Abstract
To investigate the incidence, prognosis, and treatment modality of different metastatic sites in cervical cancer. Methods: We used the surveillance epidemiology and end results (SEER) database to collect cervical cancer patients with metastasis from 2010-2016. Kaplan-Meier survival analyses and log-rank tests were used to compare overall survival between groups. Univariate and multivariate Cox proportional hazards regression analyses were used for identifying the prognostic factors in metastatic cervical cancer. Results: In total, 1347 patients with distant metastatic cervical cancer were selected for the study. The average age of patients with metastatic cervical cancer was 57 years old. Unmarried white patients were the majority. About 7.9%, 53.3%, and 64.6% patients were treated with surgery, radiation, and chemotherapy, respectively. Additionally, lungs were the most common metastatic sites. The survivals of single-site metastases were similar, which were better than multi-organ metastases. Lung metastatic patients were older than other metastatic patients, and with poorer differentiation and higher stage tumors. In terms of treatment, bone metastatic patients were more commonly treated with radiotherapy (68.4%) than other metastatic patterns. Surgery, radiation, and chemotherapy all prolonged survival months of single-site and multi-site metastatic patients. Furthermore, age, ethnicity, tumor stage, surgery, radiotherapy, chemotherapy, and metastatic sites were independent prognostic factors for patients with metastatic cervical cancer. Conclusions: This large-population based study showed that the most common metastatic site of cervical cancer is lung. Although lung metastatic patients harbor older ages and poorer differentiation and higher stage tumors than other sites, the prognosis of lung metastasis is similar to other single metastatic sites. However, the single-site metastatic patients survive longer than multi-site metastatic patients. Surgery, radiotherapy, and chemotherapy all bring benefit to patients with metastases, which may guide the treatment in metastatic cervical cancers.
Keywords: Cervical cancer, metastasis, prognosis, overall survival, SEER
Introduction
Cervical cancer is one of the most common malignancies in the female genital tract system. It is the second leading cause of cancer mortality in women aged 20 to 39 years [1]. Regardless of the several strategies for prevention, diagnosis, and treatment that are applied to the disease, the prognosis of cervical cancer patients remains poor, especially in metastatic patients. Previous studies have shown that the median survival time of metastatic cervical cancer is only 8-13 months, and the 5-year survival rate is 16.5% [2,3]. Due to the poor prognosis, metastatic cervical cancer has become one of the main challenges in the world.
Previous studies have shown that the outcome varied between different metastatic sites in breast, ovarian, liver, and pancreatic cancers et al. [4-7]. For instance, the prognosis of lung metastatic patients is worse than liver metastatic patients in ovarian cancer [4]. However, lung metastatic patients present a superior survival rate compared with liver metastatic patients in pancreatic adenocarcinoma [6]. Thus, the survival of patients with different metastatic sites is different. Assessing the prognosis of different metastatic sites can help with therapeutic strategies, as well as evaluation of prognosis. However, due to the rarity of distant metastasis in cervical cancer [8], the treatment pattern and prognosis of different metastatic sites are not fully understood. Yin (2019) is one of the first to examine the impact of metastatic sites on the survival of cervical cancer. His research of 99 Chinese metastatic cervical cancer revealed that the site of metastasis has a relationship with overall survival, with liver metastasis representing a particularly poor prognosis [9]. So far, however, there has been little discussion about metastatic cervical cancer in western countries. In addition, a large-population based study is still lacking.
In this paper, we analyzed 1347 metastatic cervical cancer patients from surveillance epidemiology and end results (SEER) database for the following aims: (1) to investigate the incidence of different metastatic sites in cervical cancer; (2) to explore the relationship among site-specific patterns of metastasis and overall survival of metastatic cervical cancer; (3) to discover the treatment pattern of different metastatic sites.
Methods and materials
Study population
The SEER program of the National Cancer Institute consists of population-based registries that collect demographic, clinical, pathology, and treatment characteristics, as well as vital status. We used SEER data released in November 2017, which included data from 18 population-based cancer registries and covered approximately 28% of US cancer patients. Patients with organ metastases (brain, liver, lung and bone) of newly diagnosed cervical cancer at SEER from 2010 to 2016 were reviewed. The inclusion criteria included primary tumor, with survival time, with newly diagnosed metastasis et al. The flow chart is shown in Figure 1.
Figure 1.
