Abstract
Extra-adrenal paraganglioma is rare, and occurs in the retroperitoneum, and head and neck. The incidence rate of paraganglioma in urogenital system is very low, especially in the spermatic cord. A case of paraganglioma of spermatic cord is reported and relevant literature is reviewed. A spermatic cord mass was found in the right scrotum in a middle-aged man 2 years ago, without hormone symptoms. Understanding of clinical and intraoperative frozen pathology was inadequate. Ultrasound images showed that there was abundant blood supply around the right spermatic cord with clear boundaries. The conventional pathology of the tumor presented a typical histologic morphology of paraganglioma. Immunohistochemistry showed that chief tumor cells were CGA (+), syn (+), CD56 (+), SDHB (+), and sertoli cells were S-100 (strong+). There are few reported cases at present, and the etiology and pathogenesis are not clear yet. The rapid frozen pathologic diagnosis during operation is very challenging, and it is easily diagnosed by routine histology combined with immunohistochemistry. Gene detection is recommended if necessary. Early diagnosis is helpful to the choice of operation mode and the prevention and control of intraoperative risk.
Keywords: Spermatic cord, paraganglioma, immunohistochemistry, SDHB
Introduction
The paraganglion is derived from embryonic neural crest cells, which are distributed in the body from the skull base to the central axis of the pelvic cavity. Most of them are distributed along the great vessels, and gradually migrate and spread all over the body with the development of the embryo. The paraganglion can synthesize and store catecholamine, whose functions vary according to the distribution of the body, but have similar histopathologic morphology in different parts: the chief cells are arranged into well-defined cell nests (Zellballen) surrounded by a thin layer of sertoli cells which are positive for S-100 protein-positive. Paraganglioma is a rare type of non-epithelial neuroendocrine tumor originating from the paraganglion system. It has the above typical histopathological characteristics, and its pathogenesis is generally the same as the distribution of paraganglion in the body. Paraganglioma mainly originates from the adrenal medulla, known as pheochromocytoma; rarely from the adrenal sympathetic and parasympathetic nervous systems, which are collectively referred to as extra-adrenal paraganglioma; the latter is usually classified according to the primary site and function. The primary extra-adrenal paraganglioma in urogenital system is very rare, mainly in bladder, urethra and other parts [1]. Primary paraganglioma of spermatic cord is more rare, first reported by Eusebi and Massarelli in 1971 [2]. At present, there are only a dozen cases reported at home and abroad [3-15]. Its etiology, genetics, and genetic characteristics need more case studies for references. A case of paraganglioma of spermatic cord is reported and relevant literature is reviewed. The clinicopathologic features, especially the intraoperative frozen pathologic features, the pathogenesis and differential diagnosis of the tumor are discussed in order to strengthen the understanding of the tumor and improve the early diagnosis and treatment effect.
Clinical data
A 40-year-old male who accidentally discovered a mass in the right scrotum two years ago, about the size of an almond, had no obvious symptoms of discomfort and was not treated at the time. In the past two years, the tumor gradually increased, and the right scrotum appeared intermittent dull pain in the last half of the year, and he was admitted to hospital in 2019. Ultrasound examination: the hypoechoic nodules were detected around the right spermatic cord, the size was about 2.9*1.8*2.9 cm, the boundaries were clear, the internal echo was not uniform, and abundant blood flow signals were visible. The right spermatic vein was widened, and the widest was about 3.6 mm (Figure 1A, 1B). Specialized examination: soft masses with clear boundaries of 3.0*2.0 size were palpated above the right scrotal testis, and no special lesions were found.
Figure 1.
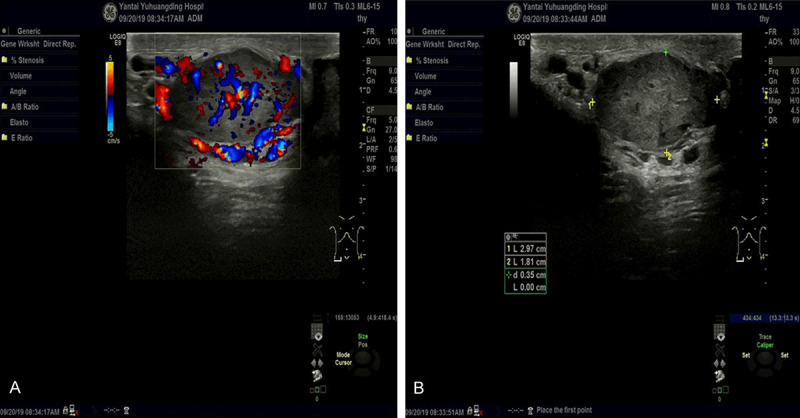
A. Ultrasound image showing abundant blood supply of the right spermatic cord mass. B. Ultrasound image showing dilation of right spermatic vein around the tumor.
