Abstract
Objective: To explore the involvement of epithelial-mesenchymal transition (EMT) and cancer stem cell (CSC) characteristics induced by Fusobacterium nucleatum (Fn) in colorectal cancer (CRC) in vitro. Methods: SW480 and HCT116 cells were co-cultivated with Fn. Western blot (WB) and real-time PCR were used for detecting EMT markers’ expression. CSC-resembling phenotypes were observed through migration, intrusion, and spherical colony formation assays. Flow cytometry was employed for sorting, based on the expression of CD44. Results: It was displayed that Fn infection was responsible for an EMT phenotype associated with an increase in mesenchymal markers (Snail1, Vimentin, and ZEB1) as well as CD44 expression. Fn treatment induced stronger expressions of such markers when MOI increased. Furthermore, infection resulted in augmented migration, intrusion, and tumorsphere formation capacities. Cell classification implicated that mere CD44high cells exhibited CSC characteristics and mesenchymal phenotype (MP) in vitro, accompanied with augmented tumor-causing capacity over CD44low cells. Finally, we demonstrated IL-6/STAT3 pathway was involved in EMT-CSC-resembling behavior of CRC cells. Conclusion: All of these data suggest that Fn reveals CSC-resembling characteristics through activating IL-6/STAT3 and eliciting EMT-resembling variations in colorectal epithelial cells (CECs).
Keywords: Fusobacterium nucleatum (Fn), colorectal cancer (CRC), epithelial-mesenchymal transition (EMT), cancer stem cell (CSC)
Introduction
CRC is the 3rd common malignancies and the 4th prevalent causative factor of cancer-related death worldwide. Five-year survival rate almost reaches 65% [1]. CRC is a complicated disease affected by genetic and environmental factors. The majority of CRC cases are intermittent. Many studies have implied that intestinal microbes assume an essential part in CRC progression [2,3].
More than one thousand diverse microbes suppress human gastrointestinal tract with high heterogeneity [4]. The gut microbes (GMs) are important to normal physical processes at homeostasis state, but likewise are connected with many diseases such as CRC and obesity [5-7]. The bacteria density in the large intestine (approximately 1012 cells/ml) is much larger than that in the small intestine (approximately 102 cells/ml). More and more evidence confirmed that GMs are relevant to CRC, which were characterized by decreased probiotics and opportunistic pathogen growth, like Campylobacter, E.coli, Enterococcus, Anaerotruncus, and Collinsella, displaying these bacteria’s latent part in CRC progression [8,9]. Further, Fn abundance in CRC was dramatically higher against that in the control group [10]. Other studies likewise demonstrated that Fn, together with several Gram-negative bacteria, such as Streptococcus and Campylobacter spp., promoted CRC occurrence [11,12].
Fn, an opportunistic symbiotic anaerobe, participated in diverse periodontitis. Furthermore, it is a particularly common extra oral infection type. More and more evidence displayed that Fn levels in tumor tissues and stool samples from CRC patients are obviously increased against those in normal controls [13]. Kostic et al. reported that Fn in Apc (Min/+) mice accelerated CRC occurrence [14]. Rubinstein et al. confirmed that Fn triggered tumor cells in CRC to grow through acting on β-catenin signaling and elicited oncogene expression through FadA adhesion virulence factor (VF) [15]. Collectively, those studies showed that Fn assumes a crucial part in initiating CRC and accelerating tumor cells’ growth, which confirmed that Fn is a causative factor instead of a outcome of CRC.
Recently, EMT has attracted much attention concerning metastatic dissemination. EMT is considered as an early event in metastasis, which participates in tumor cells’ migration and intrusion [16]. The latest evidence likewise displays that cells which receive EMT exhibit stem cell-resembling characteristics [17,18]. Particularly, Mani et al. indicated that EMT suppression in breast epithelial cells (BECs) produced a CD44+/CD24- cell subpopulation with breast CSCs-resembling phenotype and characteristics [17]. CSCs possess a capacity to induce tumor and retain tumor self-renewal. Various cell surface markers have already been depicted and characterized in CSCs among varied cancers. It’s reported that CD44 was a CSCs marker of some solid tumors, which are not confined to head and neck, breast and pancreas cancers [19]. As for CRC, CD44 has likewise been confirmed to be a classic marker, while the part performed by Fn in CSC occurrence remained to be investigated [20]. Hence, the study was directed toward delving into it in EMT and colorectal CSCs occurrence.