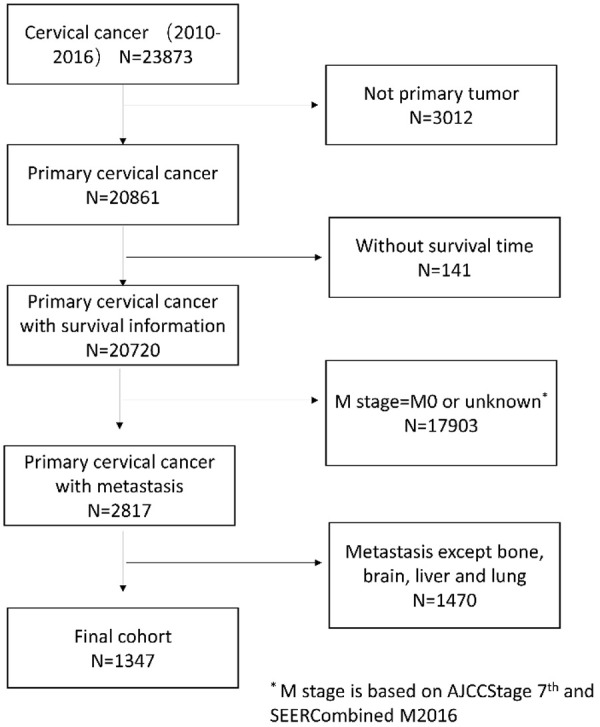
Flowchart of patient selection.
Variables
Demographic variables of interest included age at diagnosis, race, marital status, and region based on SEER registry. Tumor variables of interest included tumor grade, tumor stage, and metastatic sites. Treatment variables of interest included surgery, radiation, and chemotherapy. Vital variables of interest included vital status and survival time.
Statistical analysis
Descriptive statistics for demographic variables and tumor variables were performed by the Student’s t-test for continuous variables and the Chi-square test for categorical variables. Kaplan-Meier survival analyses and log-rank tests were used to compare overall survival between groups. Univariate and multivariate Cox proportional hazards regression analyses were used for identifying prognostic factors in metastatic cervical cancer. All statistical tests were 2-tailed and a P<0.05 was considered statistically significant. Statistical tests were performed using SPSS version 25 statistical software (IBM Corp., version 25.0; NY, USA).
Results
Demographic and tumor characteristics
In total, 1347 patients with distant metastatic cervical cancer were selected for the study. The demographic and tumor characteristics were shown in Table 1. The average age of patients with metastatic cervical cancer was 57.00±14.292 years old, with 40.5% older than 60. Whites accounted for 72.4%, while blacks accounted for 18.3%. Unmarried patients (including single, widowed, and divorced) accounted for 61.9%. Poorly differentiated tumors (40.2%), T3 stage tumors (38.2%) are mainly. Additionally, 56.9% patients were accompanied by lymph node metastasis. 7.9% patients were treated with surgery. 53.3% patients were treated with radiation. 64.6% patients were treated with chemotherapy.
Table 1.
Patient demographics and clinical characteristics (n=1347)
| Characteristics | Level | Number (%) |
|---|---|---|
| Age at diagnosis | Mean ± SD | 57.00±14.292 |
| Median (range) | 57 (16~99) | |
| ≤60 | 802 (59.5%) | |
| >60 | 545 (40.5%) | |
| Race | White | 975 (72.4%) |
| Black | 247 (18.3%) | |
| Asian or Pacific Islander | 111 (8.2%) | |
| Others/Unknown | 14 (1.0%) | |
| Marital status | Married | 449 (33.3%) |
| Unmarried | 834 (61.9%) | |
| Unknown | 64 (4.8%) | |
| Tumor grade | Well differentiated | 18 (1.3%) |
| Moderately differentiated | 242 (18.0%) | |
| Poorly differentiated | 542 (40.2%) | |
| Undifferentiated | 66 (4.9%) | |
| Unknown | 479 (35.6%) | |
| AJCC T Stage | T1 | 153 (11.4%) |
| T2 | 232 (17.2%) | |
| T3 | 515 (38.2%) | |
| T4 | 172 (12.8%) | |
| TX | 275 (20.4%) | |
| AJCC N Stage | N0 | 374 (27.8%) |
| N1 | 767 (56.9%) | |
| NX | 206 (15.3%) | |
| Surgery | Yes | 106 (7.9%) |
| None/Unknown | 1241 (92.1%) | |
| Radiation | Yes | 718 (53.3%) |
| None/Unknown | 629 (46.7%) | |
| Chemotherapy | Yes | 870 (64.6%) |
| No/Unknown | 477 (35.4%) |
Note: RT, Radiation therapy.
