Abstract
Objective: To investigate the expressions of Formin-like 2 (FMNL2) and Cortactin (CTTN) in gallbladder adenocarcinoma (GBAC) and their associations with the clinicopathological characteristics of the patients. Methods: The expressions of FMNL2 and CTTN were detected with immunohistochemistry (Max Vision) in 105 GBAC tissues and 40 normal gallbladder tissues. Results: The positive expression rates of FMNL2 and CTTN in normal gallbladder tissues were 25% and 20%, different from the positive expression rates of 84.76% and 86.67% in GBAC tissues (P < 0.001). The positive expression rate of FMNL2 and CTTN in GBAC correlated with tumor differentiation, tumor-node-metastasis (TNM), lymph node metastasis (LNM), and distant metastasis. FMNL2 expression was positively correlated with CTTN expression. Kaplan-Meier analysis showed that the overall survival time of patients with positive expressions group of FMNL2 and CTTN was significantly shorter than that of the negative expression group. Cox multivariate analysis showed that TNM, LNM, distant metastasis, and positive expression of FMNL2 and CTTN were independent factors influencing the prognosis of patients with GBAC (P < 0.05). Conclusion: The positive expression of FMNL2 and CTTN in GBAC is significantly increased, which may be related to the occurrence and development of GBAC. The combined detection of FMNL2 and CTTN may provide a scientific theoretical basis for the early diagnosis of GBAC, the development of new antitumor drugs, and the search for new targets of biotherapy.
Keywords: Gallbladder adenocarcinoma (GBAC), Formin like 2 (FMNL2), Cortactin (CTTN), immunohistochemistry, prognosis
Introduction
Gallbladder carcinoma (GC) is the most common malignant - 0 tumor of biliary tract, ranking the fifth in digestive system cancer [1]. It is considered to be an aggressive and highly fatal disease. GBAC is the most common subtype of GC, accounting for 76-90% [2]. Only 30% of GC was suspected preoperatively or postoperatively. There are usually 70% found by accident in routine cholecystectomy specimens. There are many occasional GC surgeries that give the impression of benign disease, mainly gallstones and cholecystitis [3,4]. Because of its rarity, despite the progress of hepatobiliary imaging technology, most of them are in the late stage of diagnosis. In addition, local and/or distant recurrence is often a major problem due to the aggressiveness and poor prognosis of the disease course [5].
FMNL2 is a newly discovered transfer related gene, a member of the diaphanous-related formins [6,7], act as effectors of Rho family guanosine triphosphatases (GTPases) and play critical roles in carcinogenesis [8,9]. It has been reported that the high expression of FMNL2 can promote the growth and metastasis of colon cancer. However, reducing the expression of FMNL2 can promote the invasion and metastasis of hepatocellular carcinoma, leading to poor prognosis of patients. CTTN is located on chromosome 11q13 and is considered to be a promising molecular prognostic factor in various types of cancer, which is related to the invasiveness of various cancer entities [10,11]. The clinicopathological significance of CTTN overexpression has been studied in various types of cancer, such as head and neck cancer, colorectal cancer, gastric cancer, renal cell carcinoma, breast cancer, and osteosarcoma [12].
Although efforts are being made to determine the role and expression of FMNL2 and CTTN in the development of various types of cancer, no research has been carried out in GBAC. The purpose of this study was to detect the expression of FMNL2 and CTTN in 105 GBAC tissues by immunohistochemistry, and to analyze their relationship with the clinicopathological parameters of GBAC, so as to explore their possible roles in tumor tissue infiltration, metastasis, and prognosis evaluation of GBAC patients.
Materials and methods
Specimens
105 cases of GBAC tissues and 40 cases of normal gallbladder tissues were collected from the clinical pathology department of the First Affiliated Hospital of Bengbu Medical College from January 2013 to December 2014. All patients had complete clinicopathological and follow-up data. The shortest follow-up time was 5 months and the longest was 79 months. The patients were followed up until death or until August 2019. The diagnosis of GBAC was confirmed by the pathology department of our hospital. The clinicopathological data of patients with GBAC are shown in Table 1. In the control group, 40 cases of normal gallbladder tissues were all taken from the tumor more than 5.0 cm away from the gallbladder adenocarcinoma and confirmed as normal gallbladder tissues by H & E. staining in the pathology department.