Materials and methods
Bacterial strains and culture conditions
Fusobacterium nucleatum ATCC25586 was purchased from ATCC (Manassas, VA, USA). Fn culture and co-culture assays were conducted as depicted before [21]. The number of Fn was quantified as described by Gendron et al. [22]. Fn was grown in BHI broth for 48 h. Before incubation with eukaryotic cells, BHI broth was removed by low-speed centrifugation and replaced with suitable antibiotic-free medium. Co-cultures were conducted at MOI of 10, 100, 500, respectively for 24 h in a damp 5% CO2 condition at 37°C prior to analysis.
CRC cell culture
The colon cancer epithelial cell lines SW-480 and HCT116 were grown at 37°C and 5% CO2 in the appropriate medium [23,24].
Flow cytometry (FC) analysis
Cells returned to the original state and were subjected to staining with CD44-APC antibody (1:25) (105 cells per condition) in PBS, BSA (0.5%), and EDTA (2 mmol/L). FC was conducted through DIVA and FACScan software. Cells were subjected to dual CD44 and DAPI staining (exclusive of positive dead cells), and classified for their CD44 expression levels indicated on flow cytometer.
Migration and intrusion assays
Cells returned to the normal state and were put in the upper side of Transwell insert in 24-well plates (8-mm) (5×104 cells per condition) with medium added FBS (5%). In intrusion assay, inserts were pre-covered with COL I (50 ng/ml) at 37°C for 40 min. The inserts were cultivated at 37°C for 18 h, followed by fixation in cold methanol and hematoxylin staining, as depicted before [25]. Cells passing through inserts’ lower side were quantified in 5 distinct randomly selected regions of each insert via light microscopy.
Spherical colony formation
Cells returned to the original state and were put in 96-well plates without adhesion (covered with polyHEMA solution (10%) in anhydrous ethanol and dried at 56°C overnight) (500 cells), followed by culture at 37°C for 5 d in a non-serum medium comprised of DMEM-F12 Glutamax added glucose (0.3%), N2-added 100× (1:100), EGF (0.02 mg/ml), basic-FGF (0.01 mg/ml), amphotericin B (2.5 mg/ml), gentamicin (5 mg/ml), as well as penicillin (50 IU/m). The density of spheroids was calculated.
RNA isolation and qRT-PCR
Total RNAs were isolated with Trizol and quantified by their A260. 1 µg of total RNAs was retro-transcribed through Quantification RT kit as the guidance’s provided by manufacturer. qPCR was conducted through StepOne plus real-time PCR instruments and specific primers at 0.3 µM. All used primers were obtained from Sigma. The operating procedures were summarized below: denaturation at 95°C initially for 10 min then for 60 s, annealing at 60°C for 20 s, and extension at 56°C for 1 min.
Western blotting
Western blotting was carried out essentially as described [25]. Proteins were resolved typically by either 12.5% acrylamide or gradient acrylamide (4-15%) SDS-PAGE gels. Proteins were transferred to a PVDF membrane through a semi-dry blotting apparatus. The blots were blocked with 5% dry milk without fat or 3% BSA, in PBS, followed by probing of the membrane with the appropriate primary antibody at the recommended dilution, which then was incubated overnight at 4°C. Blots were washed several times with PBS containing 0.05% v/v Tween-20, subsequently cultivated with appropriate secondary antibody at the recommended dilution for 1 h at room temperature. After washing, antibody reactivity was visualized using enhanced chemiluminescence (Thermo ECL, Shanghai, China). Briefly, PVDF membrane were immersed for 1 min in 50 ml of Tris buffer (50 mM, pH8.8) containing luminol (1.25 mM), iodophenol (400 µM), and hydrogen peroxide (0.01%, v/v). Finally the blot was exposed to Tanon 5200 (Tanon, Shanghai, China) for various periods of time to capture the image.