Frequency of organ metastasis
Distribution of metastatic sites was shown in Table 2. Single-site metastasis accounted for 68.7%, and multi-organ metastases were relatively rare. In single-site metastasis, lung metastasis was the most common, accounting for 37.9% of all patients, followed by bone metastasis (16.7%), and liver metastasis (12.5%). Brain metastases were uncommon, only accounted for 1.6%. In patients with multi-organ metastases, lung plus liver metastases and lung plus bone metastases were more common than other multi-organ metastases.
Table 2.
Frequencies of combination metastasis (n=1347)
| metastatic site | number | Percentage (%) | |
|---|---|---|---|
| One site | Lung | 511 | 37.9 |
| Bone | 225 | 16.7 | |
| Liver | 168 | 12.5 | |
| Brain | 21 | 1.6 | |
| Two sites | Lung and liver | 123 | 9.1 |
| Lung and bone | 108 | 8.0 | |
| Lung and brain | 19 | 1.4 | |
| Liver and bone | 59 | 4.4 | |
| Liver and brain | 2 | 0.1 | |
| Bone and brain | 12 | 0.9 | |
| Three sites | Lung and liver and bone | 78 | 5.8 |
| Lung and liver and brain | 6 | 0.4 | |
| Lung and bone and brain | 9 | 0.7 | |
| Liver and bone and brain | 1 | 0.1 | |
| Four sites | all | 5 | 0.4 |
Characteristics in different metastatic sites
Table 3 showed the characteristics of different metastatic organs. The age of patients with lung metastases was older than that of patients with other metastases. No difference was found between metastatic sites in marriage and ethnicity. Patients with lung metastases and multi-organ metastases had poorer differentiation and higher stage. In terms of treatment, bone metastatic patients were more commonly treated with radiotherapy (68.4%) than other metastatic patterns. There were no significant differences between metastatic sites in surgery and chemotherapy.
Table 3.
Patient characteristics by metastatic site (n=1347)
| Characteristics | Level | metastatic site | P value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Bone (n=225) | Brain (n=21) | Liver (n=168) | Lung (n=511) | >1 Site (n=422) | |||
| Age at diagnosis | Mean ± SD | 56.12±14.06 | 55.38±13.13 | 56.85±14.69 | 58.53±14.70 | 55.75±13.69 | 0.036 |
| Median (range) | 56 (24~98) | 59 (35~75) | 58 (24~96) | 58 (25~99) | 56 (16~93) | ||
| ≤60 | 138 (61.3%) | 13 (61.9%) | 103 (61.3%) | 278 (54.4%) | 270 (64.0%) | 0.047 | |
| >60 | 87 (38.7%) | 8 (38.1%) | 65 (38.7%) | 233 (45.6%) | 152 (36.0%) | ||
| Race | White | 173 (76.9%) | 15 (71.4%) | 116 (69.0%) | 376 (73.6%) | 295 (69.9%) | |
| Black | 36 (16.0%) | 4 (19.0%) | 38 (22.6%) | 90 (17.6%) | 79 (18.7%) | 0.156 | |
| Asian | 15 (6.7%) | 0 (0.0%) | 12 (7.1%) | 41 (8.0%) | 43 (10.2%) | ||
| Others/Unknown | 1 (0.4%) | 2 (9.5%) | 2 (1.2%) | 4 (0.8%) | 5 (1.2%) | ||
| Marital status | Married | 75 (33.3%) | 8 (38.1%) | 53 (31.5%) | 161 (31.5%) | 152 (36.0%) | 0.705 |