The patient had no previous operation history or related family genetic history. The blood pressure was normal at ordinary times without hormone function symptoms. The blood pressure before operation was 120/80 mm. No hormone-related biochemical indexes were detected during admission.
Clinical primary diagnosis was masses around the spermatic cord of the right scrotum and the dilation of the right spermatic vein. In the same month, the patient underwent resection of the masses around the right spermatic cord. During the operation, it was found that the masses were located in the right spermatic cord. The size of the masses was about 3.0*2.0 cm, smooth and with clear boundaries. During the operation, some tumor tissues were excised for rapid frozen pathology. The frozen pathologic results were a descriptive diagnosis, and the nature and source of the tumor were not clear. The tumor was separated completely and resected, the blood pressure was stable and the heart rate was normal during surgery.
Materials and methods
Frozen samples of intraoperative biopsy of spermatic cord masses were rapid frozen sectioned and stained. The conventional specimens were fixed with 4% neutral formaldehyde, conventionally selected, paraffin embedded, sectioned, and H&E stained. Immunohistochemical labeling adopts EnVision two-step method. Antibodies used: Synaptophysin, CD56, S-100, cytokeratin, and vimentin were purchased from Maixin Company. Chromogranin A, and α-inhibin were purchased from Zhongshan Company. Ki67 and SDHB were purchased from Roche company.
Clinicopathologic analysis
General description
Fast frozen pathologic specimens were 2 small gray-red tissues, 0.6*0.6*0.2 cm. Conventional paraffin specimens were 4 firm fragments of tissues with a total volume of 2.5*2.0*1.5 cm. Some areas were dark red, and two of them were fibrous capsule wall-like.
Microscopic characteristics
Pathologic characteristics of fast frozen and conventional paraffin under microscope: ① The main cells of the tumor were arranged into characteristic alveolar and organ like nests. The cells were polygonal or oval. The cell boundary was unclear and closely connected, and a mitotic figure was difficult to find. The cytoplasm of the cells was slightly eosinophilic or dichroic, and the nucleus was round or oval in fine granules. In the conventional section, the cytoplasm of cells was relatively abundant, the nucleus was relatively light stained, and one or more clear nucleoli could be seen. The ratio of nuclear plasma in frozen sections was relatively large, the nucleus was relatively deep stained, and the nucleolus was not obvious. ② Most neoplastic cell nests were separated by parenchyma of blood sinus, there was no obvious proliferation of vascular endothelial cells, and the collagen fibers were obviously proliferated with sclerotic degeneration in the few sections of the conventional sections. ③ There were more or less sertoli cells around the main cell nests in the area of rich blood sinuses in the stroma, but few or no Sertoli cells around the main cell nests in the area of rich sclerotic collagen in the stroma. The shape of support cells was irregular, from flat to fat. The cytoplasm of support cells was very rich, which was light brown and fine granular. Small oval granular nuclei were observed in some cells. ④ A thick fibrous capsule can be seen around the tumor (Figure 2A-E).
Figure 2.
