Abstract
This study aims to observe the effects of the combined application of rat bone marrow mesenchymal stem cells (rBMSCs) and a bioceramic material on pulp-like tissue formation. Rat incisor root fragments without pulp tissues were prepared and filled with a collagen scaffold seeded with rBMSCs, while one side of the root segment was covered by a bioceramic material (iRoot BP). After they were cultured for 12 hours, the root fragments were implanted subcutaneously for 3 months. Hematoxylin and eosin (HE) staining was applied to observe the biocompatibility and the formation of pulp-like tissues. The incisor root fragments were divided into three parts (BP1/3, M1/3, and D1/3) to analyze the areas and the number of new vessels. Immunohistochemical staining of the neuroendocrine marker PGP9.5, the dentin sialophosphoprotein (DSPP), and the vascular endothelial growth factor (VEGF) was applied to observe the formation of the pulp-like tissues. Root fragments filled with only the collagen scaffold were used as a control. Three months after the implantation, the root fragments were collected, and they were surrounded by a transparent tissue membrane with a good blood supply. The root fragment cavity was filled with pink vascularized pulp-like tissue. According to the HE results, iRoot BP had good biocompatibility with the new pulp-like tissues and a few infiltrating inflammatory cells. Increases in the number and area of the new blood vessels were observed in BP1/3 compared with the other two parts. The PGP9.5 and DSPP expressions showed that the newly formed tissues were similar to normal pulp tissues. iRoot BP has good biocompatibility and increases the number and area of new blood vessels. The combined application of stem cells and bioceramic materials may be a better method for pulp revascularization.
Keywords: Pulp regeneration, iRoot BP, Bone marrow mesenchymal stem cells, revascularization
Introduction
In recent years, with the progress in cell biology and bioengineering, pulp regeneration has become a research focus and a new trend in the field of pulp disease treatment [1,2]. The main purpose of pulp regeneration is to regenerate necrotic or infected pulp and to restore its normal activity and physiological functions, including its immune functions, the nutritional functions of its blood vessels, and the sensory functions of its nerves [3,4]. Evidence has shown that the bioceramic material iRoot BP enhances the adherence, migration, attachment, proliferation, and mineralization of dental pulp stem cells [5] and induces mineralization of human dental pulp cells and the expression of genes related to odontoblast differentiation in vitro [6,7]. However, the effects of the combined application of rat bone marrow mesenchymal stem cells (rBMSCs) and a bioceramic material on pulp-like tissue formation in vivo are not fully understood [8]. In this study, rat incisor root fragments filled with a collagen scaffold seeded with rBMSCs with one side covered by iRoot BP were implanted subcutaneously to observe the combined effect of rBMSCs and a bioceramic material on pulp-like tissue formation.
Materials and methods
Disinfection of the root segments
The mandibular incisor teeth of Wistar rats were removed completely, the pulp was first removed with a pulp extraction needle, and 5.25% sodium chloride was used to remove the remaining pulp. The incisors were ground into 3-mm long root segments. The root fragments were sterilized in 75% alcohol at room temperature. Before use, the fragments were rinsed three times with PBS containing penicillin (100 U/ml) and streptomycin (100 µg/ml). After drying, one side of the root segment was covered by iRoot BP in a thin layer for cell seeding under aseptic conditions.
BMSC culture and cell seeding
The protocol for rBMSC culture has been described previously [9]. Each collagen membrane was cut into small pieces of several triangular segments with lengths of 0.5 and 3 mm to allow maximal cell attachment under the aseptic conditions. The collagen membrane was packed into the pulp cavity compactly and then placed into a 96-well plate. The cells were suspended at a density of 1×106/ml and seeded on the collagen scaffold. The cells were cultured in a CO2 constant temperature incubator for 12 h. Scanning electron microscopy (SEM) was used to observe the attachment and morphology of the scaffolds (Figure 1).
Figure 1.