| Unmarried | 142 (63.1%) | 12 (57.1%) | 107 (63.7%) | 328 (64.2%) | 245 (58.1%) | ||
| Unknown | 8 (3.6%) | 1 (4.8%) | 8 (4.8%) | 22 (4.3%) | 25 (5.9%) | ||
| Tumor grade | Low | 54 (24.0%) | 6 (28.6%) | 28 (16.7%) | 115 (22.5%) | 57 (13.5%) | <0.001 |
| High | 87 (38.7%) | 6 (28.6%) | 67 (39.9%) | 229 (44.8%) | 219 (51.9%) | ||
| Unknown | 84 (37.3%) | 9 (42.9%) | 73 (43.5%) | 167 (32.7%) | 146 (34.6%) | ||
| AJCC T Stage | T1 | 24 (10.7%) | 2 (9.5%) | 21 (12.5%) | 45 (8.8%) | 61 (14.5%) | 0.045 |
| T2 | 39 (17.3%) | 5 (23.8%) | 34 (20.2%) | 89 (17.4%) | 65 (15.4%) | ||
| T3 | 100 (44.4%) | 5 (23.8%) | 52 (31.0%) | 208 (40.7%) | 150 (35.5%) | ||
| T4 | 27 (12.0%) | 2 (9.5%) | 28 (16.7%) | 69 (13.5%) | 46 (10.9%) | ||
| TX | 35 (15.6%) | 7 (33.3%) | 33 (19.6%) | 100 (19.6%) | 100 (23.7%) | ||
| Lymph Node Metastases | N0 | 65 (28.9%) | 4 (19.0%) | 55 (32.7%) | 142 (27.8%) | 108 (25.6%) | 0.105 |
| N1 | 135 (60.0%) | 10 (47.6%) | 82 (48.8%) | 293 (57.3%) | 247 (58.5%) | ||
| NX | 25 (11.1%) | 7 (33.3%) | 31 (18.5%) | 76 (14.9%) | 67 (15.9%) | ||
| Surgery | Yes | 17 (7.6%) | 0 (0.0%) | 19 (11.3%) | 44 (8.6%) | 26 (6.2%) | 0.155 |
| None/Unknown | 208 (92.4%) | 21 (100.0%) | 149 (88.7%) | 467 (91.4%) | 396 (93.8%) | ||
| Beam Radiation | Yes | 154 (68.4%) | 10 (47.6%) | 83 (49.4%) | 258 (50.5%) | 213 (50.5%) | <0.001 |
| None/Unknown | 71 (31.6%) | 11 (52.4%) | 85 (50.6%) | 253 (49.5%) | 209 (49.5%) | ||
| Chemotherapy | Yes | 152 (67.6%) | 12 (57.1%) | 116 (69.0%) | 332 (65.0%) | 258 (61.1%) | 0.281 |
| No/Unknown | 73 (32.4%) | 9 (42.9%) | 52 (31.0%) | 179 (35.0%) | 164 (38.9%) | ||
Prognosis
The KM curve showed that the prognosis was similar between single-site metastases. The prognosis of multi-site metastasis was worse than single-site metastasis (Figure 2). To explore the effect of treatment modality on the prognosis, we performed KM analysis. The results showed that surgery, radiation, and chemotherapy all prolonged survival months of single-site and multi-site metastatic patients (Figure 3). Further, to investigate the prognostic factors, we performed univariate and multivariate Cox regression analysis. We found that age, ethnicity, tumor stage, surgery, radiotherapy, chemotherapy, and metastatic sites were independent prognostic factors for patients with metastatic cervical cancer (Table 4).
Figure 2.
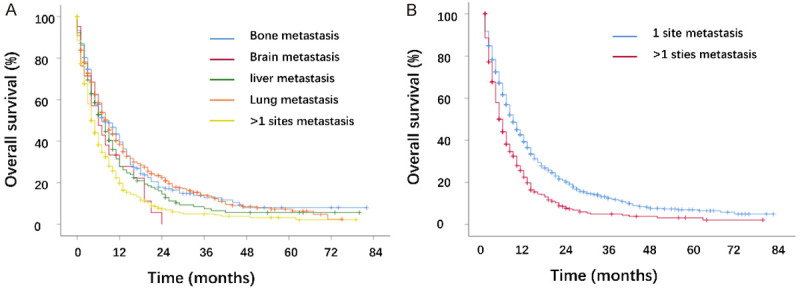
Kaplan-Meier curves of the overall survival in cervical cancer. A. Stratified by different metastatic location (bone vs. brain: P=0.107; bone vs. liver: P=0.108; bone vs. lung: P=0.983; bone vs. >1 site: P<0.001; brain vs. liver: P=0.349; brain vs. lung: P=0.068; brain vs. >1 site: P=0.636; liver vs. lung: P=0.080; liver vs. >1 site: P=0.002; lung vs. >1 site: P<0.001). B. Stratified by different metastases number (P<0.001).
