Abstract
Objective: To improve the understanding of epithelioid glioblastoma (E-GBM) and provide accurate basis for clinical diagnosis, treatment, and prognosis through the analysis of clinicopathologic characteristics, immunohistochemical expression, molecular characteristics, and prognosis of E-GBM. Methods: The clinicopathologic characteristics of 33 cases of E-GBM in our hospital from January 2015 to September 2019 were analyzed retrospectively. Kaplan Meier method was used for survival analysis. Multivariate Cox regression analysis was used to screen the independent risk factors affecting the survival time of patients. Results: Among 33 patients with E-GBM, 16 were male and 17 were female. The age ranged from 9 to 67 years old, with the median age of 36 years old and the average age of 38 years old. The tumor size (calculated by the largest diameter): 1-6 cm, average size: 3.5 cm. The ratio of smoking and non-smoking is 17:16. All the tumors were located in the cerebral hemisphere, and 26 cases (78.79%) of brain MR showed that the tumors invaded the cortex (white matter). Clinical symptoms: asymptomatic physical examination was found in 6 cases (18.18%), 5 cases (15.15%) had epilepsy history, 2 cases (6.06%) had malignant vomiting, 3 cases (9.09%) had hypertension history, and 17 cases (51.52%) had headache and dizziness. All patients received surgery (total or partial resection). Postoperative radiotherapy was given in 7 cases (21.00%), chemotherapy (TMZ temozolomide) in 3 cases (1.00%), and combined chemoradiotherapy in 16 cases (48.40%). Immunohistochemical staining: the positive rates of CK, GFAP, IDH-1, IDH-2, HMB45, Desmin, BRAF, P53, ATRX, INI-1, S-100, Ki-67 were 20/33, 30/33, 1/33, 1/33, 0/33, 0/33, 33/33, 5/33, 30/33, 33/33, 6/33, Ki-67 of all cases were higher than 40%, among which 11 cases were higher than 60%. The detection of related genes showed that 33 cases (100%) had BRAF V600E mutation. TERT mutation was found in 18 cases (54.5%); IDH1 mutation was found in 1 case (3%); MGMT promoter methylation was found in 15 cases (45.4%); EGFR amplification and 1p/19q co-deletion were not found in any cases. Conclusion: E-GBM is a highly invasive and rare malignant nervous system tumor, with poor prognosis and lack of clinical specificity. Immunohistochemically, the higher expression of CK, GFAP and Ki67 proliferation index is more conducive to the diagnosis and differential diagnosis of E-GBM. Smoking, brain MR showing tumor invasion of cortex, TERT mutation, radiotherapy, and chemotherapy are independent risk factors affecting the prognosis (survival time) of patients.
Keywords: Epithelioid, glioblastoma, clinical pathology, Cox analysis, prognosis
Introduction
Glioblastoma is the most common brain tumor. One of its subtypes, epithelioid GBM, is also known as adenoid GBM or GBM with epithelioid metaplasia. It was first reported by Kepes et al and first written into the classification of tumors of the central nervous system as a tentative isoform in 2016 [1,2]. It is mainly composed of epithelioid, melanoma like or rhabdoid cells with abundant cytoplasm, eccentric nuclei, and prominent nucleoli. Palisading and solid lamellar necrosis, high proliferative activity, more mitotic figures, and microvascular hyperplasia often occur. The median survival time is only a few months, and it is occasionally reported that it has a longer survival time [3,4]. The incidence rate of this subtype is low, and it is common in children and young adults. It is a highly malignant tumor. Generally, there is no EGFR amplification and IDH1 mutation, but BRAF V600E mutation exist in about half of the cases reported in the literature, and there are several single cases and a few case reports that E-GBM is often accompanied by the changes of TERT and MGMT methylation [3,5-8]. There was no prior report on the clinical correlation between these molecular changes and E-GBM. In this study, we summarized the clinical and pathologic characteristics of 33 cases of E-GBM, related immunohistochemistry, and molecular detection of related genes of glioma, so as to improve the further understanding of E-GBM to facilitate clinical treatment and prognosis.
Materials and methods
Clinical data
From January 2015 to September 2019, 33 patients with E-GBM were collected from Yantai Yuhuangding Hospital, who were confirmed by pathology and had follow-up data. All patients had no other tumor history, and no patients received tumor radiotherapy and chemotherapy before operation.
All cases in this study were followed up by telephone, hospital medical record room and household registration department of Public Security Bureau. Another 9 cases were not included in this study for lack of follow-up data due to reasons such as no further visit, refusal of follow-up, relocation, and telephone number change. Through the follow-up, we mainly obtain the survival time and outcome of patients, and study the time from the pathologic diagnosis to death because of tumor or the end of the study.