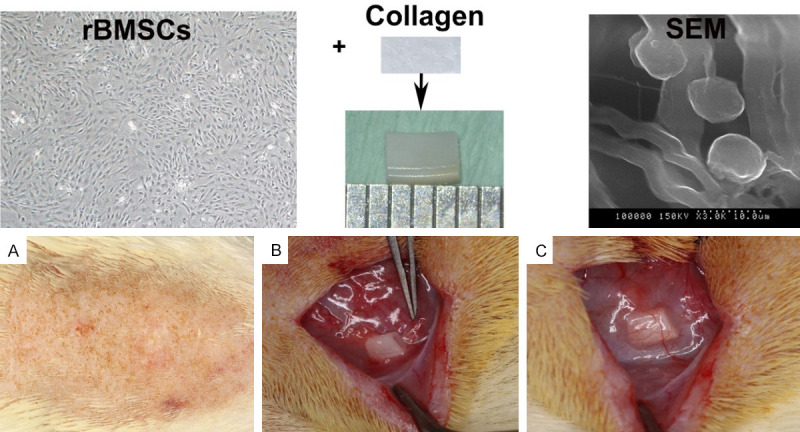
Protocol for rBMSC seeding and subcutaneous implantation. After they were cultured for 12 hours, a large number of rBMSCs had attached to the collagen scaffold. A. Skin preparation for surgery. B. Finding the location of the abundant blood supply. C. Placing the root segments in the subcutaneous mucosa.
The application of iRoot BP at one end of the root segment
One side of the root segment was covered by iRoot BP (Innovative Bioceramix, Vancouver, BC, Canada) under aseptic conditions and placed in a 37°C incubator for 2 h to harden the iRoot BP. An rBMSC suspension was placed on the root canal with the collagen membrane and incubated in the CO2 incubator for 12 h.
Establishment of a rat subcutaneous model
After inducing intraperitoneal anesthesia with 10% chloral hydrate at 4 ml/kg, the Wistar rats were routinely disinfected. A longitudinal incision of approximately 3 cm was made on the central skin of the back of the rats using surgical scissors. Blunt separation was applied on both sides with hemostatic forceps to find a location with an abundant blood supply, and the root segments were placed in the subcutaneous mucosa. The experimental groups were as follows: the rBMSC+ iRoot BP group (a collagen scaffold composite of rBMSCs covered by iRoot BP was placed in the root canal), the rBMSC group (a collagen scaffold composite of rBMSCs was placed in the root canal), the collagen group (only the collagen membrane was placed in the canal), and the blank group (only the root canal). Three months after the implantation, the specimens were gathered for histological and immunohistochemical analyses.
Tissue preparation and histological assessment
The specimens were fixed in a 4% polyformaldehyde/phosphate buffer for 24 hours. The samples were then demineralized in 10% EDTA for 2 months and embedded in paraffin. Sections with a thickness of 5 μm were prepared for HE staining, vascular area analysis, and immunohistochemistry (IHC).
Statistical analysis
All the data are expressed as the mean ± standard deviation. One-way analyses of variance and Newman-Keuls tests were conducted using GraphPad Prism 5 software.
Results
rBMSC seeding and a rat subcutaneous model
rBMSCs (passage 3) were seeded onto a collagen scaffold for 12 hours and then observed using SEM. The SEM showed that a large number of rBMSCs had attached to the collagen scaffold, and the cells and collagen membrane fiber scaffold had a certain adhesion and relatively uniform distribution. The root segments were placed in the subcutaneous mucosa (Figure 1).
General observations of the root fragments after implantation
The root fragments were placed in well blood-supplied subcutaneous areas of the rats. After 3 months, the specimens were removed from the rats for general observations. The surface was surrounded by a transparent tissue membrane with a good blood supply (Figure 2A, 2B). In the rBMSC+iRoot BP group, the pulp cavity was filled with pink or red tissues with a significant amount of blood vessels on the side without iRoot BP, and some red blood vessels covered the surface of the side where the iRoot BP was placed (Figure 2C). In the control group, both sides of the root segment had pink or red tissues with a significant amount of blood vessels filling the medullary cavity (Figure 2D).
Figure 2.
