Abstract
Paraneoplastic pemphigus (PNP) is an autoimmune bullous dermatosis associated with tumors, first described by Anhalt et al. in 1990. Reports of paraneoplastic pemphigus complicated by follicular lymphoma (FL) are rare in the medical literature. Here, we retrospectively analyze a case of PNP accompanied by FL. The patient was a 54-year-old woman who suffered from PNP associated with FL at the beginning. She had received a pathological diagnosis and was treated with R-CHOP and other drugs. Her mucosal lesions and cutaneous lesions improved, and the FL was in remission. Eleven months later, she died of BO after receiving the diagnosis of PNP. We also review most of the studies and reports about PNP accompanied by FL. We list the clinicopathologic features, therapeutic schedule, and prognosis in order to improve hematologists’ understanding and treatment of the diseases.
Keywords: Follicular lymphoma, paraneoplastic pemphigus, bronchiolitis obliterans
Introduction
PNP is a rare paraneoplastic, systemic autoimmune bullous disease characterized by severe mucosal lesions and various cutaneous lesions. Most of the basic tumors originate from the lymphatic reticular system associated with Hodgkin’s lymphoma, thymoma, and leukemia [1]. The patients suffering from PNP are characterized by autoantibodies acting against the plakin family of proteins. There are many patients with PNP who also get BO. An initial treatment with systemic corticosteroids is often attempted, and other immunosuppressive agents are also used in combination with systemic corticosteroids in patients who have PNP [2]. However, most patients have a poor prognosis independent of the status of the underlying neoplasms [3]. Though treatments for neoplasms are effective particularly in FL, in PNP associated with malignant neoplasms, the response of PNP to the treatment of the underlying neoplasm appears to be less favorable [4].
Bronchiolitis obliterans (BO) is a life-threatening form of irreversible, obstructive lung disease. Cases of BO were first reported in 1999, and BO may occur in chronic graft-versus-host disease patients undergoing allogenic hematopoietic stem cell transplantation [5]. Evidence confirms that the BO can form after PNP, and it is a major cause of death in PNP patients.
Case report
A 54-year-old woman was admitted to the hospital in March 2018 with itchy erythema on her limbs and trunk. Her eyes, mouth, and labia also had painful erosions. A dermatological examination showed blisters and erosions on her lips, tongue, mucosa, and labia (Figure 1A). Her pharynx mucosa and eyes were swollen. A purplish-red rash could be seen on her hands, feet, and torso (Figures 1B, 1C). The pimples were target-shaped, well-defined and partially colorfast when pressed. Computed tomography showed multiple enlarged lymph nodes in the bilateral axillary, mediastinal, retroperitoneal and bilateral inguinal areas. Chronic inflammation was found in the lower lobe of both lungs. A PET-CT examination showed that: 1. FDG was increased in the tongue and right tonsil; 2. Enlarged lymph nodes were found in her bilateral neck region, bilateral supraclavicular region, bilateral pectoralis minor muscle, bilateral axilla (Figure 2A), mediastinum, abdominal cavity, mesentery, bilateral inguinal (Figure 2B), pelvis, and retroperitoneal space (Figure 2C); 3. Spleen enlargement (Figure 2D); 4. The FDG metabolism increased in all the above lesions. One week after admission, the patient underwent a thigh skin biopsy. Under a microscope, mild hyperkeratosis of the skin epidermis, an irregular thickening of the granular layer, edema, and a vacuole liquefaction of the basal cells were observed. The small vessels in the superficial dermis were dilated around which the lymphocytes infiltrated densely and necrotic keratinocytes were seen in the epidermis (Figure 3A-C). Direct immunofluorescence (DIF) showed IgG deposition between the epidermal cells and the basement membrane zone (Figure 3D). One week after the skin biopsy, cervical and inguinal lymph node biopsies were performed: small atypical lymphocytes showed a nodular hyperplasic pattern in the lymph nodes. Those nodules were back to back, the nuclei were cleaved, and the chromatin was fine-grained (Figure 4A). Immunohistochemistry showed that the tumor cells were positive for CD20 (Figure 4B), CD10 (Figure 4C), Bcl-2 (Figure 4D), and Bcl-6 but negative for CD3, CD5, and CyclinD1. Meshwork showed that the CD21 (Figure 4E) and CD23 were slightly irregular, and the positive rate of Ki67 (Figure 4F) was about 20%. Combined with the morphology and the immunohistochemical results, the lesions were consistent with grade I FL. A bone marrow biopsy showed the bone marrow was involved. A diagnosis of FL with PNP was made, and the patient was then transferred to the Department of Hematology for treatment with R-CHOP (rituximab, cyclophosphamide, epirubicin, vincristine, prednisone). During her standard course of chemotherapy, she received methylprednisolone tablets, recombinant human interleukin, and sodium thiosulfate. And she also improved with the administration of recombinant bovine basic fibroblast growth factor, erythromycin eye ointment, TobraDex ophthalmic ointment, and potassium permanganate sitz baths (1:10,000). The oral mucositis and vulvar lesions were relieved during the treatment (Figure 1D), but the patient died of a pulmonary infection and bronchiolitis obliterans eleven months after her diagnosis in February 2019.