Statistical analysis
Statistical analyses were carried out through 18.0 SPSS. Pearson’s chi-square test was used for comparing categorical variables, while student’s t-test was applied to evaluate the difference in continuous variables between the groups. All values are expressed as the mean ± standard deviation (SD) of the mean. Two-sided p-values were calculated for all tests and a p-value of less than 0.05 was considered statistically significant.
Results
Infecting colon epithelial cells with Fn produces cells with mesenchymal stem cell (MSC) characteristics through epithelial-mesenchymal-resembling transition
To confirm if Fn infection was able to produce cells with CSC characteristics through epithelial-mesenchymal-resembling transition, co-culture assays with colon cancer epithelial cells were conducted and demonstrated on SW-480 and HCT116. As expected, Fn infection (at MOI of 100:1 and 500:1) elicited morphological variations, featuring polygon loss, cell cluster disruption, and the emergence of lathy cells with membrane ruffles, displaying a MP.
qPCR displayed a marked increase in mesenchymal markers, together with CD44 in SW-480 and HCT116 cell lines. Cells treated with Fn at MOI of 500:1 induced stronger Snail1, Vimentin, ZEB1, and CD44 expression when compared with the treatment with MOI of 10:1 and 100:1 (P<0.05, Figure 1A-D).
Figure 1.
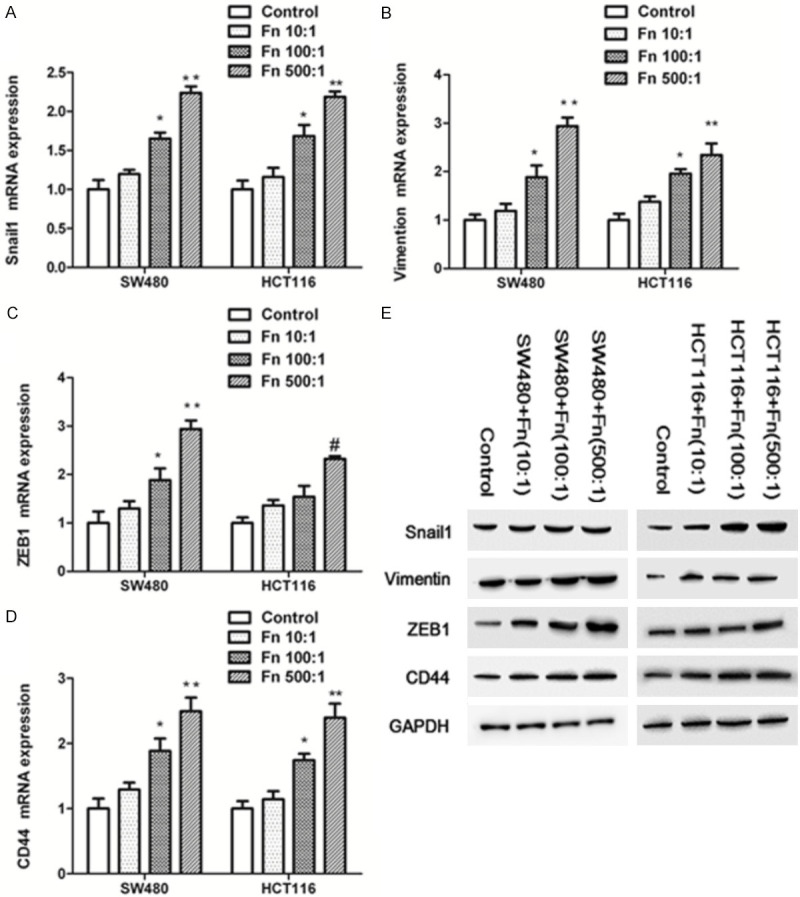
Fn Infection triggers an EMT-resembling phenotype and CD44 expression in SW480 and HCT116 cells. Those cells were exposed to Fn (MOI 10:1, 100:1 and 500:1) for 24 h. A-D. Relative expression levels of mRNA encoding mesenchymal markers and CD44 in infected cells determined by RT-qPCR (n=3 respectively, *P<0.05 vs Fn (MOI 10:1 and 500:1) and control groups, ** P<0.01 vs Fn (MOI 10:1) and control groups, # P<0.05 vs Fn (MOI 10:1) and control groups). E. WB was conducted to examine the expression levels of snail1, vimentin, ZEB1 and CD44 in SW480 and HCT116 cells.