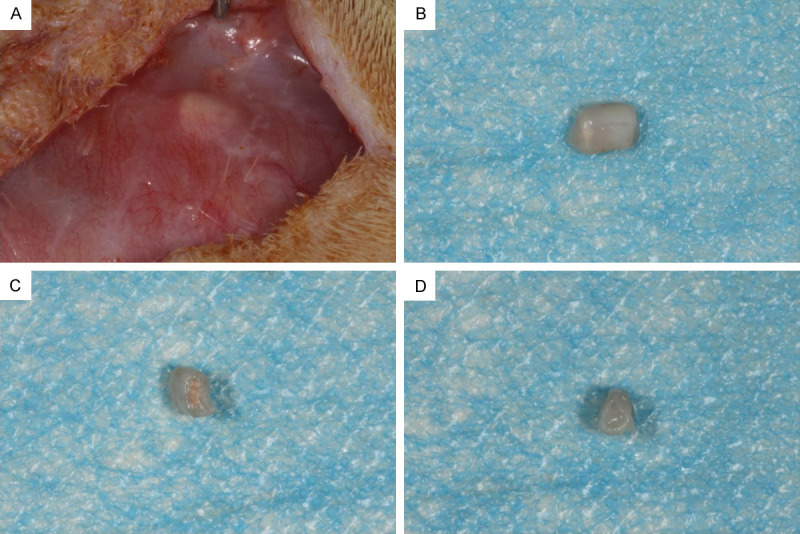
General observations of the root fragments after the 3-month implantation. A. The blood supply radiated around the tooth root. B. When the specimen was removed, the surface of the specimen was surrounded by a transparent tissue membrane. C. In the rBMSC+iRoot BP group, new tissue filled the root segment, and some red blood vessels covered the side of the BP. D. In the control group, the pulp cavity was filled with pink or red tissues with a significant number of blood vessels on both sides of the root segment.
Histological observations of the bioceramic material biocompatibility
The samples were observed using optical microscopy after the H&E staining, which showed that some collagen was not completely absorbed near the iRoot BP, and the cells in the pulp-like tissue were scattered in the iRoot BP space, contacted the BP directly, and grew well. Few infiltrating inflammatory cells were found without any adverse reactions (Figure 3A-D). In addition to root segments, cells in the tooth body were perfectly fused with the iRoot BP (Figure 3E, 3F).
Figure 3.
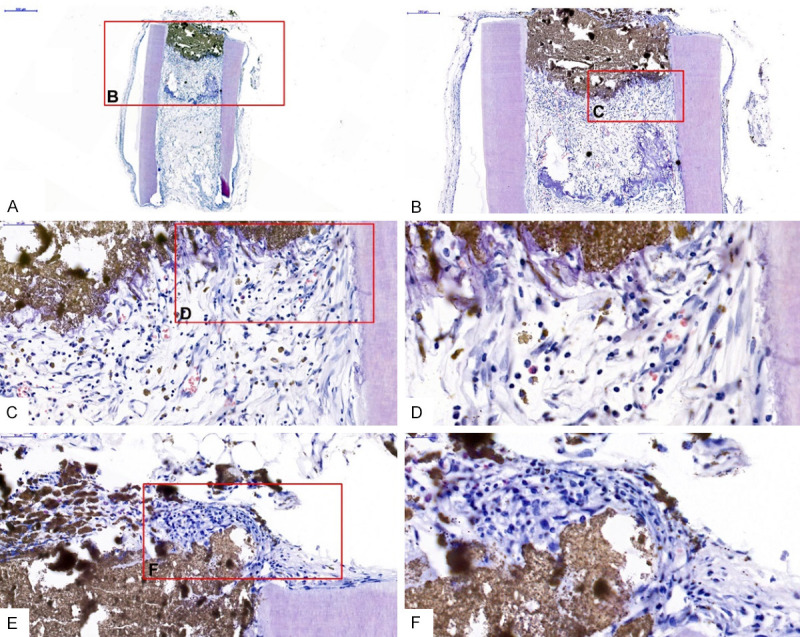
Histological observations of the iRoot BP biocompatibility. iRoot BP was in direct contact with the new dental pulp-like tissue without any adverse reactions (A-D). iRoot BP had a good compatibility with the tooth root tissue (E and F).