Figure 1.

Blisters and erosion on the lips and erosion on the tongue mucosa (A). A purplish-red rash can be seen on her hands, feet, and torso (B, C). The pimples are target-shaped, well-defined, and partially colorfast when pressed. Oral mucositis and vulvar lesions improved during the treatment (D).
Figure 2.

The PET-CT showed: enlarged lymph nodes in bilateral axilla (A), pelvic and bilateral inguinal (B), retroperitoneal (C); spleen enlargement (D).
Figure 3.

Mild hyperkeratosis of the skin epidermis, irregular thickening of the granular layer, edema, and vacuole liquefaction of the basal cells. The small vessels in the superficial dermis dilated, lymphocytes infiltrated densely around the vessels, and necrotic keratinocytes were seen in the epidermis (A-C). Direct immunofluorescence (DIF) showed IgG deposition between the epidermal cells and the basement membrane zone (D).
Figure 4.
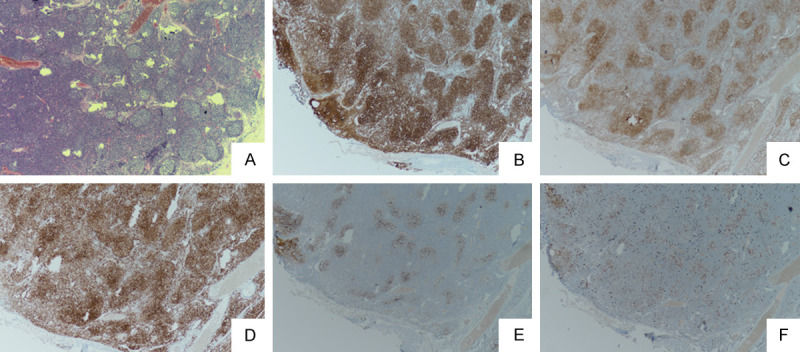
Small atypical lymphocytes showed a nodular hyperplasia pattern in the lymph nodes. Those nodules were back to back, the nuclei were cleaved and the chromatin was fine-grained (A). Immunohistochemistry showed that the tumor cells were positive for CD20 (B), CD10 (C), and Bcl-2 (D). The meshwork seen using CD21 (E) was slightly irregular, and the positive rate of Ki67 (F) was about 20%.
Discussion
Reports of PNP associated with FL are relatively rare in the medical literature. There are 16 cases of patients who suffered from FL with PNP reported in the English-language medical literature [6-21]. The clinical pathology characteristics can be drawn from these cases (Table 1): ① The patients’ ages range from 49 to 77 years old with a median age of 61; ② Gender: there are 8 males and 9 females with a ratio of M:F=8:9; ③ 6/17 of the patients’ clinical stages are IV; ④ 6/17 of the patients’ FL classifications are grade I; ⑤ PNP occurrence time: most patients (10/17, 58.8%) also had occurrences of primary tumors; ⑥ During the course of the disease, 5/17 of the patients had secondary symptoms of BO within 1 week to 11 months after the appearance of PNP, and half of 8 patients died directly from BO induced by respiratory failure (4/8, 50%); ⑦ 16 of the 17 patients received chemotherapy with rituximab (16/17, 94.1%) and 8 patients were treated with an R-CHOP regimen (8/16, 50.0%). According to the literature, the maximum survival time of the 17 patients was 42 months, and the maximum survival time of the patients with BO was only 27 months.