To further examine the effect of Fn on colonic epithelial cells, western blotting analyses were performed and revealed an increase expression of Snail1, Vimentin, ZEB1, and CD44. Also, cell treatment with Fn at an MOI of 500:1 induced stronger expression of those markers when compared with the treatment with MOI of 10:1 and 100:1 (Figure 1E).
Cells infected by Fn possess CSC characteristics
CSCs express specific markers and feature self-renewal and tumor-producing capacities as well as augmented migration and intrusion potency. The tumor sphere assay is one of the most frequently used and is ideal to identify CSC characteristics.
The spheroid-forming capacity of colonic cells was investigated in vitro in culture conditions without adhesion. SW-480 and HCT116 belong to tumor cells. While they do not exhibit obvious tumor-causing characteristics in basic conditions, they have a limited capacity of naturally forming tumors following culture. However, Fn infection resulted in the marked elevation of in spheroid formation, with similar results obtained in SW-480 and HT29 cell lines. Fn at MOI of 500:1 induced stronger ability to form tumorspheres when compared with the treatment with MOI of 10:1 and 100:1 (P<0.05, Figure 2A).
Figure 2.
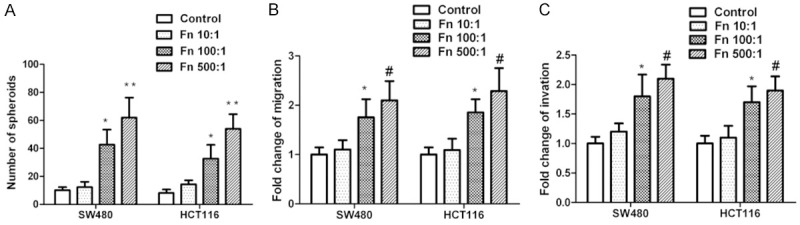
Fn infection elicits migration, intrusion, and tumorsphere formation. SW480 and HCT116 cells were exposed to Fn for 24 h at MOI of 10:1, 100:1 and 500:1, respectively, followed by recovery and inoculation in culture without adhesion for spheroid formation (A) or used for migration and intrusion assays (B, C). (A) Spheroids’ absolute number quantification 5 d later. (B) Migration quantification 18 h later. (C) Intrusion quantification 18 h later. (n=3 respectively, * P<0.05 vs Fn (MOI 10:1 and 500:1) and control groups, # P<0.05 vs Fn (MOI 10:1) and control groups).
Experiments were conducted to assess migration and intrusion characteristics. Fn infection significantly stimulated migration and intrusion characteristics compared to the basic levels indicated in control. Also, cell treatment with Fn at MOI of 500:1 induced stronger invasive behavior (P<0.05, Figure 2B, 2C).
CD44high cells isolated from Fn-infected cells exhibit MSC characteristics
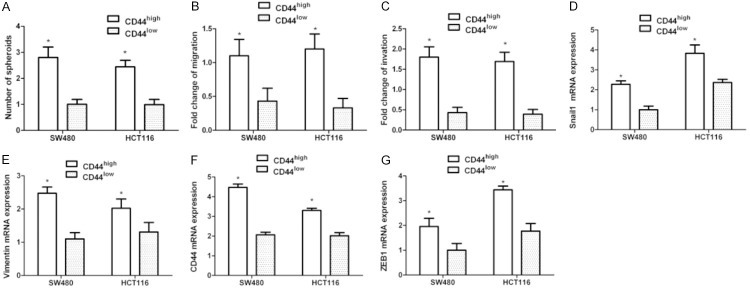
Cell classification via FC was conducted on Fn-infected SW-480 and HCT116 cells at MOI of 500:1 to disassociate CD44high and CD44low cells.