Immunohistochemistry
All the specimens were fixed with 4% neutral formaldehyde, embedded in paraffin, then sectioned continuously for 3 μm, and stained with H&E and immunohistochemistry respectively. Immunohistochemical staining was carried out by BenchMark XT automatic staining machine of Roche/Ventana company. The test steps and specifics were set in accordance with the operation manual and reagent instructions. PBS buffer was used instead of primary antibody as negative control. In addition, CK (cytokeratin), GFAP (glial fibrillary acidic protein), S-100, IDH1 (isocitrate dehydrogenase1), IDH2 (isocitrate dehydrogenase2), INI1 (SMACB1), HMB45 (Human Melanoma Black45), desmin, BRAF (v-RAF murine sarcoma viral oncogene homologueB1), P53, and ATRX (X-linked alpha thalassemia mental retardation syndrome gene) were detected by immunohistochemistry. Finally, the diagnosis of E-GBM was made by two senior pathologists. Cytokeratin, GFAP, IDH1, IDH2, HMB45, desmin, and BRAF were all cytoplasmic positive; INI1, P53 and ATRX were nuclear positive, S-100 was nuclear or cytoplasmic positive. All reagents were from Beijing Zhongshan Reagent Co., Ltd.
Genomic DNA extraction
Tissue DNA was extracted with the nucleic acid extraction kit (FFPE DNA) of Xiamen Amoy Diagnostics Co., Ltd. (China). The conventional sections were 3 µm thick; 10 of them were dewaxed and hydrated. 1 section for H&E staining was morphologically assessed for tumor cells. An area rich in tumor cells was selected, ensuring that the tumor component accounted for more than 50% of the total tissue. The tumor tissue was scraped into an EP tube based on corresponding H&E section, and treated with conventional dewaxing. An appropriate amount of lysate and protease was added, then DNA was collected and extracted and digested overnight in a 56°C water bath, and stored in a refrigerator at -20°C.
Fluorescence quantitative PCR detection of BRAF, TERT, IDH1 and EGFR genes
TERT and IDH1 gene detection kits were from Panthenon Biotechnology Co., Ltd; BRAF and EGFR gene detection kits were from Xiamen Amoy Diagnostics Co., Ltd. All the tests followed instructions of the kits. The total reaction was 50 µl, in which each reaction was set with a positive control, a negative control and external control. All reaction reagents are provided by the kits. The fluorescence quantitative PCR instrument was ABI7500, and the PCR reaction conditions of gene mutation detection were 42°C, 5 min; 94°C, 3 min; (94°C, 45 s; 60°C, 80 s), 40 cycles. After the reaction, the baseline is adjusted and threshold is manually or automatically adjusted according to the instruction of PCR instrument and the actual situation of fluorescence curve. The CT value of the mutation of each sample can be obtained by PCR instrument, and the interpretation can be carried out according to the interpretation mode of the results in the instruction manual.
Methylation specific PCR (MSP)
The extracted genomic DNA was modified by bisulfite with DNA methylation modification Kit (EZ DNA Methylation-goldTM Kit) (according to the operating instructions of the kit). After modification, the methylated cytosine (C) remained unchanged, while the unmethylated cytosine changed to uracil (U), which, in the subsequent PCR reaction, was paired with U base as template, presenting as A. The modified DNA samples were amplified with methylated and unmethylated primers, respectively, to amplify the enhancer containing nucleic acid between the promoter region-20 of MGMT gene and the first exon. Primer sequence reference [9], synthesized by Shanghai Biotechnology Co., Ltd. MGMT gene methylation primer: sense strand: 5’-TTTCGACGTTGGTAGGTTTTCGC-3’, antisense strand: 5’-GCACTCTTCCGAAAACGAAACG-3’. MGMT gene unmethylated primers: sense strand: 5’-TTTGTGTTTTGATGTTTGTAGGTTTTGT-3’, antisense strand: 5’-AACTCCACACTCTTCCAAAAACAAAACA-3’. The annealing temperature is 55°C and 63°C, respectively. For DNA amplification, Hotstart Taq DNA polymerase (TAKARA) was used. The reaction conditions were: 95°C for 5 min; 95°C for 45 s, annealing temperature of each pair of primers 72°C for 60 s; 72°C for 5 min after 35 cycles. PCR products were separated by 3% agarose gel electrophoresis, EB stained and the results were observed by UV Gel imaging system.
Fluorescence in situ hybridization (FISH)
1p/19q fracture probe and kit were from Guangzhou Anbiping Pharmaceutical Technology Co., Ltd. The experiment was carried out in strict accordance with the instructions. The cells with red signal number = green signal number were normal cells, and the cells with red signal number < green signal number were deleting cells. Deletion rate = number of gene-deleting cells ÷ total number of cells. High power visual field recorded 200 tumor cell nuclear signals. The ratio of gene-deleting signal cells to counting cells was ≥ 30%, indicating gene deletion. If the ratio of separated signal cells to counted cells was < 30%, it indicated no gene deletion.
Statistical analysis
All data were statistically analyzed by SPSS 17.0. Kaplan-Meier method was used for survival analysis, and survival curve was drawn. Single factor Log-rank method was used to analyze the influence of clinical characteristics on prognosis. Significant influencing factors in single factor Log-rank analysis were used as variables. Cox single factor and multivariate regression analysis were used to screen independent risk factors affecting the survival of patients. The difference was statistically significant if (P < 0.05).