Table 1.
Correlation of FMNL2 and CTTN expressions with clinicopathological characteristics of patients with GBAC
| Variable | FMNL2 | P | CTTN | P | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Negative | Positive | Negative | Positive | |||
| Gender | 0.070 | 0.968 | ||||
| Male | 9 | 29 | 5 | 33 | ||
| Female | 7 | 60 | 9 | 58 | ||
| Age (year) | 0.179 | 0.310 | ||||
| ≤ 60 | 3 | 32 | 3 | 32 | ||
| > 60 | 13 | 57 | 11 | 59 | ||
| Gross type | 0.739 | 0.321 | ||||
| Invasive | 12 | 60 | 12 | 60 | ||
| Ulcerative | 0 | 2 | 0 | 2 | ||
| Polypoid | 4 | 27 | 2 | 29 | ||
| Location | 0.572 | 0.618 | ||||
| Bottom | 6 | 42 | 8 | 40 | ||
| Body | 7 | 27 | 4 | 30 | ||
| Neck | 3 | 20 | 2 | 21 | ||
| Size | 0.476 | 0.215 | ||||
| D ≤ 3.0 cm | 8 | 53 | 6 | 55 | ||
| D > 3.0 cm | 8 | 36 | 8 | 36 | ||
| Depth of invasion | 0.933 | 0.707 | ||||
| Muscular layer | 3 | 15 | 2 | 16 | ||
| Serosa | 12 | 70 | 10 | 72 | ||
| Beyond serosa | 1 | 4 | 0 | 5 | ||
| TNM stage | < 0.001 | 0.005 | ||||
| I | 5 | 4 | 4 | 5 | ||
| II | 11 | 42 | 10 | 43 | ||
| III | 0 | 10 | 0 | 10 | ||
| IV | 0 | 33 | 1 | 32 | ||
| Differentiation | < 0.001 | 0.001 | ||||
| Well | 7 | 4 | 5 | 6 | ||
| Moderate | 8 | 55 | 9 | 54 | ||
| Poor | 1 | 30 | 0 | 31 | ||
| LNM | 0.048 | 0.041 | ||||
| Yes | 0 | 18 | 0 | 18 | ||
| No | 16 | 71 | 17 | 70 | ||
| Distant metastasis | 0.001 | 0.003 | ||||
| Yes | 0 | 37 | 0 | 37 | ||
| No | 16 | 52 | 14 | 54 | ||
| Gallstone | 0.915 | 0.339 | ||||
| Yes | 2 | 12 | 3 | 11 | ||
| No | 14 | 77 | 11 | 80 | ||
Reagents
Both Rabbit anti human FMNL2 polyclonal antibody (ab72105) and Rabbit anti human CTTN monoclonal antibody (ab81208) were purchased from Abcam Company of the United States. The max vision kit and DAB color development kit were purchased from Fuzhou Maixin biological company.
Methods
The paraffin embedded tissue was sliced continuously with a thickness of 4 μm. It was then baked and de-waxed in the corresponding solution until washed. The procedure of immunohistochemistry was carried out according to the instructions of the kit. The known positive tablets were used as the control and PBS solution as the blank control.
Result determination
The positive expression of FMNL2 and CTTN in GBAC tissues was brown granules, which were localized in the cytoplasm. The results of immunohistochemistry were determined by the method of second score [13], if the score ≥ 3, it was positive, and < 3, it was negative. The results of immunohistochemical staining were determined by two senior pathologists through independent double-blind method. Each section was observed under high-power microscope (400×) for at least 10 non repetitive visual fields.
Statistical analysis
Spss25.0 was used for statistical analysis. Kaplan-Meier method was used for survival analysis of FMNL2 and CTTN protein expression positive and negative groups. The log-rank test was used for comparison between groups. Cox multivariate regression model was used for multivariate analysis. The correlation between the expression of FMNL2 and CTTN and the normal gallbladder tissues and the clinicopathological parameters was examined by χ2 and Spearman rank correlation test, P < 0.05 was statistically significant.