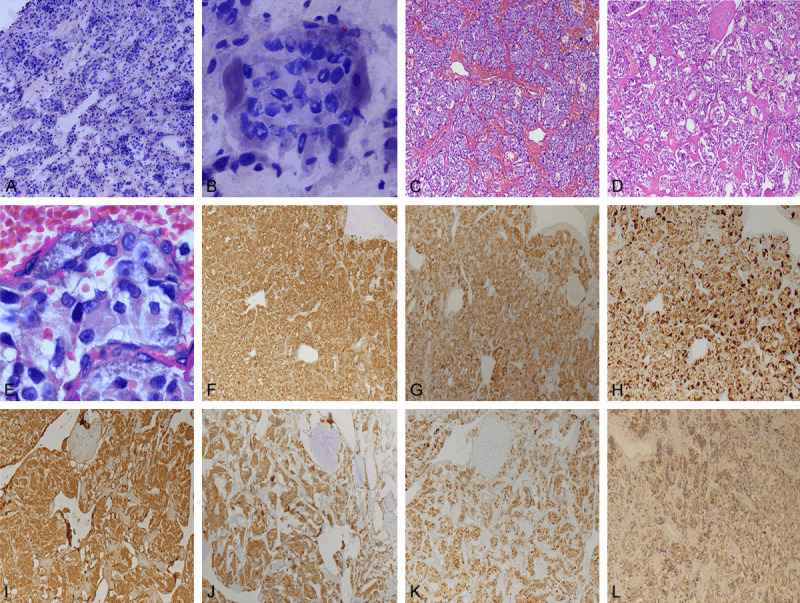
(A) Low power image of frozen pathologic section of tumor (4×). (B) Clear sertoli cells could be seen around the main cell nests in frozen pathological section (40×). (C) Low power image of conventional pathologic section of vascular rich area in stroma of tumor (4×). (D) Low power image of conventional pathological section of sclerotic area in stroma of tumor (4×). (E) Sertoli cells could be seen around the main cell nests in conventional pathologic section (40×). (F) Main cells in (C) with positive expression of CgA by immunohistochemistry. (G) Main cells in (C) with positive expression of Syn by immunohistochemistry. (H) More or less sertoli cells strongly positive for S-100 protein around the main cell nests in (C). (I) Main cells in (D) with positive expression of CgA by immunohistochemistry. (J) Main cells in (D) with positive expression of Syn by immunohistochemistry. (K) Few or no sertoli cells strongly positive for S-100 protein around the main cell nests in (D). (L) Immunohistochemical positive expression of SDHB in tumor cells.
Immunohistochemical results: chief tumor cells were CGA (+), Syn (+), CD56 (+), SDHB (+), and sertoli cells were S-100 (strong+); Vim (focal+), CK (-), α-inhibin (-); ki67 positive rate about 5% (Figure 2F-L).
Final pathologic diagnosis: right spermatic cord paraganglioma. The patient’s blood pressure and heart rate were stable after the surgery, with no obvious discomfort. He recovered well and was discharged with follow-up. There was no sign of recurrence or metastasis at the time of writing this article.
Summary of clinical characteristics
Paraganglioma of spermatic cord is a rare clinical case. The clinical characteristics of one case in this article and more than ten cases reported in the literature are summarized as follows (Table 1). The patients ranged in age from 18 to 69, most of them were middle-aged men. The manifestations were masses in scrotal spermatic cord with or without swelling and pain. The incidence rate of bilateral spermatic cord tumors is similar. Ultrasound images showed hypervascular solid nodules; some of them could be accompanied by cystic changes, and most of the tumor boundaries were still clear. The maximum diameter of the tumor ranged from 1.5 cm to 10 cm. The interval of diagnosis varied from 0 to 20 years. A few were functional, showing hypertension, palpitations, sweating, chest tightness, headache, tachycardia and supraventricular arrhythmias. One case presented at multiple sites, with history of bilateral carotid body tumors and bilateral adrenal pheochromocytomas resection. Paraganglioma of the spermatic cord was found after a 5 year interval. It is difficult to determine whether the case was multiple or metastatic. One case was confirmed as malignant by histopathology, but no metastasis was found during follow-up. In one case of nonfunctional disease, the negative expression of SDHB protein was detected by immunohistochemistry, and the mutation analysis showed the mutation of SDHD gene. One functional case was diagnosed as testicular and spermatic cord paraganglioma with retroperitoneal and right lung metastasis, with genetic gene mutation of SDHB from the father. This case showed positive expression of SDHB protein by immunohistochemistry.
Table 1.