Results
Clinicopathologic features
Among 33 patients with E-GBM, 16 were male and 17 were female. The ratio of male patients and female patients was 16:17, and the age ranged from 9 to 67 years old, with the median age of 36 years old and the average age of 38 years old. The tumor size (calculated by the largest diameter): 1-6 cm, average size: 3.5 cm, the ratio of smoking and non-smoking is 17:16. All the tumors were located in the cerebral hemisphere, and 26 cases (79%) of brain MRs showed that the tumors invaded the cortex (Figure 1). Clinical symptoms included: asymptomatic physical examination found 6 cases (18.18%), 5 cases (15.15%) of epilepsy history, 2 cases (6.06%) of malignant vomiting, 3 cases (9.09%) of hypertension history, 17 cases (51.52%) of headache and dizziness. All patients received surgery (total or partial resection), postoperative radiotherapy in 7 cases (21.00%), chemotherapy in 3 cases (1.00%), and combined chemoradiotherapy in 16 cases (48.40%). 25 (76.00%) died.
Figure 1.
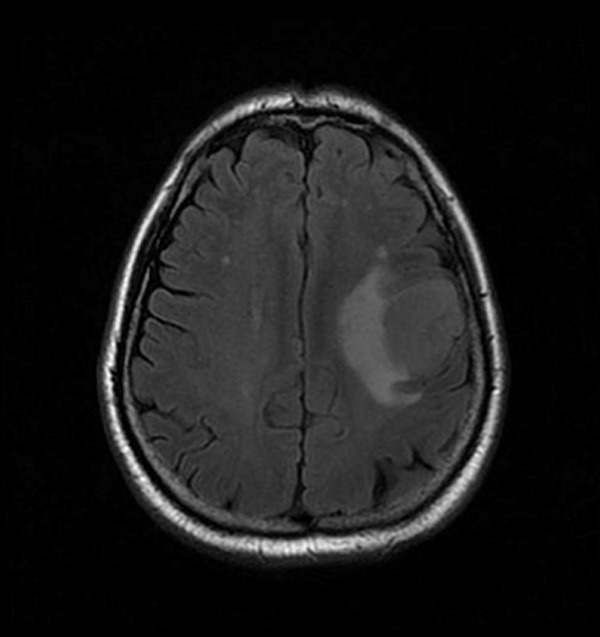
Brain MR indicating tumor invading cortex.
H&E morphology showed that E-GBM contained epithelioid and rhabdoid cells, with poor cell adhesion, abundant pink cytoplasm, prominent nucleoli, and increased necrosis and mitosis (Figure 2A, 2B). Immunohistochemistry showed that CK and GFAP were positive (Figure 2C, 2D), INI1 was positive (Figure 2E), and Ki67 proliferation index was high (Figure 2F).
Figure 2.
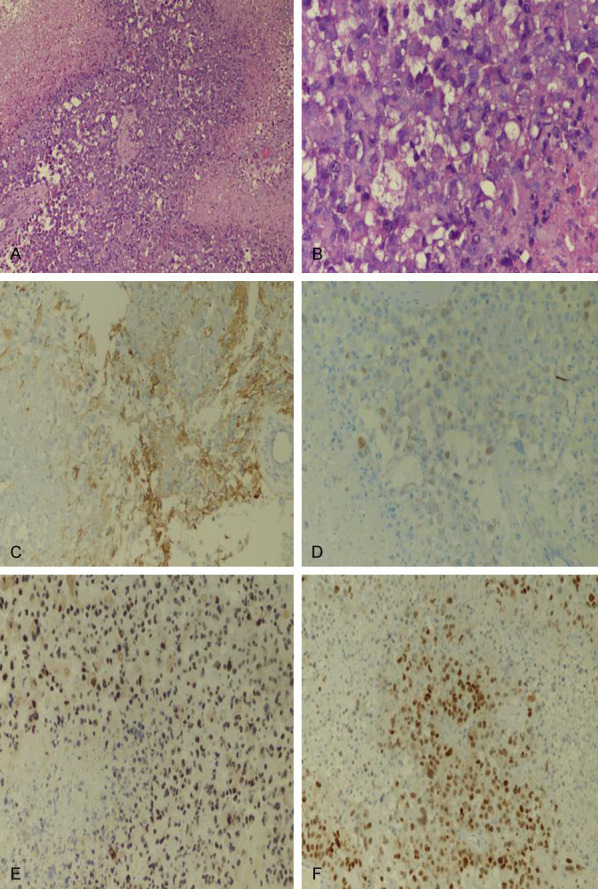
A. Tumor necrosis and epithelioid cells (magnification, ×200). B. High magnification of rhabdoid cells (magnification, ×400). C. GFAP focal weakly positive (magnification, ×200). D. CK focal weakly positive (magnification, ×200). E. No deletion of INI-1 (magnification, ×200). F. Higher Ki67 proliferation index (magnification, ×200).