Figure 3.
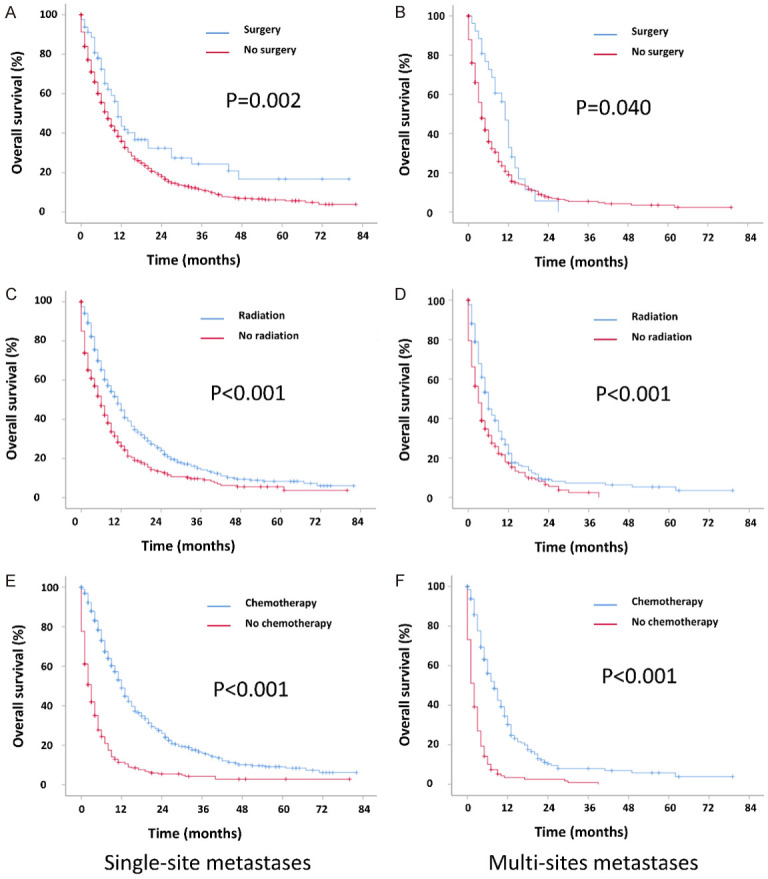
Kaplan-Meier curves of the overall survival in cervical cancer when stratified by treatment and metastases number. A. Surgery in single metastatic patients; B. Surgery in multi-site metastatic patients; C. Radiation in single metastatic patients; D. Radiation in multi-site metastatic patients; E. Chemotherapy in single metastatic patients; F. Chemotherapy on multi-site metasta patients.
Table 4.
Univariate and multivariate analyses for metastasis of patients (n=1347)
| Variables | Level | Univariate | Multivariate | ||
|---|---|---|---|---|---|
|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| metastatic site | Bone | 1 | 1 | ||
| Brain | 1.464 (0.921~2.326) | 0.107 | 0.900 (0.563~1.438) | 0.900 | |
| Liver | 1.182 (0.946~1.478) | 0.141 | 1.075 (0.858~1.348) | 0.529 | |
| Lung | 1.001 (0.837~1.198) | 0.990 | 0.828 (0.690~0.995) | 0.044 | |
| >1 site | 1.604 (1.337~1.924) | <0.001 | 1.399 (1.162~1.684) | <0.001 | |
| Age | ≤60 | 1 | 1 | ||
| >60 | 1.329 (1.178~1.500) | <0.001 | 1.152 (1.017~1.304) | 0.026 | |
| Race | white | 1 | 1 | ||
| black | 1.204 (1.033~1.402) | 0.017 | 1.103 (0.946~1.287) | 0.210 | |
| Asian or Pacific Islander | 0.813 (0.649~1.019) | 0.072 | 0.728 (0.579~0.914) | 0.006 | |
| Others/Unknown | 1.073 (0.591~1.945) | 0.818 | 1.085 (0.596~1.976) | 0.789 | |
| Marital status | Married | 1 | |||
| Unmarried | 1.132 (0.995~1.287) | 0.061 | |||
| Unknown | 1.118 (0.836~1.497) | 0.451 | |||
| Tumor grade | Low | 1 | 1 | ||
| High | 1.281 (1.088~1.507) | 0.003 | 1.224 (1.038~1.444) | 0.016 | |
| Unknown | 1.234 (1.040~1.463) | 0.016 | 1.048 (0.880~1.249) | 0.596 | |
| AJCC T Stage | T1 | 1 | |||
| T2 | 0.861 (0.682~1.086) | 0.206 | |||
| T3 | 1.076 (0.878~1.319) | 0.478 | |||
| T4 | 1.172 (0.918~1.497) | 0.202 | |||
| TX | 1.449 (1.158~1.812) | 0.001 | |||
| Lymph Node metastases | N0 | 1 | |||
| N1 | 1.035 (0.901~1.190) | 0.625 | |||
| NX | 1.366 (1.131~1.649) | 0.001 | |||
| Surgery | Yes | 1 | 1 | ||
| No/unknown | 1.551 (1.223~1.967) | <0.001 | 1.529 (1.201~1.947) | 0.001 | |
| Beam Radiation | Yes | 1 | 1 | ||
| No/unknown | 1.567 (1.389~1.767) | <0.001 | 1.379 (1.216~1.565) | <0.001 | |
| Chemotherapy | Yes | 1 | 1 | ||
| No/unknown | 3.137 (2.764~3.560) | <0.001 | 2.964 (2.594~3.387) | <0.001 | |
Discussion and conclusions
This large-population based study, which include 1347 patients with distant metastatic cervical cancer, revealed the following phenomenon: (1) The most common metastatic site of cervical cancer was the lung. (2) The prognosis of single-site metastasis was similar. However, the single-site metastatic patients survived longer than multi-site metastatic patients. (3) Surgery, radiotherapy, and chemotherapy all brought benefit to patients with metastases. (4) Age, race, tumor stage, surgery, radiotherapy, chemotherapy, and metastatic sites were independent prognostic factors for patients with metastatic cervical cancer.