Clinical characteristics of fifteen cases with paraganglioma of spermatic cord reported
| Report time | Country | Age | Site | Hormonal symptoms | Interval between diagnosis | Maximum diameter | Gene | Metastases |
|---|---|---|---|---|---|---|---|---|
| 1971 | Italy | 37 | Right | N/A | 10 years | 2.5 cm | N/A | N/A |
| 1977 | Japan | 52 | Legt | I/A | 10 years | 4.5 cm | N/A | N/A |
| 1990 | Brazil | 18 | Right | N/A | 2 years | - | N/A | N/A |
| 1993 | Pakistan | 37 | Right | N/A | 9 months | 10 cm | N/A | N/A |
| 1996 | Iran | 40 | Legft | N/A | 0 | 1.5 cm | N/A | N/A |
| 1999 | UK | 52 | Right | N/A | 1 month | 1.5 cm | N/A | N/A |
| 2000 | Japan | 55 | Left | I/A | 1 month | 2.0 cm | N/A | Uncertain |
| 2007 | China | 38 | Left | N/A | More than 20 years | 2.5 cm | N/A | N/A |
| 2008 | UK | 69 | Right | N/A | 0 | 2.0 cm | N/A | N/A |
| 2009 | China | 66 | Right | I/A | 20 years | 3.5 cm | N/A | N/A |
| 2010 | Greece | 45 | Left | N/A | Several months | 4.8 cm | I/A | N/A |
| 2016 | Korea | 40 | Left | N/A | 0 | 1.8 cm | N/A | N/A |
| 2019 | USA | 55 | Left | I/A | 1 year | 2.5 cm | N/A | N/A |
| 2019 | China | 28 | Right | I/A | 3 years | 3 cm | I/A | I/A |
| This case | China | 40 | Right | N/A | 2 years | 3 cm | N/A | N/A |
Discussion
The paraganglion system spreads all over the body. It is most developed in fetus and infants. After puberty, except for adrenal medulla, it mostly atrophies or even disappears. Therefore, extra-adrenal primary paraganglioma is rare, and common in areas corresponding to the distribution of paraganglion. However, during the development of the body, paraganglioma can also occur sporadically in some special parts in extraadrenal locations, which is extremely rare and often causes some difficulties in clinical diagnosis and treatment due to lack of understanding. Primary paraganglioma of spermatic cord belongs to one of the above-mentioned cases. At present, there are few reported cases, which need our attention. Only timely and accurate diagnosis and treatment can make patients benefit the most.
Histology of extra-adrenal paraganglioma is similar to pheochromocytoma, but the incidence rate and metastasis rate are higher. Its clinical manifestations are sporadic and familial, mainly sporadic. It has been found that at least 30% of tumors are genetically related and have been found in a variety of tumor syndromes. Previous studies have confirmed that a large proportion of extra-adrenal paragangliomas have gene mutations. The common ones are SDHB, SDHC, SDHD, VHL and other gene mutations [16,17], among which SDHB and SDHD mutations are more common, while patients with SDHB mutations have a higher risk of metastasis [18]. At present, the etiology of this kind of tumor is unknown. The pathogenesis of this kind of tumor is mainly related to hypoxia signal pathway and kinase signal pathway, and the mutation gene is mainly involved in the regulation of hypoxia signal pathway, which indirectly suggests that hypoxia signal pathway may play an important role in the pathogenesis of extra-adrenal paraganglioma. Neumann et al. found that VHL gene mutation was the most common gene of the above susceptibility genes in children younger than 18 years old. Therefore, it is recommended that VHL gene detection should be preferred in children with tumor and SHDx gene detection should be preferred in adults older than 18 years old [19]. In addition, it has been reported that extra-adrenal paraganglioma may be the most hereditary human tumor known [20]. Therefore, it is necessary to selectively detect related genes in all confirmed patients [21,22]. The cost of gene detection is high and limited by the conditions. Alatak et al. proposed that the results of SDHB immunohistochemistry could guide the subsequent DNA mutation analysis. No matter what gene mutation is related to SDHx, the immunohistochemical staining of SDHB is negative. SDHB immunohistochemical staining is a simple and useful index for reference value before SDHx gene detection [12].