Results
Expression of FMNL2 in GBAC and its relationship with clinicopathological parameters
The positive rate of FMNL2 was 25% (10/40) in the normal gallbladder tissues group and 84.76% (89/105) in the GBAC tissues group. The difference between the two groups was statistically significant (P < 0.001, Figure 1A, 1B). In the GBAC tissues, with the increase of the positive expression rate of FMNL2, the higher the TNM, the worse the differentiation, the easier the LNM and the more likely the distant metastasis occurred (P < 0.05). The positive expression of FMNL2 was not related to gender, age, tumor size, tumor gross type, tumor location, tumor invasion depth, and gallstone of patients with GBAC (P > 0.05, Table 1).
Figure 1.
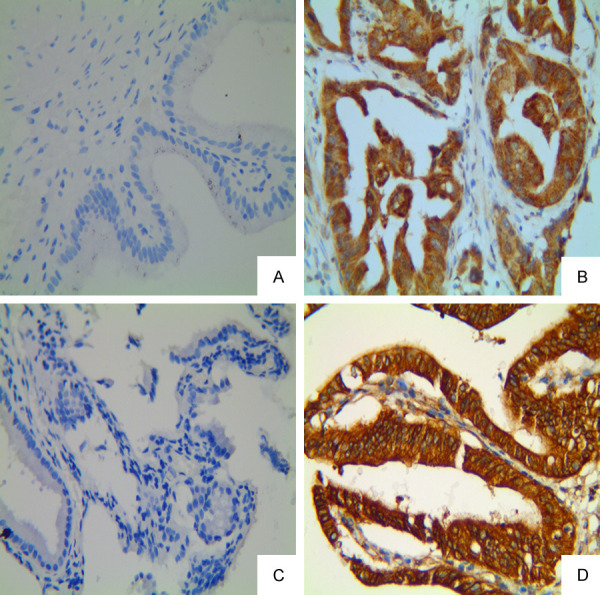
Expression of FMNL2 and CTTN in GBAC and normal gallbladder tissue (Max Vision, original magnification: ×400). A, B: Negative staining of FMNL2 in the normal gallbladder tissue and positive staining in the cytoplasm of GBAC, respectively; C, D: Negative staining of CTTN in the normal gallbladder tissue specimen and positive staining in the cytoplasm of GBAC, respectively.
Expression of CTTN in GBAC and its relationship with clinicopathological parameters
The positive expression rate of CTTN in the normal gallbladder group and the GBAC group was 20% (8/40) and 86.67% (91/105) (Figure 1C, 1D). The difference between the two groups was statistically significant (P < 0.001). With the worse differentiation of GBAC, the higher the positive expression rate of CTTN, the difference was statistically significant (P < 0.05). The positive rate of CTTN was related to LNM and distant metastasis (P < 0.05). The positive expression rate was 55.56% (5/9), 81.13% (43/10), 100% (10/10), and 96.97 (32/33) in I, II, IV, and IV stage respectively (Table 1). The difference between the four groups was statistically significant (P < 0.05). In addition, there was no significant difference in CTTN expression rate among different age, gender, tumor size, tumor gross type, tumor location, tumor invasion depth, and gallstone.
Relationship of FMNL2 and CTTN expressions in GBAC
Spearman correlation analysis showed that there was a positive correlation between the expression of FMNL2 and CTTN in GBAC (r=0.535, P < 0.001, Table 2).
Table 2.
Relationship of FMNL2 and CTTN expressions in GBAC
| Variable | FMNL2 expression | p | |
|---|---|---|---|
|
| |||
| Negative | Positive | ||
| CTTN | < 0.001@ | ||
| Negative | 9 | 5 | |
| Positive | 7 | 84 | |
Positive correlation.