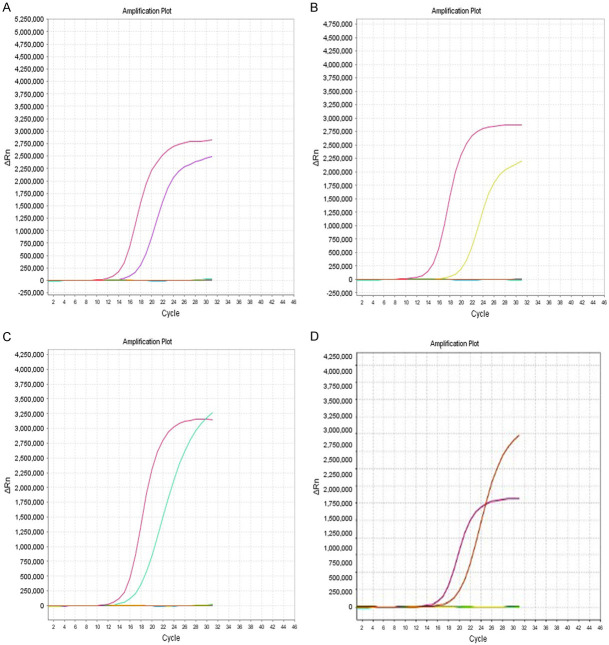
Related gene detection showed 33 cases (100%) of BRAF V600E mutation (Figure 3A), 18 cases (54.5%) of TERT mutation (16 cases of C228T; 2 cases of C250T) (Figure 3B, 3C), 1 case (3%) of IDH1 mutation (Figure 3D), and 15 cases (45.4%) of MGMT promoter methylation (Figure 4). EGFR amplification, PTEN mutation, and 1p/19q co-deletion were not found in all cases.
Figure 3.
A. BRAF V600E mutation. B. TERT (C228T) mutation. C. TERT (C250T) mutation. D. IDH1 mutation.
Figure 4.
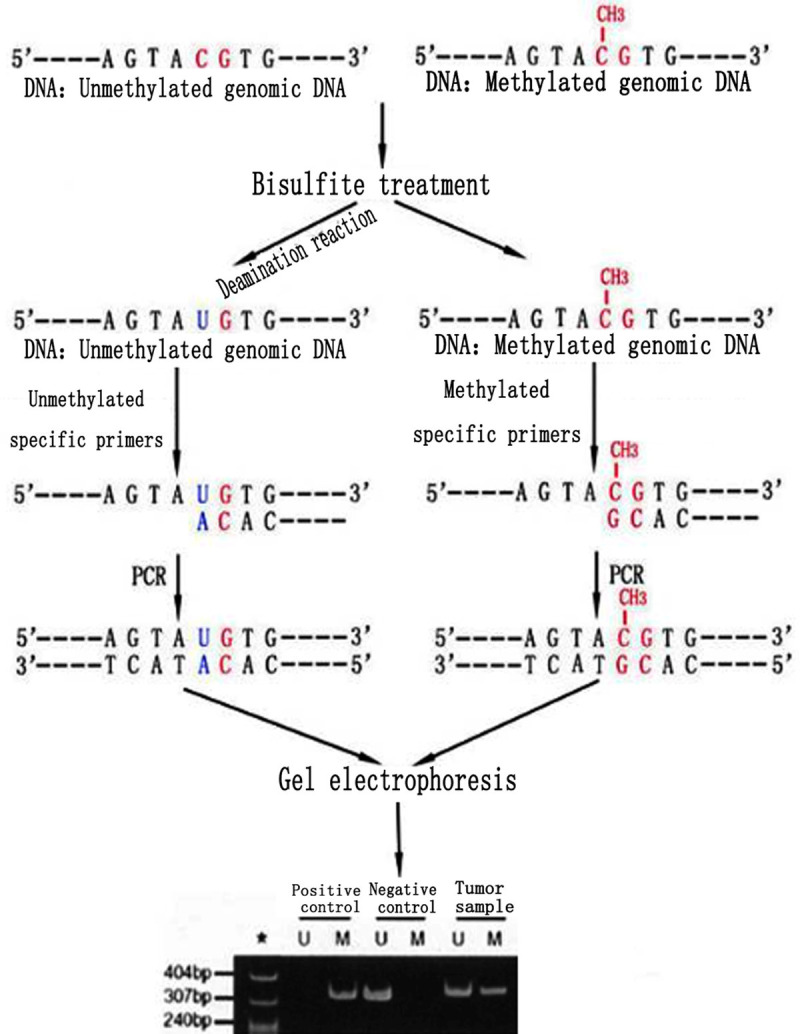
Methylation of tumor cells.
The relationship between clinicopathologic features and prognosis. The follow-up period is from the date of diagnosis to September 4, 2019, with a follow-up period of 2-32 months and a median follow-up period of 11 months. The follow-up data of 33 patients were obtained. 25 patients died during the follow-up period, with an average survival time of 13 months. The total survival rates at 1, 3 and 5 years were 63.63%, 39.39% and 0%, respectively.
The impact of TERT gene on the survival of patients
Of the 18 patients with mutation of TERT gene, 12 (66.67%) died; of the 15 patients without mutation of TERT gene, 13 (86.67%) died. The difference of total survival time between TERT mutations and non-mutations was significant (P = 0.018) Figure 5A.
Figure 5.