Table 1.
The main clinical features of the 17 FL patients with PNP or BO
| Case Num. | References | Age | Gender | Clinical stage | FL Level | Diagnostic Time of PNP | Diagnostic Time of BO | Treatment Plan | Curative effect | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Moustafa, et al. [6] | 68 | M | Unknown | I | Simultaneous diagnosis with tumor | Unknown | B*/R*→R*/Prednisone | Improvement of skin lesion | Died of infectious complications 10 months after diagnosis. |
| Tumor shrinking | ||||||||||
| The symptoms of infection are still repeated. | ||||||||||
| 2 | Lee, et al. [7] | 53 | M | IVA | Unknown | Simultaneous diagnosis with tumor | Simultaneous diagnosis with PNP | R-CHOP* | Complete remission of tumor, stomatitis, other skin lesions and BO are persistent existence. | Died of respiratory failure caused by BO 27 months after diagnosis. |
| 3 | Borradori, et al. [8] | 61 | F | Unknown | I | Simultaneous diagnosis with tumor | Unknown | P*→CHOP*/antibiotics/Cyclosporin/0.5% Retinaldehyde/Sucralfate→R* | The tumor was relieved and there was no progress. Stomatitis and palmar erythema were relieved and the patient was well tolerated by rituximab. | Unknown |
| 4 | Aoi, et al. [9] | 60 | M | Unknown | Unknown | Simultaneous diagnosis with tumor | Unknown | P*→P*/R*→R* combined with chemotherapy | The condition improved and the skin lesion recovered. The FL was relieved partly. | Unknown |
| 5 | Lim, et al. [10] | 58 | M | Unknown | Unknown | Simultaneous diagnosis with tumor | 1 week after PNP diagnosis | Before BO: R-CHOP* | Atypical pneumonia caused by cytomegalovirus infection one week after treatment. | Died of septic shock one month after diagnosis of BO. |
| After BO: R-CHOP* | ||||||||||
| 6 | Lachenal, et al. [11] | 52 | F | Unknown | Unknown | Simultaneous diagnosis with tumor | Unknown | R-CHOP* | The skin lesions were improved obviously, the oral erosion was improved, but the tumor reaction was unknown. | Unknown |
| 7 | Higo, et al. [12] | 77 | F | Unknown | Unknown | Simultaneous diagnosis with tumor | Unknown | R-CHOP*→Bendamustine/R*→Prednisone | The skin lesion recovered and the mucosal rash did not improve. | Unknown |
| 8 | Morikawa, et al. [13] | 65 | F | IIIA | Unknown | Simultaneous diagnosis with tumor | 5 months after FL diagnosis | Before BO: R-CHOP*→R*→R-CVP* | The tumor shrank and the symptoms such as conjunctivitis, oral mucosal ulcer and the rash improved. | Unknown |
| After BO: Palliative and symptomatic treatment | ||||||||||
| 9 | Jain, et al. [14] | 60 | M | IVB | I | 29 months after FL diagnosis | Unknown | Bendamustine/R*→R* | The tumor was remission completely at the beginning and relapsed after 18 months. | Unknown |
| 10 | Hirano, et al. [15] | 60 | M | Unknown | Unknown | Simultaneous diagnosis with tumor | 3 months after FL diagnosis | Before BO: R*/Prednisone | The blisters disappeared completely and the autoantibodies decreased to the normal level. | Died 7 months after the diagnosis of BO. |
| After BO: Plasma exchange | But the BO appeared later. | |||||||||
| →R-CHOP*→Bendamustine/R* | ||||||||||
| 11 | Hoque, et al. [16] | 49 | F | Unknown | Unknown | 9 years after FL diagnosis | Unknown | CHOP*→Radiotherapy/F*/Mitoxanone/Prednisone→F→R-CVP* | The tumor was completely relieved, the rash improved after treatment, but the general condition of the patient was poor. | After the occurrence of PNP, the condition worsened and the patient died after palliative treatment. |
| 12 | Martínez, et al. [17] | 70 | F | IVA | I | 4 years after FL diagnosis | Unknown | CHOP*→Chlorambutyronitrile→P | The tumor was completely relieved, the skin blisters disappeared, but the lesions of oral mucosa persisted. | 40 months after the diagnosis of PNP, there was no recurrence of skin and mucosal lesions. |
| 13 | Prodanovic, et al. [18] | 73 | M | IV | I | 3 years after FL diagnosis | Unknown | R*/F*/D*/Mitoxantrone/Granisetron→R* | The tumor was relieved, the skin blisters were cured and there was no recurrence during the follow-up period. | Unknown |