Paraganglioma of spermatic cord is one of the rare extra-adrenal paragangliomas. It is speculated that it may originate from the residual chromaffin cells in the embryo that enter the scrotum along the testis [2,3]. Combined with the case analysis, most of the patients were middle-aged men, and most of them visited a doctor for scrotal masses. The largest diameter of the tumors reported was 10 cm, and the longest interval of diagnosis was more than 20 years. Most of the imaging findings were hypervascular masses with clear boundaries, and the boundaries of some tumors were not clear. Five of them were functional (5/15, accounting for 33.3%); most of them were nonfunctional, which may be related to the tumor being too small to secrete enough catecholamine. There were a few reports of histopathologic malignancy and vascular invasion, but no metastasis. In addition to the multiple or metastatic uncertainty of one case, further gene detection and close follow-up were needed to provide more basis. A single case showed SDHB protein expression deletion with SDHD gene mutation. A case of multiple malignancies was genetically related with SDHB gene mutation. All of these findings are consistent with the previous reports of extra-adrenal paraganglioma. The special feature of this case lies in the rapid frozen pathologic biopsy during the operation. During the operation, the morphological knowledge was insufficient and the diagnosis was not clear. After the routine pathologic diagnosis, we reviewed the frozen pathological sections and found clear alternative sertoli cells around the nests of some tumor main cells, which played a certain warning role for us. However, if the sertoli cells are not obvious, intraoperative frozen pathology is easily misdiagnosed. Because of the special location of tumor lesions, it is a good choice to delay the diagnosis when necessary, and wait for the paraffin section to be fully taken and further confirmed by immunohistochemistry, so as to avoid misdiagnosis during the operation leading to overtreatment of clinical operation. In addition, the tumor immunohistochemistry in this case did not show the lack of SDHB protein expression. According to the above theory, it is speculated that there is no mutation in the SDHx gene, and the patient did not conduct further gene detection and analysis. At present, there is limited reported cases of paraganglioma of spermatic cord, its clinical characteristics and heritability are not clear, and the related pathogenesis is rarely studied, which needs further research and discussion.
The ultrasonic image and pathological morphology of paraganglioma of spermatic cord have some overlap and similarity with other tumors that it should be differentiated from. (1) Ultrasound differential diagnosis: ultrasound image shows that the tumor was solid nodule with abundant blood supply, and most of the boundaries were still clear. The following neuroendocrine tumors, schwannomas, and solitary fibrous tumors can all show similar ultrasound images, so we should pay attention to distinguishing them. (2) Pathologic differential diagnosis: ① neuroendocrine tumors such as carcinoid: Neuroendocrine tumor stroma is rich in blood vessels. When tumor cells are in acinar and organ like arrangement, there is a great overlap with paraganglioma which is rich in blood sinuses. The former showed a kind of tumor cells in frozen pathology. In addition to positive immunohistochemical neuroendocrine markers, epithelial markers were also expressed, such as CK (+). ② Sertoli cell tumor: When the tumor cells are solid nests and tubular structures distributed between the dense collagen connective tissue, they should be differentiated from paraganglioma which is rich in sclerotic collagen. Pathology of the former showed that there were vacuoles in the cytoplasm of some cells, and a nuclear sulcus could be observed. Immunohistochemistry expressed α-inhibin, calretinin, CD99, vimentin and so on, but not neuroendocrine markers. ③ Multiple or metastatic paraganglioma: it depends on the clinical data, pathologic characteristics, and gene detection to be comprehensively analyzed.
Paraganglioma of spermatic cord is a special neuroendocrine tumor. Based on the literature review and summary of this case, we conclude: (1) most of the patients are nonfunctional, the tumor is occult and grows slowly, which is easily ignored. It is suggested that patients should see a doctor as soon as possible when suspicious masses are found. Patients with hormonal symptoms should be aware of the possibility of this disease, and if necessary, relevant biochemical indicators should be detected. (2) Because of the lack of characteristics in imaging, it is often difficult to distinguish from other tumors with similar images. In general, when a tumor shows solid nodules with abundant blood supply, clear boundaries, with or without cystic change, then the possibility of this disease should be considered. (3) The frozen pathologic diagnosis during tumor operation is challenging. Attention should be paid to the special shape of sertoli cells around the main cell nests, which can be an important clue for diagnosis. (4) Serious complications such as severe fluctuation of blood pressure and massive hemorrhage may occur during operation. Preoperative biopsy is an effective method for diagnosis and guiding the operation. (5) Tumor morphology is not consistent with biological behavior. It is difficult to identify benign and malignant tumors only by histological morphology. The detection of related genes is helpful for the diagnosis and the evaluation of clinical risk prognosis.