Cox multivariate analysis
The age, gender, tumor size, tumor location, tumor gross type, invasion depth, differentiation degree, TNM, LNM, distant metastasis, FMNL2, and CTTN expression of patients with GBAC were grouped. The parameters were introduced into Cox multivariate model for analysis. Analysis results showed that: TNM, LNM, distant metastasis, and positive expression of FMNL2 and CTTN were independent factors affecting the prognosis of patients with GBAC (P < 0.05) (Table 3).
Table 3.
Multivariate survival analysis of 105 patients with GBAC
| Covariate | B | SE | sig | Exp (B) | 95% CI |
|---|---|---|---|---|---|
| FMNL2 | 1.614 | 0.516 | 0.002 | 5.022 | 1.827-13.801 |
| CTTN | 1.411 | 0.515 | 0.006 | 4.100 | 1.493-11.259 |
| TNM | 1.409 | 0.350 | < 0.001 | 4.092 | 2.062-8.123 |
| LNM | 0.698 | 0.243 | 0.004 | 2.010 | 1.247-3.240 |
| STI | 1.181 | 0.242 | < 0.001 | 3.259 | 2.028-5.236 |
Survival analysis
The overall 5-year survival rate was 29.5% (31/105). Kaplan-Meier survival analysis showed that the overall survival time of FMNL2 positive expression group was significantly lower than that of its negative expression group (P < 0.05, Figure 2A). The overall survival time of CTTN positive expression group was significantly shorter than that of its negative expression group (P < 0.05, Figure 2B). The overall survival time of patients with TNM stage (II, IV) was significantly lower than that of patients with TNM stage (I, II), the difference was statistically significant (P < 0.001, Figure 2C). The overall survival time of patients with LNM was significantly lower than that of patients without LNM (P < 0.05, Figure 2D). The overall survival time of patients with distant metastasis was significantly lower than that of patients without distant metastasis (P < 0.001, Figure 2E).
Figure 2.
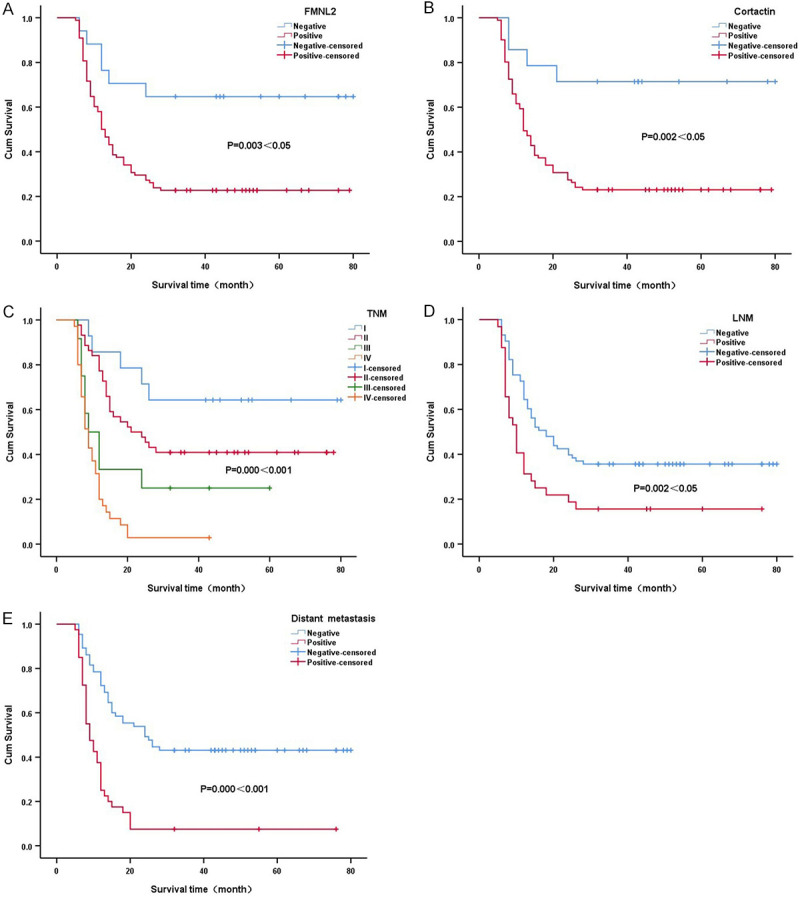
Survival curves of GBAC patients with positive or negative expression of FMNL2 (A), CTTN (B), TNM (C), LNM (D) and Distant metastasis (E).