| 14 | Maruta, et al. [19] | 70 | M | IVB | Unknown | 5 years after FL diagnosis | Unknown | Chlorambutyronitrile/C*/O*→P*/F*/Chlorambutyronitrile→P*/Radiotherapy→Chlorambutyronitrile/P*→D*/Azathioprine→R*/P* | The tumor was relieved, the skin lesions were improved, but the oral mucosal lesions were relieved very little. | Died of left heart failure and pulmonary edema 9 months after the diagnosis of PNP. |
| 15 | Heizmann, et al. [20] | 73 | F | Unknown | Unknown | Simultaneous diagnosis with tumor | Unknown | P*/C*→R*/P* | The tumor was relieved partly and conjunctival and mucosal erosion recovered completely. | Unknown |
| 16 | Barnadas, et al. [21] | 77 | F | IIB | Unknown | Later than FL diagnosis time, the certain time is unknown | Unknown | Chlorophenylbutyronitrile→P*/Valaciclovir→R*/P*→P*/Cyclosporin | The tumor was relieved partly and the oral ulcer was completely cured. | The patient died 42 months after the FL diagnosis. The cause of death is unknown, but acid-fast bacilli is active. |
| 17 | Present case | 54 | F | IV | I-II | Simultaneous diagnosis with tumor | 11 months after FL diagnosis | R-CHOP* and so on | The FL was relieved and mucositis and vulvar lesions were improved. | Died of respiratory failure caused by BO 11 months after the diagnosis of PNP. |
R: Rituximab; C: Cyclophosphamide; O/V: Vincristine; P: Prednisone; D: Dexamethasone; F: Fludarabine.
PNP is a rare and lethal type of paraneoplastic autoimmune skin vesicular disease [1]. The diagnosis of paraneoplastic pemphigus depends on a histopathological examination and a direct or indirect immunofluorescence examination. The clinical features include painful mucosal erosions and pleomorphic skin lesions which can be involved in the skin and mucosa of the whole body, and the damage is severe and extensive. There are also large patches of purple erythema, blisters, moss-like, keratinizing lesions, and special serum immunoprecipitation. The histopathological characteristics include acantholysis, the formation of intraepithelial blisters, necrosis of the keratinocytes and an infiltration of inflammatory cells dominated by lymphocytes in the superficial dermis. IgG and C3 depositions between the epidermal cells and/or the basement membrane can be seen using a direct immunofluorescence (DIF) examination, and positive pemphigus antibodies based on mouse bladder epithelia is seen using indirect immunofluorescence (IIF) [22].
FL is a type of indolent B cell lymphoma derived from follicular central cells and central blasts. The mechanism of incorporating PNP is not yet clear, but PNP is closely related to the primary tumor. Published studies indicate that after the primary tumor is treated, the PNP symptoms improve. Takashi and others [12] believe that the use of bendamustine can trigger PNP because the skin toxicity of the drug causes a well-known adverse reaction. But the patient Jain et al. reported on [14] was treated with bendamustine/rituximab, and the PNP didn’t appear until 29 months after the treatment. Therefore, the relationship between bendamustine and PNP needs to be explored further. Braess [23] believed that fludarabine seems to induce or worsen the process of PNP. But summarizing the prognosis of three patients treated with fludarabine, he indicated that the induction of fludarabine cannot be denied, for two of the patients did not achieve a longer survival time from this treatment plan. The immunological features of patients with PNP include the presence of autoantibody-recognizing proteins in the stratified squamous epithelium, the transitional columnar epithelium, and the monolayer epithelium in sera. So they were positive for DIF and IIF. Complement and immunoglobulin have been confirmed to have antitumor activity in a variety of tumors [24,25]. Increased immunoglobulins and complements in cancer patients are self-protective responses. But most of the tumors that are associated with PNP originate from the lymphatic reticulum system, and whether they are related to the chemotherapeutic drugs or to the characteristics of the tumor itself needs further research.