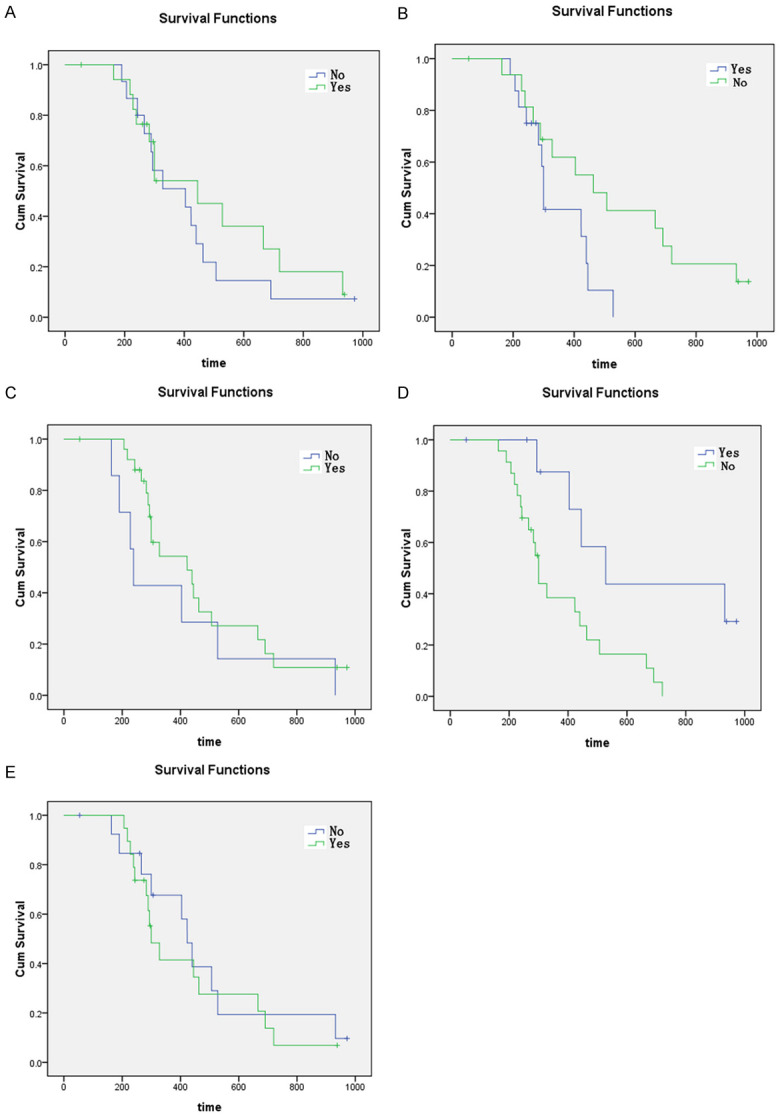
A-E. Survival curve of E-GBM patients with different pathologic findings.
The impact of smoking on the survival of patients
In 17 patients with smoking, 13 (76.47%) died; in 16 patients without smoking, 12 (75%) died; there was a significant difference in total survival between smoking and non-smoking (P = 0.004) Figure 5B.
The impact of MR showing tumor invasion of cortex on the survival of patients
In 26 patients with tumor invasion, 18 (69.23%) died; in 7 patients without tumor invasion, 7 (100%) died; there was a significant difference in the total survival time between patients with and without tumor invasion (P = 0.000) Figure 5C.
The impact of radiotherapy on survival
In 23 patients with radiotherapy, 20 patients (86.96%) died; in 10 patients without radiotherapy, 5 patients (50%) died; the difference of total survival time between patients with radiotherapy and without radiotherapy was significant (P = 0.004) Figure 5D.
Impact of chemotherapy on the survival of patients
In 19 patients with chemotherapy, 15 patients (78.95%) died; in 14 patients without chemotherapy, 10 patients (71.43%) died; the difference in total survival time between patients with and without chemotherapy was significant (P = 0.004) Figure 5E.
Prognosis of patients with E-GBM was assessed by multivariate Cox regression analysis with variable selection method. Smoking, MR showing cortical invasion, TERT mutation, radiotherapy and chemotherapy were independent risk factors for prognosis (survival time) (Table 1).
Table 1.