Our study showed that the most common single metastatic site for cervical cancer was the lung (37.9%), followed by bone (16.7%). Consistent with our study, Carlson et al. studied 341 patients who developed distant metastasis and discovered that lung metastasis (36.3%) and bone metastasis (16.3%) were the most prevalent metastasis in cervical cancer [10]. The preference of lung and bone metastases in cervical cancer was also observed by Hwang and Zighelboim [11,12]. Referring to the mechanism of the frequent lung metastasis, “seed and soil” hypothesis may be an explanation [13]. In other words, lung may have a favorable microenvironment which may benefit cervical cancer cell survival and proliferation. Cervical cancer cells may transform themselves to adapt to lung. For instance, a study showed that cervical cancer cells can promote lung metastasis by upregulating CXCR4, a receptor of chemokine [13]. Surprisingly, one study from China reported that bone metastasis in cervical cancer is more common than lung metastasis [9], which may be explained by the ethnicity discrepancy and different usage rate of bone scans. It would be interesting to assess the effects of ethnicity of metastatic pattern in cervical cancer.
In terms of clinical characteristics of the various metastatic sites, about half of the patients with lung metastases were older than 60, while less than 40% of the patients with other metastases were older than 60. This is consistent with Yamamoto’s work which showed that half of the patients with lung metastases were older than 60 years old [14]. Since lung is the most common metastatic site in cervical cancer, we suspected that the clinician may screen the lung rather than other sites for older patients. As a result, the lung metastatic patients showed older than other site metastatic patients. Another phenomenon of lung metastasis was the poorer differentiation and higher tumor grade compared with other oligometastatic patients. One speculation concerns the features of high malignancy of cervical cancer cells which metastases to lung.
Although cervical cancer patients with lung metastases were older and with higher malignancy, the prognosis of lung metastasis and other single metastases were similar, with a median survival of 9 months in lung metastasis, 7 months in liver metastasis, 6 months in brain metastasis, and 8 months in bone metastasis. It is consistent with previous research which showed that cervical cancer patients have 7-10 months of survival months with lung, bone, or brain metastases [15-17]. However, it is somewhat surprising since several previous studies indicate that liver metastasis may exhibit a worse outcome (Yin et al., 2019; Kim et al., 2017). For example, Yin’s study, containing 99 Chinese cervical cancer patients with metastasis showed that liver metastatic patients have signifying particularly poor overall survival than other metastasis [9]. Kim’s research also found that patients with recurrence in lung after treatment have a better outcome than with recurrence in liver after treatment [18]. One explanation for this variation is that the racial or post-treatment metastasis may play a role. Further study is needed to find whether the liver metastatic patients have poorer prognosis.