The treatment for PNP mainly includes the treatment for the primary tumors, skin and mucosal damage, and for the complications of the affected organs [22]. The treatment of primary tumors is critical, and the involved skin and mucosa can be improved after treating the primary tumors [26]. R-CHOP is a first line chemotherapy regimen for FL and is effective for patients with PNP [21,27]. The application of rituximab in this scheme can kill tumor cells and B cells. Based on the summary of the current relevant literature, FL patients with PNP treated with rituximab or R-CHOP can achieve tumor remission or even complete remission. But the rash and mucosal lesions cannot be completely cured. This phenomenon may be caused by the continuous release (or helpful release) of a specific antibody in the patient. For skin and mucosal lesions, we often chose a large dose of glucocorticoid and maintain it in small doses. Although this can alleviate skin and mucosal lesions to a certain extent, the patient’s resistance is severely damaged. Regarding the above patients, most did not have a good survival time during the follow up period. Regardless of whether BO appears, the patients often die from respiratory infections from which we can infer that the use of chemotherapeutics and glucocorticoids has a direct relationship between the suppression of the patients’ autoimmune function and atelectasis.
About 30% of patients with PNP also suffer from BO, and the mechanism [28,29] involves the infiltration of T cells below the bronchial tubes, as determined by autopsies. In addition, immunoglobulin and complement are deposited in the bronchial columnar epithelium, resulting in the exfoliation of the respiratory tract ciliated columnar epithelium from the basal cell layer. Mixed inflammatory infiltration composed of lymphocytes, neutrophils, eosinophils, and plasma cells can be seen in the submucosa. Nousari’s experiment [28] confirmed that the acantholysis of the respiratory epithelium caused by Plakin proteins can lead to distal bronchial occlusion and scar formation, which gradually block the bronchioles. Then they cause breathing difficulties and aggravate respiratory infections. Therefore, we believe that the secondary BO symptoms in patients with PNP are irreversible pathological changes caused by the deposition of autoimmune antibodies associated with tumor tissues in the bronchiolar mucosa.
BO usually occurs quickly and irreversibly in the terminal stage of the disease, eventually leading to fatal respiratory failure. Patients with BO have a poor prognosis [30] and BO has become the leading cause of death in patients with PNP. Theoretically, patients can receive a lung transplant after the appearance of BO. But due to the lack of available lungs, the anti-host response after transplantation, and the chance that the BO will reappear after the transplantation, many clinical studies are needed to confirm this theory. Bronchoscopic transmural or open lung biopsies are often missed due to the lesions mainly involving the terminal or respiratory bronchioles and showing an uneven patchy or patch distribution. Therefore, clinical doctors often based their examinations on symptoms, pulmonary function, and imaging manifestations in order to reach a comprehensive diagnosis [31,32].
For such patients who may have BO, pulmonary function measurement and fiberoptic bronchoscopy should be started earlier. They should determine whether the patient has diffuse lung retention (in the expiration phase), bronchial thickening, or secondary dilatation using HRCT as soon as possible [32-35]. As for the absence of specific medicines, it is necessary to find out whether the patients have BO in time in order to strengthen the management of the respiratory tract and to inhale oxygen earlier, control the increase of inflammation, and improve their breathing difficulties.
Summary
FL with PNP is rare. Grade 1 and clinical stage IV of FL are more common. The emergence of PNP indicates the potential of tumors and then the need to actively look for primary lesions. The mechanism of BO in PNP patients associated with FL is still unclear, and preventing the occurrence of BO is still a clinical challenge.
Acknowledgements
The present study was supported by the Yantai Key Research and Development Project (grant no. 2017WS101).
Disclosure of conflict of interest
None.