Multivariate Cox regression analysis of total survival time of E-GBM
| Variables | HR (95% CI) | P |
|---|---|---|
| TERT | ||
| Mutation | 0.189 | |
| No mutation | 0.012-4.789 | 0.018 |
| Smoking | ||
| Yes | 0.023 | |
| No | 0.011-1.967 | 0.004 |
| MR invasion of cortex | ||
| Yes | 0.013 | |
| No | 0.006-2.978 | 0.000 |
| Radiotherapy | ||
| Yes | 23.707 | |
| No | 1.457-109.34 | 0.004 |
| Chemotherapy | ||
| Yes | 11.774 | |
| No | 2.537-30.129 | 0.008 |
Discussion
GBM is the most common brain tumor with high risk and short survival time. As a new subtype of GBM, E-GBM was first called adenoid GBM or GBM with epithelioid metaplasia. It was first written into the classification of tumors of the central nervous system in 2016, which are more common in children and adolescents, and can also occur in the elderly [1]. The morphology is characterized by eosinophilic, nonadhesive epithelioid cells or rhabdoid cells, with prominent nucleoli, more necrosis and mitotic images, usually accompanied by BRAF mutation. It is a highly invasive tumor, with a median survival period of only a few months, occasionally reported with a longer survival period [2-5]. As of 2017, nearly 40 cases of E-GBM have been reported. The average age of onset is about 26.3 years, and the ratio of male to female is about 5:3 [8]; the data in this case showed that the incidence rate of male and female was near 1:1 (16:17). Age was reported as 9-67 years old, median age: 36 years. Tumor size (calculated according to the largest diameter) was 1-6 cm; average tumor size: 3.5 cm. The ratio of smoking to non-smoking was 17:16, and the relationship between tumor size and smoking has not been discussed and reported in the literature previously, so the sample size should be expanded for further verification. All the tumors were located in the cerebral hemisphere, and 26 cases (79%) showed cortical involvement on preoperative MR (Figure 1A), which was similar to the previous report. Clinical symptoms included asymptomatic physical examination found 6 cases (18.18%), 5 cases (15.15%) of epilepsy history, 2 cases (6.06%) of malignant vomiting, 3 cases (9.09%) of hypertension history, 17 cases (51.52%) of headache and dizziness. The clinical symptoms were similar to those of other brain tumors. All patients received surgery (total or partial resection), postoperative radiotherapy in 7 cases (21%), chemotherapy (temozolomide TMZ) in 3 cases (1%), and combined chemoradiotherapy in 16 cases (48.4%). 25 (76%) died. Combined with the analysis of 64 cases and 14 cases in the two groups, the mortality rates were 60% and 70% respectively, which further suggested that E-GBM was a highly malignant tumor [3,10].
Differentiation must be made between E-GBM and many kinds of tumors with epithelioid or rhabdoid cell morphology in the brain. (1) Unlike the pleomorphism, pseudopalisading necrosis and glomerular vascular hyperplasia of classical glioblastoma, E-GBM usually has the same cell morphology, and often has map-like necrosis and microvascular hyperplasia. The classical reactivity for GFAP is mostly positive, while CK and BRAF are both negative. However, among these immunostains, CK and GFAP were positive in different degrees, and BRAF was positive, which helped distinguish them [2]. (2) Anaplastic polymorphic yellow astrocytoma and E-GBM are common in young people, and both have a high mutation rate of BRAF. However, the latter lacks classical low-level regions, such as foam cells and eosinophilic granular bodies, and cells are usually single epithelioid [4]. (3) Atypical teratoid/rhabdoid tumor, AT/RT and E-GBM are all common in children or adolescents, and rhabdoid cells can be found in morphology, but the former may be accompanied by gene changes of SMARCB1 (INI-1) or SMARCB4 (BRG1), and immunohistochemistry shows that GFAP is negative, INI-1 is negative, in contrast to INI-1 being positive in E-GBM [7,11]; (4) Malignant melanoma may also have rhabdomyoid cells, and when S-100 and BRAF are positive, it should be differentiated from E-GBM. The former usually has melanin markers such as HMB-45 and Melan-A positive, while the latter is GFAP positive [7]. (5) E-GBM shows epithelioid cells, and when the epithelial marker CK is positive, it should be differentiated from metastatic cancer. Usually, this positive expression is focal weak expression, and the expression of glial cell markers GFAP and Olig-2 can help to distinguish the two [2,7].
At present, there are many single case reports on E-GBM, and the largest number of medical records is 64 cases, mainly focusing on morphologic and molecular genetic characteristics, and lacking relevant survival analysis [3,8,10]. In this study, we collected 33 cases, and multivariate analysis revealed that TERT mutation, smoking, brain MR of tumor invading cortex, radiotherapy and chemotherapy were correlated with prognosis. Previously there was no relevant literature report, because of the limited sample size. Verification will require expanding the sample.
In the literature, E-GBM is usually not accompanied by EGFR amplification, and there are few IDH1 mutations [3,12]. In this study, EGFR of 33 cases were not amplified, and only one patient had IDH1 mutation, which is consistent with the literature. In contrast, BRAF V600E mutations are reported in about half of the cases in the literature. In addition, it has been reported that E-GBM may be accompanied by the methylation of TERT and MGMT, but there are only a few cases reported [2,7,10,13]. Data of this group showed that the mutation of BRAF V600E was 100%, higher than the previously reported positive rate of 50%, which also shows that our study has high homogeneity and the samples should be expanded for further verification. It has been reported that TERT can maintain the length of chromosome by increasing the termini, and has the function of increasing cell stability, promoting cell proliferation, and avoiding apoptosis. At present, the reports of TERT mutation in gliomas are mainly concentrated in common gliomas, especially in low-grade gliomas. For example, there are reports that TERT mutation can enhance the sensitivity of WHO grade II and III gliomas with IDH wild-type to postoperative adjuvant treatment. In low-grade gliomas, under the condition of IDH mutation, TERT mutation has a relatively good prognosis. On the contrary, under the condition of no IDH mutation, TERT mutation has a poor prognosis [13,14]. One case in this study showed that there were mutations in TERT and IDH, and the prognosis was better than that of 16 cases without mutations in TERT and IDH. However, 78 cases of common adult GBM without TERT mutation were reported to have a long survival time [15], so the relationship between the mutation of TERT in glioma and its prognosis is not uniform. Although there are two reports about the mutation of TERT in E-GBM, Nakajima reported that the mutation rate of TERT in 14 cases of E-GBM was 71%, and the co-mutation rate of TERT and BRAF was 50% [10], there is no report about the correlation between the mutation and prognosis. Our data showed that TERT had a mutation rate of 54.5%, and survival analysis showed that TERT mutation had a relatively good prognosis. Hence, our study enriches the report of TERT mutation in E-GBM.