In the new edition of WHO (2016) classification of urological tumors, paraganglioma of spermatic cord was established as ICD-O code 8963/1, which is believed to be a borderline tumor with malignant potential [23]. Most of the tumors showed benign biological behavior, only one case was reported to be metastatic, with genetic correlation [15]. At present, the biologic behavior of this kind of tumor is mainly determined according to the standards established by PASS classification system and GAPP classification system. In addition, tumor size, microRNAs and genetic results can also help predict the biologic behavior of this tumor. However, metastasis is still the only criterion for the diagnosis of malignancy. Surgical resection of paraganglioma of spermatic cord is the first treatment plan. The rapid frozen pathologic diagnosis during the operation is difficult. The operation mode depends on the size of the tumor, the nature of the lesion, and the fertility requirements. Whether radiotherapy or chemotherapy is needed after the operation is still uncertain, but long-term close follow-up is necessary.
Disclosure of conflict of interest
None.
References
- 1.Purnell S, Sidana A, Maruf M, Grant C, Agarwal PK. Genitourinary paraganglioma: demographic, pathologic, and clinical characteristics in the surveillance, epidemiology, and end results database (2000-2012) Urol Oncol. 2017;35:457.e9–457.e14. doi: 10.1016/j.urolonc.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eusebi V, Massarelli G. Phaeochromocytoma of the spermatic cord: report of a case. J Pathol. 1971;105:283–284. doi: 10.1002/path.1711050407. [DOI] [PubMed] [Google Scholar]
- 3.Soejima H, Ogawa O, Nomura Y, Ogata J. Pheochromocytoma of the spermatic cord: a case report. J Urol. 1977;118:495–496. doi: 10.1016/s0022-5347(17)58081-7. [DOI] [PubMed] [Google Scholar]
- 4.Bacchi CE, Schmidt RA, Brandão M, Scapulatempo R, Costa JC, Schmitt FC. Paraganglioma of the spermatic cord. Report of a case with immunohistochemical and ultrastructural studies. Arch Pathol Lab Med. 1990;114:899–901. [PubMed] [Google Scholar]
- 5.Mashat F, Meccawi A, Garg S, Christian E. Paraganglioma of the spermatic cord. Ann Saudi Med. 1993;83:208–210. doi: 10.5144/0256-4947.1993.208. [DOI] [PubMed] [Google Scholar]
- 6.Attaran SY, Shakeri S, Sobhani AR. Paraganglioma of the spermatic cord: report of a case. J Urol. 1996;155:651. [PubMed] [Google Scholar]
- 7.Young IE, Nawroz IM, Aitken RJ. Phaeochromocytoma of the spermatic cord. J Clin Pathol. 1999;52:305–306. doi: 10.1136/jcp.52.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abe T, Matsuda H, Shindo J, Nonomura K, Koyanagi T. Ectopic pheochromocytoma arising in the spermatic cord 5 years after removal of bilateral carotid body tumors and adrenal pheochromocytomas. Int J Urol. 2000;7:110–111. doi: 10.1046/j.1442-2042.2000.00143.x. [DOI] [PubMed] [Google Scholar]
- 9.Cai YB, Zhan HL, Wang DJ, Qiu JG, Situ J, Wen XQ, Zhou XF, Gao X. Paraganglioma of the spermatic cord: a case report and literature review. Chinese Journal of Urology. 2007;28:778–781. [Google Scholar]
- 10.Garaffa G, Muneer A, Freeman A, Abdel Raheem AM, Ralph DJ, Minhas S, Rees RW. Paraganglioma of the spermatic cord: case report and review of the literature. ScientificWorldJournal. 2008;8:1256–1258. doi: 10.1100/tsw.2008.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Liu LH, Guo C, et al. Paraganglioma of the spermatic cord: a case report and literature review. West China Medical Journal. 2009;24:1836–1837. [Google Scholar]