Discussion
FMNL2, located on chromosome 2q23.3, is the most effective actin nucleating factor [14]. FMNL2 has actin binding domains that affect actin polymerization and actin filament formation [15]. It serves as an upstream modulator and downstream effector of Rho family GTPases. FMNL2, as a new target of colorectal cancer invasion and metastasis [16], is a positive regulatory factor in colorectal cancer metastasis [17]. It has been reported that the abnormal expression of FMNL2 is closely related to the invasion, metastasis, and prognosis of various tumors [18,19]. In this study, the expression of FMNL2 tissues in GBAC and normal gallbladder tissues was detected by immunohistochemistry. It was found that the positive expression rate of FMNL2 in GBAC tissues (84.76%) was significantly higher than that in normal gallbladder tissues (25%). The difference was statistically significant. Further analysis showed that the worse the differentiation of GBAC, the higher the positive rate of FMNL2; the higher the positive rate of FMNL2 in LNM group and distant metastasis group; and the higher the TNM stage, the higher the positive rate, the difference has statistical significance. Kaplan-Meier survival analysis showed that the survival time of patients with FMNL2 positive expression group was significantly lower than that of patients with negative expression group. The above results showed that the abnormal increase of FMNL2 expression was involved in the progression, invasion, and metastasis of GBAC, which meant that the prognosis of patients was poor, consistent with the literature [8,9,18-20].
CTTN was found in Thomas Parson’s laboratory in 1993. It was identified as the main substrate of v-src kinase. It is a kind of protein that can gather in subcellular cortex and bind to filamentous actin. Therefore, CTTN is also known as cortical actin binding protein [21,22]. Previous studies have confirmed that CTTN is the main functional protein of invasive pseudopodia of tumor cells [23], which regulates cytoskeleton mainly through cell movement, adhesion, polarization, contraction, etc, and promotes tumor cell invasion and metastasis [24]. CTTN overexpression was significantly associated with various indicators of poor prognosis, including high TNM stage, LNM, increased recurrence rate, and decreased overall survival rate in cancer patients [12]. In this study, the positive expression rate of CTTN in the normal gallbladder tissues group was 20%, while that in the GBAC group was 86.67%. The difference between the two groups was statistically significant. Further analysis showed that with the increase of CTTN positive expression rate, the higher the stage of pTNM, the worse the differentiation, the more prone to LNM and distant metastasis. Survival analysis also showed that the survival time of CTTN positive expression group was significantly lower than that of its negative expression group. These results indicate that the increased expression of CTTN may be involved in the occurrence, development, invasion, and metastasis of GBAC, and the increased expression may mean poor prognosis [10-12,24].
It has been reported that in colorectal cancer, CTTN is directly combined with FMNL2 to improve the activity of actin polymerization and recovery of endogenous motility. The interaction of CTTN and FMNL2 can further promote the formation of Chlamydia and matrix degradation. In vivo, the formation of invadopodia induced by CTTN is crucial to the ability of FMNL2 to promote colorectal cancer metastasis [25]. In this study, Spearman grade correlation analysis showed that FMNL2 and CTTN expression were positively correlated, suggesting that FMNL2 expression was closely related to CTTN expression in GBAC.
Conclusions
In conclusion, the abnormal expression of FMNL2 and CTTN promotes the occurrence and malignant progression of GBAC. Therefore, early combined detection of FMNL2 and CTTN expression can be used as a predictor of invasion, metastasis, and prognosis in patients with GBAC.
Acknowledgements
This study was supported by the Bengbu Medical College Graduate Research Innovation Program (Byycxz1926). We would like to thank the patients for agreeing to our report and for providing a detailed medical history. The source and publication of the information have been approved by the patients.
Disclosure of conflict of interest
None.