MGMT has no definite diagnostic significance in gliomas, but detection of MGMT methylation status in gliomas can predict its chemosensitivity to alkylating agents such as TMZ. Methylation of MGMT promoter can cause MGMT gene silencing, thus enhancing its sensitivity to TMZ [16]. There are only a few reports about the methylation status of MGMT in epithelioid glioblastoma, among which Tanaka reported that the methylation status of MGMT in a 55 year old male patient with a mixture of common GBM and E-GBM was the opposite, and the component of E-GBM was methylated [8]. Alexandrescu reported two cases of MGMT methylation in E-GBM [4]. At present, there are no more reports on MGMT methylation in E-GBM. The data in our study showed that the methylation of MGMT in 15 patients was positive (45.4%), which enriched the reporting of MGMT methylation in E-GBM.
As a highly invasive brain tumor, the median survival time of E-GBM is only a few months [2,11], although it has been treated with various therapies. At present, the treatment of E-GBM also uses that of common GBM. Surgical resection to the greatest extent and radiotherapy with TMZ are considered as the classic treatment of GBM, but despite the application of various treatment methods, the prognosis of GBM is still poor. In addition, literature has reported that because of its high local recurrence rate, including the self-renewal and multiple differentiation potential of tumor cells in the irradiated area, it is often resistant to radiotherapy and chemotherapy. GBM is considered to be a tumor resistant to radiotherapy [5,13]. In our study, the multivariate Cox regression analysis showed that chemotherapy (P = 0.008) and radiotherapy (P = 0.004) could improve the prognosis, but the overall prognosis of the patients was still poor. The median overall survival time was only 10 months (range from 6 to 31 months). Our conclusion also showed that radiotherapy and chemotherapy could not prolong the overall survival time of patients, so we need to find new treatment strategies, especially targeted drug therapy. It has been reported that the combination of radiation sensitizer Poly (ADP-ribose), polymerase inhibitors (PARPi), and radiotherapy with TMZ can improve the prognosis of GBM, which provides an auxiliary treatment for E-GBM radiotherapy [16,17]. In addition, for a variety of tumors with BRAF mutations, BRAF mutations have not only diagnostic value, but also targeted therapeutic significance. In patients with malignant melanoma with a V600E point mutation of the BRAF gene, the application of BRAF kinase inhibitors has achieved significant curative effect [18,19]. In the treatment of E-GBM, it is reported that BRAF kinase inhibitors dabrafenib was used as a remedy, and the clinical and radiologic stable period of ten months was achieved [20]; Burger and colleagues reported that treated with BRAF kinase inhibitors dabrafenib, the GBM patients with BRAF V600E mutated had the longest clinical and radiologic stable period of 27 months [21]. At present, a study on vemurafenib (NCT01748149) or darafib (NCT02684058) in children with BRAF V600E mutation brain tumor is in clinical trial stage (https://www.clinicaltrials.gov). Therefore, since the significance of radiotherapy and chemotherapy in E-GBM is not clear, surgery combined with BRAF inhibitor targeted therapy is expected to become a new treatment strategy for E-GBM, pending more evidence.
Disclosure of conflict of interest
None.
References
- 1.Kepes JJ, Fulling KH, Garcia JH. The clinical significance of “adenoid” formations of neoplastic astrocytes, imitating metastatic carcinoma, in gliosarcomas. A review of five cases. Clin Neuropathol. 1982;1:139–50. [PubMed] [Google Scholar]
- 2.Malzkorn B, Reifenberger G. Practical implications of integrated glioma classification according to the World Health Organization classification of tumors of the central nervous system 2016. Curr Opin Oncol. 2016;28:494–501. doi: 10.1097/CCO.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 3.Korshunov A, Chavez L, Sharma T, Ryzhova M, Schrimpf D, Stichel D, Capper D, Sturm D, Kool M, Habel A, Kleinschmidt-DeMasters BK, Rosenblum M, Absalyamova O, Golanov A, Lichter P, Pfister SM, Jones DTW, Perry A, von Deimling A. Epithelioid glioblastomas stratify into established diagnostic subsets upon integrated molecular analysis. Brain Pathol. 2018;28:656–662. doi: 10.1111/bpa.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexandrescu S, Korshunov A, Lai SH, Dabiri S, Patil S, Li R, Shih CS, Bonnin JM, Baker JA, Du E, Scharnhorst DW, Samuel D, Ellison DW, Perry A. Epithelioid glioblastomas and anaplastic epithelioid pleomorphic xanthoastrocytomas--same entity or first cousins? Brain Pathol. 2016;26:215–23. doi: 10.1111/bpa.