Analysis of the neovascularization in the new pulp-like tissues
In the rBMSC+ iRoot BP group, a large number of new blood vessels were seen near the BP site - significantly more than in the middle and opening of the root (Figure 4A-C). The number and area of new blood vessels were greater in BP1/3 than in M1/3 and D1/3, which further indicated that iRoot BP significantly promoted the formation of neovascularization in pulp-like tissue (Figure 4D, 4E). In addition, closure by BP affected the absorption rate of the collagen scaffold to some extent. The collagen scaffolds in the middle of the root were not absorbed as thoroughly as those at the opening (Figure 4).
Figure 4.
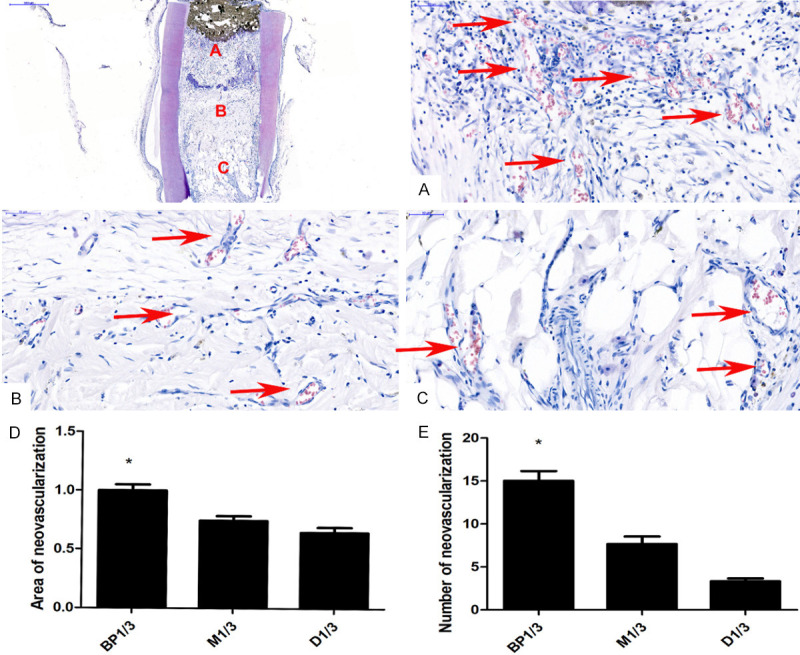
Analysis of the neovascularization in the new pulp-like tissues. A. The number of new blood vessels in BP1/3. B. The number of new blood vessels in M1/3. C. The number of new blood vessels in the opening. D. Comparison of the BP1/3, M1/3, and D1/3 neovascularization areas. *P < 0.05. E. Comparison of the amount of neovascularization in BP1/3, M1/3, and D1/3. *P < 0.05.
The neuroendocrine marker PGP9.5 and DSPP expressions in the newly formed tissues
DSPP (the complex of DSP and DPP) is a kind of non-collagen, which plays an important role in dentin mineralization. After the DSPP immunohistochemical staining, the immunoreactive cells were mainly distributed in the newly formed pulp-like tissues, especially cells arranged with the newly formed dentin-like tissues. The results revealed that immunoreactivity for DSPP in the rBMSC group was significant throughout the regenerated soft tissues, appearing as a light-brown, unevenly stained area (Figure 5A). Overall moderate PGP9.5 staining was also seen in the regenerated pulp-like tissues as a non-uniform area of cable-like tan (shown by the red arrow), which was particularly intense in the nerve-like cells (Figure 5B). This observation suggested the formation of nerve endings in the pulp-like tissues. The immunoreactivity for VEGF in the rBMSC+ iRoot BP group was significant throughout the regenerated soft tissues, appearing as a light-brown, unevenly stained area (Figure 5C). This observation suggested angiogenesis in the new pulp tissues of the teeth.
Figure 5.
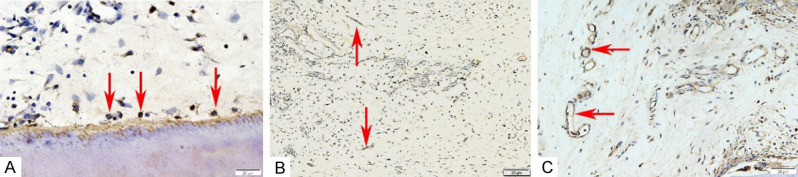
The expressions of the neuroendocrine marker PGP9.5 and DSPP in the newly formed tissues. A. The arrow points to DSPP positive staining. B. The PGP9.5 immunohistochemical staining (The arrow points to a positive stain). C. The VEGF immunohistochemical staining (The arrow points to a positive stain).
