10.1200/EDBK_175666. [DOI] [PubMed] [Google Scholar]
- 3.Hoang LN. Immunophenotypic features of dedifferentiated endometrial carcinoma insights from BRGI/INI1-deficient tumors. Histopathology. 2016;69:560–9. doi: 10.1111/his.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie DG. Keeping the home fires burning: AMP-activated protein kinase. J R Soc Interface. 2018;15 doi: 10.1098/rsif.2017.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Liu H, Liu J. Akt activation: a potential strategy to ameliorate insulin resistance. Diabetes Res Clin Pract. 2019;156:107092. doi: 10.1016/j.diabres.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg GR. Cellular energy sensing and metabolism-implications for treating diabetes: the 2017 outstanding scientific achievement award lecture. Diabetes. 2018;67:169–179. doi: 10.2337/dbi17-0039. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Liu Z, Li X. Shikonin causes apoptosis by disrupting intracellular calcium homeostasis and mitochondrial function in human hepatoma cells. Exp Ther Med. 2018;15:1484–1492. doi: 10.3892/etm.2017.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusaczuk M, Krętowski R, Naumowicz M. Silica nanoparticle-induced oxidative stress and mitochondrial damage is followed by activation of intrinsic apoptosis pathway in glioblastoma cells. Int J Nanomedicine. 2018;13:2279–2294. doi: 10.2147/IJN.S158393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoxhaj G, Hughes-Hallett J, Timson RC. The mTORC1 signaling network senses changes in cellular purine nucleotide levels. Cell Rep. 2017;21:1331–1346. doi: 10.1016/j.celrep.2017.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Troncone M, Cargnelli SM, Villani LA. Targeting metabolism and AMP-activated kinase with metformin to sensitize non-small cell lung cancer (NSCLC) to cytotoxic therapy: translational biology and rationale for currentclinical trials. Oncotarget. 2017;8:57733–57754. doi: 10.18632/oncotarget.17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Jing Z, Lv J. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed Pharmacother. 2017;95:18–24. doi: 10.1016/j.biopha.2017.08.045. [DOI] [PubMed] [Google Scholar]