Abbreviations
- GC
Gallbladder carcinoma
- GBAC
Gallbladder adenocarcinoma
- FMNL2
Formin-like 2
- CTTN
Cortactin
References
- 1.Utsumi M, Aoki H, Kunitomo T, Mushiake Y, Yasuhara I, Arata T, Katsuda K, Tanakaya K, Takeuchi H. Evaluation of surgical treatment for incidental gallbladder carcinoma diagnosed during or after laparoscopic cholecystectomy: single center results. BMC Res Notes. 2017;10:56. doi: 10.1186/s13104-017-2387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Li Y, Jiang W, Yan J, Dai J, Jiao B, Yin Z, Zhang Y. Simple cholecystectomy is adequate for patients with T1b gallbladder adenocarcinoma < 1 cm in diameter. Front Oncol. 2019;9:409. doi: 10.3389/fonc.2019.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelbaum R, Alvarado FJ, Blackham AU, Brodsky J. A case of acute pancreatitis associated with early-stage adenocarcinoma of the gallbladder. Am J Case Rep. 2019;20:957–960. doi: 10.12659/AJCR.915543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geramizadeh B, Kashkooe A. Incidental gall bladder adenocarcinoma in cholecystectomy specimens; a single center experience and review of the literature. Middle East J Dig Dis. 2018;10:249–253. doi: 10.15171/mejdd.2018.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim TH, Woo SM, Lee WJ, Oh ES, Youn SH, Moon SH, Kim SS, Han SS, Park SJ, Kim DY. Benefit of adjuvant chemoradiotherapy in resected gallbladder carcinoma. Sci Rep. 2019;9:11770. doi: 10.1038/s41598-019-48099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katoh M, Katoh M. Identification and characterization of human FMNL1, FMNL2 and FMNL3 genes in silico. Int J Oncol. 2003;22:1161–1168. [PubMed] [Google Scholar]
- 7.Gardberg M, Talvinen K, Kaipio K, Iljin K, Kampf C, Uhlen M, Carpen O. Characterization of Diaphanous-related formin FMNL2 in human tissues. BMC Cell Biol. 2010;11:55. doi: 10.1186/1471-2121-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Y, Xie H, Qiao Y, Wang J, Zhu X, He G, Li Y, Ren X, Wang F, Liang L, Ding Y. Formin-like 2 regulates Rho/ROCK pathway to promote actin assembly and cell invasion of colorectal cancer. Cancer Sci. 2015;106:1385–1393. doi: 10.1111/cas.12768. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Li Y, Zhu X, Zeng Y, Wang J, Zhang X, Ding YQ, Liang L. FMNL2 enhances invasion of colorectal carcinoma by inducing epithelial-mesenchymal transition. Mol Cancer Res. 2010;8:1579–1590. doi: 10.1158/1541-7786.MCR-10-0081. [DOI] [PubMed] [Google Scholar]
- 10.Horn D, Gross M, Dyckhoff G, Fuchs J, Grabe N, Weichert W, Herpel E, Herold-Mende C, Lichter P, Hoffmann J, Hess J, Freier K. Cortactin expression: association with disease progression and survival in oral squamous cell carcinoma. Head Neck. 2018;40:2685–2694. doi: 10.1002/hed.25515. [DOI] [PubMed] [Google Scholar]
- 11.Zhang LH, Tian B, Diao LR, Xiong YY, Tian SF, Zhang BH, Li WM, Ren H, Li Y, Ji JF. Dominant expression of 85-kDa form of cortactin in colorectal cancer. J Cancer Res Clin Oncol. 2006;132:113–120. doi: 10.1007/s00432-005-0046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu L, Cho E, Zhao G, Roh MR, Zheng Z. The pathogenic effect of cortactin tyrosine phosphorylation in cutaneous squamous cell carcinoma. In Vivo. 2019;33:393–400. doi: 10.21873/invivo.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu S, Yu L, Wang D, Zhou L, Cheng Z, Chai D, Ma L, Tao Y. Aberrant expression of CD133 in non-small cell lung cancer and its relationship to vasculogenic mimicry. BMC Cancer. 