In terms of treatment, our results showed that surgery, radiotherapy, and chemotherapy can improve the prognosis of single-site and multi-site metastases. These results reflected those of Ning et al. (1992) who also found that radiation therapy can local control oligometastatic cervical cancer and improve survival [19]. A review from li et al. showed that surgery, chemotherapy, and the combination of surgery and chemotherapy are valuable treatments of lung metastatic patients [3,12,20,21]. Chemotherapy and radiotherapy in bone are promising for patients with bone or brain metastasis [3]. Accordingly, our results showed that multi-organ metastatic cervical cancer can also benefit from radiotherapy, which suggest a proactive treatment in those multisite metastatic patients. Further investigation and experimentation into the effect of treatment in multisite metastatic cervical cancer patients is strongly recommended.
As for prognostic factors, Basta showed that age, clinical stage, grade, and treatment are significant factors in survival of cervical cancer patients [22]. Consistent with these factors, we also found that age, tumor stage, surgery, radiotherapy, and chemotherapy were independent prognostic factors. Additionally, we further discovered that race and metastatic sites are also prognostic factors. The Asian or Pacific Islander has lower motility compared with white patients, which further support the idea of Asian survival advantage in cervical cancer [23].
Similar to other studies using SEER as a data source, there are also some flaws in our study. First, the data we analyzed is retrospective in nature and there is inherent selection bias despite our effort to control for confounding variables. Furthermore, the metastatic sites other than brain, lung, liver, and bone were not included. Additionally, there is no detailed chemotherapy drug information and detailed dose of radiation, thus we cannot further analyze the precise effect of chemo-drugs. Finally, the misclassification may be a concern of the SEER database for years. Despite these limitations, our results showed that a single metastasis harbor similar outcome regardless of the metastatic site; and multi-organ metastasis showed a worse prognosis than single metastasis. Surgery, radiation, and chemotherapy can prolong survival time of metastatic patients, either in single metastasis or multi-organ metastasis. These results may contribute to the treatment chosen and prognosis prediction in the clinical practice.
Acknowledgements
This work is supported by the Zhejiang Provincial Public Welfare Technology Research Program (LGF18H160028).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Li H, Wu X, Cheng X. Advances in diagnosis and treatment of metastatic cervical cancer. J Gynecol Oncol. 2016;27:e43. doi: 10.3802/jgo.2016.27.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng K, Yang C, Tan Q, Song W, Lu M, Zhao W, Lou G, Li Z, Li K, Hou Y. Sites of distant metastases and overall survival in ovarian cancer: a study of 1481 patients. Gynecol Oncol. 2018;150:460–465. doi: 10.1016/j.ygyno.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Wu W, He X, Andayani D, Yang L, Ye J, Li Y, Chen Y, Li L. Pattern of distant extrahepatic metastases in primary liver cancer: a SEER based study. J Cancer. 2017;8:2312–2318. doi: 10.7150/jca.19056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oweira H, Petrausch U, Helbling D, Schmidt J, Mannhart M, Mehrabi A, Schöb O, Giryes A, Decker M, Abdel-Rahman O. Prognostic value of site-specific metastases in pancreatic adenocarcinoma: a surveillance epidemiology and end results database analysis. World J Gastroenterol. 2017;23:1872–1880. doi: 10.3748/wjg.v23.i10.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen MT, Sun HF, Zhao Y, Fu WY, Yang LP, Gao SP, Li LD, Jiang HL, Jin W. Comparison of patterns and prognosis among distant metastatic breast cancer patients by age groups: a SEER population-based analysis. Sci Rep. 2017;7:9254. doi: 10.1038/s41598-017-10166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn MA, Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, Ngan HY, Pecorelli S. Carcinoma of the cervix uteri. FIGO 26th annual peport on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S43–S103. doi: 10.1016/S0020-7292(06)60030-1. [DOI] [PubMed] [Google Scholar]