References
- 1.Anhalt GJ, Kim SC, Stanley JR, Korman NJ, Jabs DA, Kory M, Izumi H, Ratrie H 3rd, Mutasim D, Ariss-Abdo L, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729–35. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 2.Frew JW, Murrell DF. Current management strategies in paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome) Dermatol Clin. 2011;29:607–12. doi: 10.1016/j.det.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Leger S, Picard D, Ingen-Housz-Oro S, Arnault JP, Aubin F, Carsuzaa F, Chaumentin G, Chevrant-Breton J, Chosidow O, Crickx B, D’incan M, Dandurand M, Debarbieux S, Delaporte E, Dereure O, Doutre MS, Guillet G, Jullien D, Kupfer I, Lacour JP, Leonard F, Lok C, Machet L, Martin L, Paul C, Pignon JM, Robert C, Thomas L, Weiller PJ, Ferranti V, Gilbert D, Courville P, Houivet E, Benichou J, Joly P. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol. 2012;148:1165–72. doi: 10.1001/archdermatol.2012.1830. [DOI] [PubMed] [Google Scholar]
- 4.Sehgal VN, Srivastava G. Paraneoplastic pemphigus/paraneoplastic autoimmune multiorgan syndrome. Int J Dermatol. 2009;48:162–9. doi: 10.1111/j.1365-4632.2009.03995.x. [DOI] [PubMed] [Google Scholar]
- 5.Nousari HC, Deterding R, Wojtczack H, Aho S, Uitto J, Hashimoto T, Anhalt GJ. The mechanism of respiratory failure in paraneo-plastic pemphigus. N Engl J Med. 1999;340:1406–10. doi: 10.1056/NEJM199905063401805. [DOI] [PubMed] [Google Scholar]
- 6.Moustafa MA, Seningen JL, Jouni H, Singh PP, el-Azhary RA, Witzig TE. A skin rash and what lies beneath: paraneoplastic pemphigus, an atypical presentation of follicular cell lymphoma. Am J Hematol. 2013;88:822–823. doi: 10.1002/ajh.23471. [DOI] [PubMed] [Google Scholar]
- 7.Lee S, Yamauchi T, Ishii N, Hashimoto T, Kinoshita K, Imamura S, Kamiya K. Achievement of the longest survival of paraneoplastic pemphigus with bronchiolitis obliterans associated with follicular lymphoma using R-CHOP chemotherapy. Int J Hematol. 2017;106:852–859. doi: 10.1007/s12185-017-2305-2. [DOI] [PubMed] [Google Scholar]
- 8.Borradori L, Lombardi T, Samson J, Girardet C, Saurat JH, Hügli A. Anti-CD20 monoclonal antibody (rituximab) for refractory erosive stomatitis secondary to CD20(+) follicular lymphoma-associated paraneoplastic pemphigus. Arch Dermatol. 2001;137:269–272. [PubMed] [Google Scholar]
- 9.Aoi J, Makino K, Sakai K, Masuguchi S, Fukushima S, Jinnin M, Inoue Y, Koga H, Hashimoto T, Ihn H. Case of paraneoplastic pemphigus with follicular lymphoma treated with rituximab. J Dermatol. 2013;40:285–286. doi: 10.1111/1346-8138.12095. [DOI] [PubMed] [Google Scholar]
- 10.Lim JM, Kim JH, Hashimoto T, Kim SC. Lichenoid paraneoplastic pemphigus associated with follicular lymphoma without detectable autoantibodies. Clin Exp Dermatol. 2018;43:613–615. doi: 10.1111/ced.13563. [DOI] [PubMed] [Google Scholar]
- 11.Lachenal F, Amini M, Salino S, Biron P. Paraneoplastic pemphigus associated with follicular lymphoma. Br J Haematol. 2009;144:458. doi: 10.1111/j.1365-2141.2008.07388.x. [DOI] [PubMed] [Google Scholar]
- 12.Higo T, Miyagaki T, Nakamura F, Shinohara A, Asano H, Abe H, Senda N, Yoshizaki A, Fukayama M, Kurokawa M. Paraneoplastic pemphigus occurring after bendamustine and rituximab therapy for relapsed follicular lymphoma. Ann Hematol. 2015;94:683–5. doi: 10.1007/s00277-014-2202-1. [DOI] [PubMed] [Google Scholar]