The CD44high cells formed more tumorspheres (Figure 3A) and displayed stronger migration and intrusion abilities over CD44low (Figure 3B, 3C). The same CD44high classified cells (Fn infected) had an increased mesenchymal marker and CD44 levels relative to the CD44low cells (Figure 3D-F). The findings collectively confirm that CD44high cells elicited by Fn infection displayed MSC characteristics.
Figure 3.
The CD44high cells produced by Fn infection shared EMT and CSC characteristics. SW480 and HCT116 cells were co-cultivated with Fn at MOI of 500:1 for 1 d, and then stained with DAPI, and CD44-APC antibodies, along with DAPI-negative/CD44high or CD44low cells were classified through FACS as depicted. A. Spheroids’ quantification following 5-day culture of classified cells in conditions without adhesion. B. Migration quantification 18 h later. C. Intrusion quantification 18 h later. D-G. Relative expression levels of mRNA encoding mesenchymal markers, CD44 in CD44high vs CD44low classified cells detected through RT-qPCR. Data were expressed as the average proportion of CD44high/CD44low cells. (n=4 respectively, *P<0.05 vs CD44low groups).
IL-6/STAT3 signal participated in Fn-induced EMT-CSC like changes
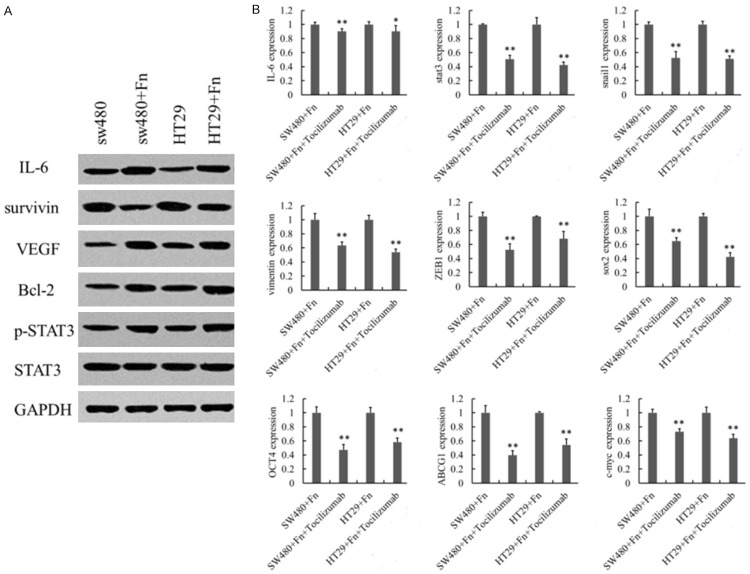
To determine the molecular mechanism on which Fn infection depended, western blot was employed to analyze IL-6/STAT3 pathway. The elevated levels of IL-6, pSTAT3, as well as downstream VEGF, survivin and Bcl-2 confirmed that Fn treatment could activate IL-6/STAT3 signaling pathway (Figure 4A). After blockage of IL-6/STAT3 signal, the levels of EMT markers Snail1, Vimentin, ZEB1, and CSC markers SOX2, OCT4, c-MYC, as well as ABCG2 decreased (Figure 4B), suggesting that IL-6/STAT3 signaling pathway can regulate EMT-like effect and CSC formation in colorectal cancer cells.
Figure 4.
Role of IL-6/STAT3 signal in Fn-induced EMT-CSC like changes of CRC cells. SW480 and HCT116 cells were treated with Fn only, or pre-treated with IL-6/STAT3 inhibitor for 24 h at MOI of 500:1. A. Expressions of IL-6, STAT3 and downstream target genes (VEGF, Survivin, and Bcl-2) after Fn infection. B. Expressions of EMT markers Snail1, Vimentin, ZEB1, and CSC markers sox2, OCT4, ABCG2, c-myc, after blocking IL-6/STAT3 signaling pathway. (n=3 respectively, *P<0.05 vs control, **P<0.01 vs control).