- 12.Alataki D, Triantafyllidis A, Gaal J, Rodiou C, Vouros J, Papathanasiou A, Papanicolaou A, Rombis V, de Krijger RR. A non-catecholamine-producing sympathetic paraganglioma of the spermatic cord: the importance of performing candidate gene mutation analysis. Virchows Arch. 2010;457:619–622. doi: 10.1007/s00428-010-0966-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon AY, Kang H, An HJ, Kim G, Kim TH, Heo JH, Lee HJ, Hong YK. Spermatic cord paraganglioma with histologically malignant feature. Urology. 2016;93:e7–e8. doi: 10.1016/j.urology.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Gontarz B, Hegde P, McFadden D. Paraganglioma of the spermatic cord: a case report and literature review. Int J Surg Case Rep. 2019;60:368–370. doi: 10.1016/j.ijscr.2019.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo S, Liu Z, Zhou Z. A rare hereditary and metastatic paraganglioma involved in both spermatic cord and testis. Endocrine. 2019;65:217–218. doi: 10.1007/s12020-019-01872-6. [DOI] [PubMed] [Google Scholar]
- 16.Yao L, Schiavi F, Cascon A, Qin Y, Inglada-Pérez L, King EE, Toledo RA, Ercolino T, Rapizzi E, Ricketts CJ, Mori L, Giacchè M, Mendola A, Taschin E, Boaretto F, Loli P, Iacobone M, Rossi GP, Biondi B, Lima-Junior JV, Kater CE, Bex M, Vikkula M, Grossman AB, Gruber SB, Barontini M, Persu A, Castellano M, Toledo SP, Maher ER, Mannelli M, Opocher G, Robledo M, Dahia PL. Spectrum and prevalence of FP/TMEM127 gene mutations in pheochromocytomas and paragangliomas. JAMA. 2010;304:2611–2619. doi: 10.1001/jama.2010.1830. [DOI] [PubMed] [Google Scholar]
- 17.Burnichon N, Cascón A, Schiavi F, Morales NP, Comino-Méndez I, Abermil N, Inglada-Pérez L, de Cubas AA, Amar L, Barontini M, de Quirós SB, Bertherat J, Bignon YJ, Blok MJ, Bobisse S, Borrego S, Castellano M, Chanson P, Chiara MD, Corssmit EP, Giacchè M, de Krijger RR, Ercolino T, Girerd X, Gómez-García EB, Gómez-Graña A, Guilhem I, Hes FJ, Honrado E, Korpershoek E, Lenders JW, Letón R, Mensenkamp AR, Merlo A, Mori L, Murat A, Pierre P, Plouin PF, Prodanov T, Quesada-Charneco M, Qin N, Rapizzi E, Raymond V, Reisch N, Roncador G, Ruiz-Ferrer M, Schillo F, Stegmann AP, Suarez C, Taschin E, Timmers HJ, Tops CM, Urioste M, Beuschlein F, Pacak K, Mannelli M, Dahia PL, Opocher G, Eisenhofer G, Gimenez-Roqueplo AP, Robledo M. MAX mutations cause hereditary and sporadic pheochromocytoma and paraganglioma. Clin Cancer Res. 2012;18:2828–2837. doi: 10.1158/1078-0432.CCR-12-0160. [DOI] [PubMed] [Google Scholar]
- 18.Strajina V, Dy BM, Farley DR, Richards ML, McKenzie TJ, Bible KC, Que FG, Nagorney DM, Young WF, Thompson GB. Surgical treatment of malignant pheochromocytoma and paraganglioma: retrospective case series. Ann Surg Oncol. 2017;24:1546–1550. doi: 10.1245/s10434-016-5739-5. [DOI] [PubMed] [Google Scholar]
- 19.Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peçzkowska M, Szmigielski C, Eng C Freiburg-Warsaw-Columbus Pheochromocytoma Study Group. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–66. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- 20.Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer. 2014;14:108–19. doi: 10.1038/nrc3648. [DOI] [PubMed] [Google Scholar]
- 21.Fishbein L, Merrill S, Fraker DL, Cohen DL, Nathanson KL. Inherited mutations in pheochromocytoma and paraganglioma: why all patients should be offered genetic testing. Ann Surg Oncol. 2013;20:1444–50. doi: 10.1245/s10434-013-2942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NGS in PPGL (NGSnPPGL) Study Group. Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP, Welander J, Tops CM, Firth H, Dwight T, Ercolino T, Mannelli M, Opocher G, Clifton-Bligh R, Gimm O, Maher ER, Robledo M, Gimenez-Roqueplo AP, Dahia PL. Consensus statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol. 2017;13:233–247. doi: 10.1038/nrendo.2016.185. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 2016:252. [Google Scholar]