Histological observations of the rBMSC and collagen groups
In the rBMSC group, the root was filled with new pulp-like tissues, and the regeneration of the blood vessels was clearly seen (Figure 6A, 6B). In the collagen group, only the collagen membrane was placed on the lumen. After the staining, most of the collagen on the lumen was not degraded completely, and fibrous tissue grew on both sides of the root canal, which was continuous with the lateral tissue (Figure 6C). In the blank group, only the root was implanted subcutaneously in rats. There was fibrous tissue growing in the opening of both sides of the root, but no new tissue formation was found in the middle part (Figure 6D).
Figure 6.
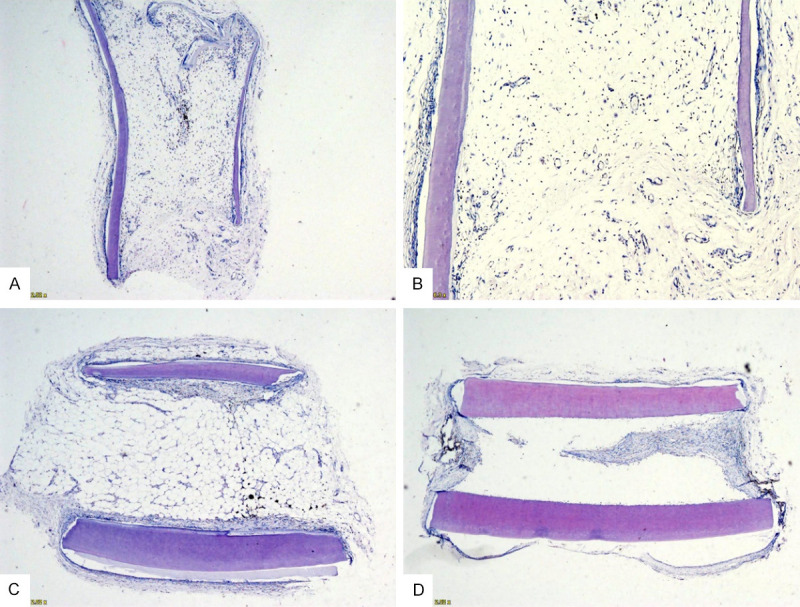
A histological observation of the control groups. Pulp-like tissue filled the lumen, and new blood vessels were seen at the opening in the rBMSC group (A and B). (C) An incomplete absorption of a collagen membrane was observed in the collagen group (C). A few fibrous tissues were found growing into the opening on both sides of the root in the blank group (D).
Discussion
There are two strategies for pulp regeneration at present: cell transplantation and homing. The former method is based on cells by transplanting exogenous stem cells onto scaffolds [10]. Root fragment models for pulp regeneration have been discussed in many studies, which show that pulp-like tissues can be produced in vivo by the transplantation of dental pulp or other stem cells into tooth slices or fragments [11-14]. Previous studies have selected human single premolar fragments as carriers for implantation into animals. In our study, we chose the rat incisor root as a carrier to avoid the immune rejection of the experimental animals to the greatest extent. Rat bone marrow mesenchymal stem cells (rBMSCs) combined with collagen membrane scaffolds were implanted into the rat incisor teeth fragments, and new tissue filling the pulp cavity was observed in the root segment of the rat incisor teeth. H&E and immunohistochemical staining showed the appearance of odontoblast-like cells and neovascularization.
iRoot BP, a nano-bioceramic material, is a recently-developed bioceramic-based endodontic cement with improved performance compared with MTA [15]. Its main components include calcium trisilicate, calcium disilicate, calcium phosphate, tantalum oxide, and zirconi [16]. Compared with MTA, iRoot BP plus has the same cytotoxicity, apical closure, and antimicrobial activity [17]. In addition, it overcomes the shortcomings of MTA. Therefore, iRoot BP plus has recently been considered an alternative to MTA.