2012;12:535. doi: 10.1186/1471-2407-12-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhn S, Erdmann C, Kage F, Block J, Schwenkmezger L, Steffen A, Rottner K, Geyer M. The structure of FMNL2-Cdc42 yields insights into the mechanism of lamellipodia and filopodia formation. Nat Commun. 2015;6:7088. doi: 10.1038/ncomms8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghorbanpour E, Pasalar P, Yazdani S, Moazzeni H, Elahi E. FMNL2 with functions related to the cytoskeleton is partially regulated by PAX6. J Ophthalmic Vis Res. 2017;12:407–412. doi: 10.4103/jovr.jovr_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peladeau C, Heibein A, Maltez MT, Copeland SJ, Copeland JW. A specific FMNL2 isoform is up-regulated in invasive cells. BMC Cell Biol. 2016;17:32. doi: 10.1186/s12860-016-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang SS, Li XM, Yang M, Ren XL, Hu JL, Zhu XH, Wang FF, Zeng ZC, Li JY, Cheng ZQ, Liao WT, Ding YQ, Guan J, Liang L. FMNL2 destabilises COMMD10 to activate NF-kappaB pathway in invasion and metastasis of colorectal cancer. Br J Cancer. 2017;117:1164–1175. doi: 10.1038/bjc.2017.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardberg M, Heuser VD, Koskivuo I, Koivisto M, Carpen O. FMNL2/FMNL3 formins are linked with oncogenic pathways and predict melanoma outcome. J Pathol Clin Res. 2016;2:41–52. doi: 10.1002/cjp2.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Arjonen A, Pouwels J, Ta H, Pausch P, Bange G, Engel U, Pan X, Fackler OT, Ivaska J, Grosse R. Formin-like 2 promotes beta1-integrin trafficking and invasive motility downstream of PKCalpha. Dev Cell. 2015;34:475–483. doi: 10.1016/j.devcel.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Zhong B, Wang K, Xu H, Kong F. Silencing Formin-like 2 inhibits growth and metastasis of gastric cancer cells through suppressing internalization of integrins. Cancer Cell Int. 2018;18:79. doi: 10.1186/s12935-018-0576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weaver AM. Cortactin in tumor invasiveness. Cancer Lett. 2008;265:157–166. doi: 10.1016/j.canlet.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stock K, Borrink R, Mikesch JH, Hansmeier A, Rehkamper J, Trautmann M, Wardelmann E, Hartmann W, Sperveslage J, Steinestel K. Overexpression and Tyr421-phosphorylation of cortactin is induced by three-dimensional spheroid culturing and contributes to migration and invasion of pancreatic ductal adenocarcinoma (PDAC) cells. Cancer Cell Int. 2019;19:77. doi: 10.1186/s12935-019-0798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng DF, Xie P, Peng LX, Sun R, Luo DH, Chen QY, Lv X, Wang L, Chen MY, Mai HQ, Guo L, Guo X, Zheng LS, Cao L, Yang JP, Wang MY, Mei Y, Qiang YY, Zhang ZM, Yun JP, Huang BJ, Qian CN. Erratum to: CDC42-interacting protein 4 promotes metastasis of nasopharyngeal carcinoma by mediating invadopodia formation and activating EGFR signaling. J Exp Clin Cancer Res. 2017;36:33. doi: 10.1186/s13046-016-0483-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkbride KC, Sung BH, Sinha S, Weaver AM. Cortactin: a multifunctional regulator of cellular invasiveness. Cell Adh Migr. 2011;5:187–198. doi: 10.4161/cam.5.2.14773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ren XL, Qiao YD, Li JY, Li XM, Zhang D, Zhang XJ, Zhu XH, Zhou WJ, Shi J, Wang W, Liao WT, Ding YQ, Liang L. Cortactin recruits FMNL2 to promote actin polymerization and endosome motility in invadopodia formation. Cancer Lett. 2018;419:245–256. doi: 10.1016/j.canlet.2018.01.023. [DOI] [PubMed] [Google Scholar]