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broniscer A, Tatevossian RG, Sabin ND, Klimo P Jr, Dalton J, Lee R, Gajjar A, Ellison DW. Clinical, radiological, histological and molecular characteristics of paediatric epithelioid glioblastoma. Neuropathol Appl Neurobiol. 2014;40:327–36. doi: 10.1111/nan.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleinschmidt-DeMasters BK, Aisner DL, Birks DK, Foreman NK. Epithelioid GBMs show a high percentage of BRAF V600E mutation. Am J Surg Pathol. 2013;37:685–98. doi: 10.1097/PAS.0b013e31827f9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khanna G, Pathak P, Suri V, Sharma MC, Chaturvedi S, Ahuja A, Bhardwaj M, Garg A, Sarkar C, Sharma R. Immunohistochemical and molecular genetic study on epithelioid glioblastoma: series of seven cases with review of literature. Pathol Res Pract. 2018;214:679–685. doi: 10.1016/j.prp.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka S, Nakada M, Hayashi Y, Nakada S, Sawada-Kitamura S, Furuyama N, Suzuki T, Kamide T, Hayashi Y, Yano S, Hamada J. Epithelioid glioblastoma changed to typical glioblastoma: the methylation status of MGMT promoter and 5-ALA fluorescence. Brain Tumor Pathol. 2011;28:59–64. doi: 10.1007/s10014-010-0009-x. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Kim WH, Jung HY, Yang MH, Kang GH. Aberrant CpG island methylation of multiple genes in intrahepatic cholangiocarcinoma. Am J Pathol. 2002;161:1015–22. doi: 10.1016/S0002-9440(10)64262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakajima N, Nobusawa S, Nakata S, Nakada M, Yamazaki T, Matsumura N, Harada K, Matsuda H, Funata N, Nagai S, Nakamura H, Sasaki A, Akimoto J, Hirato J, Yokoo H. BRAF V600E, TERT promoter mutations and CDKN2A/B homozygous deletions are frequent in epithelioid glioblastomas: a histological and molecular analysis focusing on intratumoral heterogeneity. Brain Pathol. 2018;28:663–673. doi: 10.1111/bpa.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu R, Hatef J, McLendon RE, Cummings TJ, Sampson JH, Friedman AH, Adamson C. Clinicopathological characteristics and treatment of rhabdoid glioblastoma. J Neurosurg. 2013;119:412–9. doi: 10.3171/2013.3.JNS121773. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez FJ, Scheithauer BW, Giannini C, Bryant SC, Jenkins RB. Epithelial and pseudoepithelial differentiation in glioblastoma and gliosarcoma: a comparative morphologic and molecular genetic study. Cancer. 2008;113:2779–89. doi: 10.1002/cncr.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumura N, Nakajima N, Yamazaki T, Nagano T, Kagoshima K, Nobusawa S, Ikota H, Yokoo H. Concurrent TERT promoter and BRAF V600E mutation in epithelioid glioblastoma and concomitant low-grade astrocytoma. Neuropathology. 2017;37:58–63. doi: 10.1111/neup.12318. [DOI] [PubMed] [Google Scholar]
- 14.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih IeM, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110:6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu VM, George ND, Brown DA, Akinduro OO, Raghunathan A, Jentoft M, Quinones-Hinojosa A, Chaichana KL. Confirming diagnosis and effective treatment for rare epithelioid glioblastoma variant: an integrated survival analysis of the Literature. World Neurosurg. 2019;131:243–251. e2. doi: 10.1016/j.wneu.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Chen X, Zhang M, Gan H, Wang H, Lee JH, Fang D, Kitange GJ, He L, Hu Z, Parney IF, Meyer FB, Giannini C, Sarkaria JN, Zhang Z. A novel enhancer regulates MGMT expression and promotes temozolomide resistance in glioblastoma. Nat Commun. 2018;9:2949. doi: 10.1038/s41467-018-05373-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lesueur P, Lequesne J, Grellard JM, Dugué A, Coquan E, Brachet PE, Geffrelot J, Kao W, Emery E, Berro DH, Castera L, Goardon N, Lacroix J, Lange M, Capel A, Leconte A, Andre B, Léger A, Lelaidier A, Clarisse B, Stefan D. Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol. BMC Cancer. 2019;19:198. doi: 10.1186/s12885-019-5413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, Garbe C, Schadendorf D, Krajsova I, Gutzmer R, Chiarion Sileni V, Dutriaux C, de Groot JWB, Yamazaki N, Loquai C, Moutouh-de Parseval LA, Pickard MD, Sandor V, Robert C, Flaherty KT. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315–1327. doi: 10.1016/S1470-2045(18)30497-2. [DOI] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceccon G, Werner JM, Dunkl V, Tscherpel C, Stoffels G, Brunn A, Deckert M, Fink GR, Galldiks N. Dabrafenib Treatment in a patient with an epithelioid glioblastoma and BRAF V600E mutation. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burger MC, Ronellenfitsch MW, Lorenz NI, Wagner M, Voss M, Capper D, Tzaridis T, Herrlinger U, Steinbach JP, Stoffels G, Langen KJ, Brandts C, Senft C, Harter PN, Bähr O. Dabrafenib in patients with recurrent, BRAF V600E mutated malignant glioma and leptomeningeal disease. Oncol Rep. 2017;38:3291–3296. doi: 10.3892/or.2017.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]