Several studies have shown that iRoot BP can be used for direct pulp capping without pulp inflammation [18]. However, there is no report on its application to pulp regeneration. In our study, one side of the root segment was closed by iRoot BP, which showed that iRoot BP had good biocompatibility with new dental pulp tissues, and odontoblast-like cells and neovascularization were seen in the new tissues.
Angiogenesis is a crucial cellular morphogenesis process through which new blood vessels grow from existing blood vessels, penetrate the extracellular matrix, and generate new blood vessels to meet local metabolic needs [19,20]. Promoting angiogenesis has been emphasized as a key strategy in regenerative medicine, which stimulates the repair of damaged tissues, such as bone, cartilage, muscles, and nerves, by providing effective nutrition and oxygen [21,22]. Angiogenesis is thought to be an important factor in pulp regeneration, because only blood vessels are generated in the canal space, leading to the long-term stability of the newly formed tissues [5]. In the iRoot BP+rBMSC group, iRoot BP slowed the absorption of collagen, but the neovascularization near BP1/3 was significantly higher than it was at the other sites, indicating that iRoot bp significantly promotes the regeneration of blood vessels.
Markers such as DMP1, DSPP, ALP, OCN, and RUNX2 are commonly used to evaluate the odontogenic differentiation of stem cells from different sources [23,24]. In our study, we chose DSPP as a marker of odontogenic differentiation. VEGF plays an important role in angiogenesis by promoting endothelial cell proliferation, increasing vascular permeability, and changing the biological effects of the extracellular matrix [25]. In this study, the PGP9.5 protein was selected to reflect the nervous system, because it is a specific marker of neurons and nerve fibers and reflects the degree of damage and repair of neurons and nerve fibers [26]. The IHC of DSPP, VEGF, and PGP9.5 showed that iRoot BP promotes the differentiation of mesenchymal stem cells into dentin and generated pulp-like tissue with blood vessels and nerves.
In summary, this study shows that iRoot BP has a significant role in promoting pulp regeneration and the formation of new blood vessels, and it has a good biocompatibility with the pulp-like tissues in the body.
Acknowledgements
This work was supported by the Jinan Health Science Development Fund (2017-1-14), the Dean’s Research Fund of Jinan Stomatological Hospital (2017-02), and the Clinical Medicine Science and Technology Innovation Plan of Jinan (201907099).
Disclosure of conflict of interest
None.
References
- 1.Rombouts C, Giraud T, Jeanneau C, About I. Pulp Vascularization during tooth development, regeneration, and therapy. J Dent Res. 2017;96:137–144. doi: 10.1177/0022034516671688. [DOI] [PubMed] [Google Scholar]
- 2.Hashemi-Beni B, Khoroushi M, Foroughi MR, Karbasi S, Khademi AA. Tissue engineering: dentin - pulp complex regeneration approaches (a review) Tissue Cell. 2017;49:552–564. doi: 10.1016/j.tice.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Fu H, Wu X, Duan Y, Zhang S, Hu H, Liao Y, Wang T, Yang Y, Chen G, Li ZH, Tian WD. Regeneration of pulpo-dentinal-like complex by a group of unique multipotent CD24a(+) stem cells. Sci Adv. 2020;6:eaay1514. doi: 10.1126/sciadv.aay1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ercal P, Pekozer GG. A current overview of scaffold-based bone regeneration strategies with dental stem cells. Adv Exp Med Biol. 2020 doi: 10.1007/5584_2020_505. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Zhu LX, Cheng X, Lin Y, Yan P, Peng B. Promotion of dental pulp cell migration and pulp repair by a bioceramic putty involving fgfr-mediated signaling pathways. J Dent Res. 2015;94:853–862. doi: 10.1177/0022034515572020. [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Yang X, Fan M. BioAggregate and iRoot BP Plus optimize the proliferation and mineralization ability of human dental pulp cells. Int Endod J. 2013;46:923–929. doi: 10.1111/iej.12082. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed GM, Abouauf EA, AbuBakr N, Dörfer CE, El-Sayed KF. Tissue engineering approaches for enamel, dentin, and pulp regeneration: an update. Stem Cells Int. 2020;2020:5734539. doi: 10.1155/2020/5734539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eramo S, Natali A, Pinna R, Milia E. Dental pulp regeneration via cell homing. Int Endod J. 2018;51:405–419. doi: 10.1111/iej.12868. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LX, Shen LL, Ge SH, Wang LM, Yu XJ, Xu QC, Yang PS, Yang CZ. Systemic BMSC homing in the regeneration of pulp-like tissue and the enhancing effect of stromal cell-derived factor-1 on BMSC homing. Int J Clin Exp Pathol. 2015;8:10261–10271. [PMC free article] [PubMed] [Google Scholar]
- 10.Mao JJ, Kim SG, Zhou J, Ye L, Cho S, Suzuki T, Fu SY, Yang R, Zhou X. Regenerative endodontics: barriers and strategies for clinical translation. Dent Clin North Am. 2012;56:639–649. doi: 10.1016/j.cden.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33:2109–2118. doi: 10.1016/j.biomaterials.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima M, Iohara K. Regeneration of dental pulp by stem cells. Adv Dent Res. 2011;23:313–319. doi: 10.1177/0022034511405323. [DOI] [PubMed] [Google Scholar]
- 14.Iohara K, Zheng L, Ito M, Ishizaka R, Nakamura H, Into T, Matsushita K, Nakashima M. Regeneration of dental pulp after pulpotomy by transplantation of CD31(-)/CD146(-) side population cells from a canine tooth. Regen Med. 2009;4:377–385. doi: 10.2217/rme.09.5. [DOI] [PubMed] [Google Scholar]
- 15.Mukhtar-Fayyad D. Cytocompatibility of new bioceramic-based materials on human fibroblast cells (MRC-5) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112:e137–142. doi: 10.1016/j.tripleo.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 16.Shi S, Bao ZF, Liu Y, Zhang DD, Chen X, Jiang LM, Zhong M. Comparison of in vivo dental pulp responses to capping with iRoot BP Plus and mineral trioxide aggregate. Int Endod J. 2016;49:154–160. doi: 10.1111/iej.12439. [DOI] [PubMed] [Google Scholar]
- 17.Lu J, Li Z, Wu X, Chen Y, Yan M, Ge X, Yu J. iRoot BP Plus promotes osteo/odontogenic differentiation of bone marrow mesenchymal stem cells via MAPK pathways and autophagy. Stem Cell Res Ther. 2019;10:222. doi: 10.1186/s13287-019-1345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Wang S, Dong Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J Endod. 2015;41:652–657. doi: 10.1016/j.joen.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 20.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Sun X, Lee JH, Kim HW, Fu X, Leong KW. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv Drug Deliv Rev. 2019;146:209–239. doi: 10.1016/j.addr.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Torres AL, Bidarra SJ, Pinto MT, Aguiar PC, Silva EA, Barrias CC. Guiding morphogenesis in cell-instructive microgels for therapeutic angiogenesis. Biomaterials. 2018;154:34–47. doi: 10.1016/j.biomaterials.2017.10.051. [DOI] [PubMed] [Google Scholar]
- 23.Baldion PA, Velandia-Romero ML, Castellanos JE. Odontoblast-like cells differentiated from dental pulp stem cells retain their phenotype after subcultivation. Int J Cell Biol. 2018;2018:6853189. doi: 10.1155/2018/6853189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JH, Kang MS, Mahapatra C, Kim HW. Effect of aminated mesoporous bioactive glass nanoparticles on the differentiation of dental pulp stem cells. PLoS One. 2016;11:e0150727. doi: 10.1371/journal.pone.0150727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schertl P, Volk J, Perduns R, Adam K, Leyhausen G, Bakopoulou A, Geurtsen W. Impaired angiogenic differentiation of dental pulp stem cells during exposure to the resinous monomer triethylene glycol dimethacrylate. Dent Mater. 2019;35:144–155. doi: 10.1016/j.dental.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Miyahara K, Kato Y, Koga H, Dizon R, Lane GJ, Suzuki R, Akazawa C, Yamataka A. Visualization of enteric neural crest cell migration in SOX10 transgenic mouse gut using time-lapse fluorescence imaging. J Pediatr Surg. 2011;46:2305–2308. doi: 10.1016/j.jpedsurg.2011.09.020. [DOI] [PubMed] [Google Scholar]