Discussion
Reportedly, high levels of Fn may be connected to the poor outcomes of CRC. Li et al. showed that lymph node metastases lie in 52/88 subjects with a high Fn abundance and in 0/13 with a low Fn abundance, indicating the Fn enrichment is relevant to CRC progression and metastasis [26]. Furthermore, several studies have found that Fn DNA load is related to higher cancer-relevant mortality and Fn DNA was possibly a latent unfavorable prognosis indicator [27]. Fn was accumulated in microbiota that could stick to and intrude human epithelial and endothelial cells [28]. Nowadays, a couple of studies have displayed that Fn is a nosogenic bacterium which enhances CRC occurrence [15,29]. Some studies displayed that its VF had a close correlation with colorectal lesions. Fn has been confirmed to be able to intrude human epithelial cells, act on β-catenin signaling, elicit oncogene expression and accelerate CRC cells’ growth [29]. Another VF, an auto transporter protein, Fap2, could enhance CRC progression through suppressing the activities of immune cells [30]. However, numerous diverse risk factors existed in CRC and the relationship of Fn and CRC remains to be investigated. Since EMT is a stage that precedes carcinogenesis, it was of great interest to examine the ability of Fn to influence EMT to drive inflammation and malignant cell transformation that enables neoplastic progression. The work was aimed at exploring the carcinogenic potential of Fn to induce EMT-like behavior and the emergence of colorectal CSCs.
EMT has gained much attention concerning metastatic dissemination. It is considered an early event in metastasis, which participates in tumor cells’ migration and intrusion. It is associated with the modification of gene expression involved in inhibiting epithelial phenotype (EP) and activating MP [31]. In addition, EMT induces cell proliferation that mimics the progression of invasive CRC, which correlates with CRC progression in humans [32]. Such processes can be observed in other cancer including gastric cancer, breast cancer, and cholangiocarcinoma [33-35]. Also, deregulation of tumor suppressor, epigenetic reprograming, and the activation of CSC associated pathways elicited by bacteria, may be the causative factor of cancer occurrence and progression [36]. Lately, Fn has been reported to be able to elicit a EMT-resembling process, our principal objective was to investigate if Fn participated in CSC occurrence through EMT [37]. In consequence, CRC cells naturally tend to cover EMT. We found that Fn-infected SW480 and HCT116 cells were diverse and consisted of cells that preserved a polygonal phenotype and harbored ‘hummingbird’ phenotype. Fn infection triggered a notable improvement in mesenchymal markers, as previously stated [37]. The findings demonstrated that Fn infection influences epithelial differentiation.
A close link between CSCs and EMT-relevant gene expression has been confirmed recently. EMT transcriptional mediators snail or twist in BECs exhibited a stem cell-relevant CD44+/CD24- expression way and a 30-fold augmented sphere formation against control cells [17]. CD44, a cell adhesion molecule, can accomplish cell-ECM and cell-cell interactions by connecting with corresponding major ligand, hyaluronic acid. CD44+ CRC cells possess stronger colony-forming and tumor-causing capacities relative to CD44- cells. Further, mere CD44+, while not confined to CD44- CRC cells can keep the morphological and phenotypic features of tumor lesions where they originated after serial transplantations [38]. CD44 has already been identified as CSCs markers of colon cancers. It is likewise seductive that Fn-infected cells have a pretty high CD44 expression. Also, cells infected by Fn possessed CSCs characteristics and shared characteristics of high tumorigenic and migration or intrusion. Furthermore, the findings indicated that the CD44high cell subpopulation triggered by Fn infection exhibited CSC-resembling characteristics regarding phenotype along with migration, intrusion, and tumor-forming capacities against their CD44low counterparts. Seductively, the identical CD44high infected cells displayed increased mesenchymal marker levels than the CD44low cells. At last, we suggested IL-6/STAT3 pathway may be implicated in EMT behavior as well as CSC-resembling changes of CRC cells. Fn probably assumes a part in EMT-CSC cross talk in CRC progression.
In brief, we first reported that Fn infection can trigger EMT-resembling actions and the appearance of CD44high cells with CSC characteristics via IL-6/STAT3 signal. These findings, together with additional literatures, enhance the knowledge of the cellular mechanisms concerning cancer-causing behaviors in the course of Fn infection.