- 9.Yin Z, Tang H, Li L, Ni J, Yuan S, Lou H, Chen M. Impact of sites versus number of metastases on survival of patients with organ metastasis from newly diagnosed cervical cancer. Cancer Manag Res. 2019;11:7759–7766. doi: 10.2147/CMAR.S203037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson V, Delclos L, Fletcher GH. Distant metastases in squamous-cell carcinoma of the uterine cervix. Radiology. 1967;88:961–966. doi: 10.1148/88.5.961. [DOI] [PubMed] [Google Scholar]
- 11.Hwang JH, Lim MC, Seo SS, Kang S, Park SY, Kim JY. Outcomes and toxicities for the treatment of stage IVB cervical cancer. Arch Gynecol Obstet. 2012;285:1685–1693. doi: 10.1007/s00404-011-2173-6. [DOI] [PubMed] [Google Scholar]
- 12.Zighelboim I, Taylor NP, Powell MA, Gibb RK, Rader JS, Mutch DG, Grigsby PW. Outcomes in 24 selected patients with stage IVB cervical cancer and excellent performance status treated with radiotherapy and chemotherapy. Radiat Med. 2006;24:625–630. doi: 10.1007/s11604-006-0082-6. [DOI] [PubMed] [Google Scholar]
- 13.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto K, Yoshikawa H, Shiromizu K, Saito T, Kuzuya K, Tsunematsu R, Kamura T. Pulmonary metastasectomy for uterine cervical cancer: a multivariate analysis. Ann Thorac Surg. 2004;77:1179–1182. doi: 10.1016/j.athoracsur.2003.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Kim GE, Lee SW, Suh CO, Park TK, Kim JW, Park JT, Shim JU. Hepatic metastases from carcinoma of the uterine cervix. Gynecol Oncol. 1998;70:56–60. doi: 10.1006/gyno.1998.5037. [DOI] [PubMed] [Google Scholar]
- 16.Hayashi N, Takahashi H, Hasegawa Y, Higuchi F, Takahashi M, Makino K, Takagaki M, Akimoto J, Okuda T, Okita Y, Mitsuya K, Hirashima Y, Narita Y, Nakasu Y Committee of Brain Tumor Registry of Japan Supported by the Japan Neurosurgical Society. A nationwide multi-institutional retrospective study to identify prognostic factors and develop a graded prognostic assessment system for patients with brain metastases from uterine corpus and cervical cancer. BMC Cancer. 2017;17:397. doi: 10.1186/s12885-017-3358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim H, Lee KK, Heo MH, Kim JY. The prognostic factors influencing overall survival in uterine cervical cancer with brain metastasis. Korean J Intern Med. 2019;34:1324–1332. doi: 10.3904/kjim.2018.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim TH, Kim MH, Kim BJ, Park SI, Ryu SY, Cho CK. Prognostic importance of the site of recurrence in patients with metastatic recurrent cervical cancer. Int J Radiat Oncol Biol Phys. 2017;98:1124–1131. doi: 10.1016/j.ijrobp.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Ning MS, Ahobila V, Jhingran A, Stecklein SR, Frumovitz M, Schmeler KM, Eifel PJ, Klopp AH. Outcomes and patterns of relapse after definitive radiation therapy for oligometastatic cervical cancer. Gynecol Oncol. 2018;148:132–138. doi: 10.1016/j.ygyno.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 20.Luvero D, Plotti F, Aloisi A, Capriglione S, Ricciardi R, Miranda A, Lopez S, Scaletta G, De Luca G, Benedetti-Panici P, Angioli R. Patients treated with neoadjuvant chemotherapy + radical surgery + adjuvant chemotherapy in locally advanced cervical cancer: long-term outcomes, survival and prognostic factors in a single-center 10-year follow-up. Med Oncol. 2016;33:110. doi: 10.1007/s12032-016-0830-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen CC, Wang L, Lin JC, Jan JS. The prognostic factors for locally advanced cervical cancer patients treated by intensity-modulated radiation therapy with concurrent chemotherapy. J Formos Med Assoc. 2015;114:231–237. doi: 10.1016/j.jfma.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Basta T, Gawron I, Mirocki K, Babczyk D, Jach R. Analysis of histological type and staging of cervical cancer as prognostic factors among women treated in the department of gynecology and oncology, Jagiellonian university in the years 2001-2014. Przegl Lek. 2017;74:13–20. [PubMed] [Google Scholar]
- 23.Hou Y, Guo S, Lyu J, Lu Z, Yang Z, Liu D, Chen Z. Prognostic factors in Asian and white American patients with cervical cancer, considering competing risks. Curr Oncol. 2019;26:e277–e285. doi: 10.3747/co.26.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]