Acknowledgements
This work was supported by Natural Science Foundation of Jiangsu Province (BK20151017), Six Talent Peaks Project in Jiangsu Province (WSW-050), and National Natural Science Foundation of China (81502052).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jobin C. Colorectal cancer: looking for answers in the microbiota. Cancer Discov. 2013;3:384–387. doi: 10.1158/2159-8290.CD-13-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobhani I, Tap J, Roudot-Thoraval F, Roperch JP, Letulle S, Langella P, Corthier G, Tran Van Nhieu J, Furet JP. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6:e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobhani I, Amiot A, Le Baleur Y, Levy M, Auriault ML, Van Nhieu JT, Delchier JC. Microbial dysbiosis and colon carcinogenesis: could colon cancer be considered a bacteria-related disease? Therap Adv Gastroenterol. 2013;6:215–229. doi: 10.1177/1756283X12473674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–1119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hold GL, Smith M, Grange C, Watt ER, El-Omar EM, Mukhopadhya I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: what have we learnt in the past 10 years? World J Gastroenterol. 2014;20:1192–1210. doi: 10.3748/wjg.v20.i5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu YN, Yu TC, Zhao HJ, Sun TT, Chen HM, Chen HY, An HF, Weng YR, Yu J, Li M, Qin WX, Ma X, Shen N, Hong J, Fang JY. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget. 2015;6:32013–32026. doi: 10.18632/oncotarget.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukugaiti MH, Ignacio A, Fernandes MR, Ribeiro Junior U, Nakano V, Avila-Campos MJ. High occurrence of Fusobacterium nucleatum and Clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz J Microbiol. 2015;46:1135–1140. doi: 10.1590/S1517-838246420140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA. Co-occurrence of anaerobic bacteria in colorectal carcinomas. Microbiome. 2013;1:16. doi: 10.1186/2049-2618-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Feng Q, Wong SH, Zhang D, Liang QY, Qin Y, Tang L, Zhao H, Stenvang J, Li Y, Wang X, Xu X, Chen N, Wu WK, Al-Aama J, Nielsen HJ, Kiilerich P, Jensen BA, Yau TO, Lan Z, Jia H, Li J, Xiao L, Lam TY, Ng SC, Cheng AS, Wong VW, Chan FK, Yang H, Madsen L, Datz C, Tilg H, Wang J, Brunner N, Kristiansen K, Arumugam M, Sung JJ. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2017;66:70–78. doi: 10.1136/gutjnl-2015-309800. [DOI] [PubMed] [Google Scholar]
- 13.Wong SH, Kwong TNY, Chow TC, Luk AKC, Dai RZW, Nakatsu G, Lam TYT, Zhang L, Wu JCY, Chan FKL, Ng SSM, Wong MCS, Ng SC, Wu WKK, Yu J, Sung JJY. Quantitation of faecal fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut. 2017;66:1441–1448. doi: 10.1136/gutjnl-2016-312766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guarino M, Rubino B, Ballabio G. The role of epithelial-mesenchymal transition in cancer pathology. Pathology. 2007;39:305–318. doi: 10.1080/00313020701329914. [DOI] [PubMed] [Google Scholar]
- 17.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci U S A. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanali C, Lucchetti D, Farina M, Corbi M, Cufino V, Cittadini A, Sgambato A. Cancer stem cells in colorectal cancer from pathogenesis to therapy: controversies and perspectives. World J Gastroenterol. 2014;20:923–942. doi: 10.3748/wjg.v20.i4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, Chen H, Zhang P, Wang F, Han H, Wu W, Gao R, Gasche C, Qin H, Ma Y, Goel A. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2016;65:1470–1481. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 22.Gendron R, Plamondon P, Grenier D. Binding of pro-matrix metalloproteinase 9 by Fusobacterium nucleatum subsp. nucleatum as a mechanism to promote the invasion of a reconstituted basement membrane. Infect Immun. 2004;72:6160–6163. doi: 10.1128/IAI.72.10.6160-6163.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fardini Y, Wang X, Temoin S, Nithianantham S, Lee D, Shoham M, Han YW. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol Microbiol. 2011;82:1468–1480. doi: 10.1111/j.1365-2958.2011.07905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang P, Guo A, Possemato A, Wang C, Beard L, Carlin C, Markowitz SD, Polakiewicz RD, Wang Z. Identification and functional characterization of p130Cas as a substrate of protein tyrosine phosphatase nonreceptor 14. Oncogene. 2013;32:2087–2095. doi: 10.1038/onc.2012.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrand J, Lehours P, Schmid-Alliana A, Megraud F, Varon C. Helicobacter pylori infection of gastrointestinal epithelial cells in vitro induces mesenchymal stem cell migration through an NF-kappaB-dependent pathway. PLoS One. 2011;6:e29007. doi: 10.1371/journal.pone.0029007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen B, Wan YJ, Nie YQ. Association of Fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J Gastroenterol. 2016;22:3227–3233. doi: 10.3748/wjg.v22.i11.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mima K, Nishihara R, Qian ZR, Cao Y, Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, Kostic AD, Giannakis M, Bullman S, Milner DA, Baba H, Giovannucci EL, Garraway LA, Freeman GJ, Dranoff G, Garrett WS, Huttenhower C, Meyerson M, Meyerhardt JA, Chan AT, Fuchs CS, Ogino S. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2016;65:1973–1980. doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han YW, Shi W, Huang GT, Kinder Haake S, Park NH, Kuramitsu H, Genco RJ. Interactions between periodontal bacteria and human oral epithelial cells: fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000;68:3140–3146. doi: 10.1128/iai.68.6.3140-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan CW, Ma X, Paranjpe A, Jewett A, Lux R, Kinder-Haake S, Shi W. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun. 2010;78:4773–4778. doi: 10.1128/IAI.00567-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, Shussman N, Almogy G, Cuapio A, Hofer E, Mevorach D, Tabib A, Ortenberg R, Markel G, Miklic K, Jonjic S, Brennan CA, Garrett WS, Bachrach G, Mandelboim O. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bates RC, Mercurio AM. The epithelial-mesenchymal transition (EMT) and colorectal cancer progression. Cancer Biol Ther. 2005;4:365–370. doi: 10.4161/cbt.4.4.1655. [DOI] [PubMed] [Google Scholar]
- 32.Lamouille S, Subramanyam D, Blelloch R, Derynck R. Regulation of epithelial-mesenchymal and mesenchymal-epithelial transitions by microRNAs. Curr Opin Cell Biol. 2013;25:200–207. doi: 10.1016/j.ceb.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moyret-Lalle C, Ruiz E, Puisieux A. Epithelial-mesenchymal transition transcription factors and miRNAs: “Plastic surgeons” of breast cancer. World J Clin Oncol. 2014;5:311–322. doi: 10.5306/wjco.v5.i3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaquero J, Guedj N, Claperon A, Nguyen Ho-Bouldoires TH, Paradis V, Fouassier L. Epithelial-mesenchymal transition in cholangiocarcinoma: from clinical evidence to regulatory networks. J Hepatol. 2017;66:424–441. doi: 10.1016/j.jhep.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Zong W, Yu C, Wang P, Dong L. Overexpression of SASH1 inhibits TGF-beta1-induced EMT in gastric cancer cells. Oncol Res. 2016;24:17–23. doi: 10.3727/096504016X14570992647203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofman P, Vouret-Craviari V. Microbes-induced EMT at the crossroad of inflammation and cancer. Gut Microbes. 2012;3:176–185. doi: 10.4161/gmic.20288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye X, Wang R, Bhattacharya R, Boulbes DR, Fan F, Xia L, Adoni H, Ajami NJ, Wong MC, Smith DP, Petrosino JF, Venable S, Qiao W, Baladandayuthapani V, Maru D, Ellis LM. Fusobacterium nucleatum subspecies animalis influences proinflammatory cytokine expression and monocyte activation in human colorectal tumors. Cancer Prev Res (Phila) 2017;10:398–409. doi: 10.1158/1940-6207.CAPR-16-0178. [DOI] [PubMed] [Google Scholar]
- 38.Du L, Wang H, He L, Zhang J, Ni B, Wang X, Jin H, Cahuzac N, Mehrpour M, Lu Y, Chen Q. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14:6751–6760. doi: 10.1158/1078-0432.CCR-08-1034. [DOI] [PubMed] [Google Scholar]