- 13.Morikawa K, Tsuji T, Yamasaki H, Egashira S, Kaguchi A, Kido M, Tsuda H. Paraneoplastic pemphigus occurs most commonly in indolent B cell lymphoma. Acta Haematol. 2014;132:73–4. doi: 10.1159/000357109. [DOI] [PubMed] [Google Scholar]
- 14.Jain A, Prakash G, Nampoothiri RV, De D, Bal A, Khadwal A, Lad D, Malhotra P, Varma S. Peri-anal paraneoplastic pemphigus heralding the relapse of follicular lymphoma and its successful management by rituximab: a short correspondence. Indian J Hematol Blood Transfus. 2016;32:519–521. doi: 10.1007/s12288-016-0689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano T, Higuchi Y, Yuki H, Hirata S, Nosaka K, Ishii N, Hashimoto T, Mitsuya H, Okuno Y. Rituximab monotherapy and rituximab-containing chemotherapy were effective for paraneoplastic pemphigus accompanying follicular lymphoma, but not for subsequent bronchiolitis obliterans. J Clin Exp Hematop. 2015;55:83–8. doi: 10.3960/jslrt.55.83. [DOI] [PubMed] [Google Scholar]
- 16.Hoque SR, Black MM, Cliff S. Paraneoplastic pemphigus associated with CD20-positive follicular non-Hodgkin’s lymphoma treated with rituximab: a third case resistant to rituximab therapy. Clin Exp Dermatol. 2007;32:172–5. doi: 10.1111/j.1365-2230.2006.02331.x. [DOI] [PubMed] [Google Scholar]
- 17.Martínez De Pablo MI, Iranzo P, Mascaró JM, Llambrich A, Baradad M, Herrero C. Paraneoplastic pemphigus associated with non-Hodgkin B-cell lymphoma and good response to prednisone. Acta Derm Venereol. 2005;85:233–5. doi: 10.1080/00015550510025542. [DOI] [PubMed] [Google Scholar]
- 18.Prodanovic EM, Korman NJ. A case of non-Hodgkin’s lymphoma-associated pemphigus foliaceus and use of rituximab for treatment. J Dermatolog Treat. 2008;19:1–3. doi: 10.1080/09546630802032603. [DOI] [PubMed] [Google Scholar]
- 19.Maruta CW, Miyamoto D, Aoki V, Carvalho RGR, Cunha BM, Santi CG. Paraneoplastic pemphigus: a clinical, laboratorial, and therapeutic overview. An Bras Dermatol. 2019;94:388–398. doi: 10.1590/abd1806-4841.20199165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heizmann M, Itin P, Wernli M, Borradori L, Bargetzi MJ. Successful treatment of paraneoplastic pemphigus in follicular NHL with rituximab: report of a case and review of treatment for paraneoplastic pemphigus in NHL and CLL. Am J Hematol. 2001;66:142–4. doi: 10.1002/1096-8652(200102)66:2<142::AID-AJH1032>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 21.Barnadas M, Roe E, Brunet S, Garcia P, Bergua P, Pimentel L, Puig L, Francia A, García R, Gelpí C, Sierra J, Coll P, Alomar A. Therapy of paraneoplastic pemphigus with rituximab: a case report and review of literature. J Eur Acad Dermatol Venereol. 2006;20:69–74. doi: 10.1111/j.1468-3083.2005.01345.x. [DOI] [PubMed] [Google Scholar]
- 22.Kimyai-Asadi A, Jih MH. Paraneoplastic pemphigus. Int J Dermatol. 2001;40:367–72. doi: 10.1046/j.1365-4362.2001.01169.x. [DOI] [PubMed] [Google Scholar]
- 23.Braess J, Reich K, Willert S, Strutz F, Neumann C, Hiddemann W, Wörmann B. Mucocutaneous autoimmune syndrome following fludarabine therapy for low-grade non-Hodgkin’s lymphoma of B-cell type (B-NHL) Ann Hematol. 1997;75:227–230. doi: 10.1007/s002770050347. [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Kuwahara A, Kinoshita T, Shigemitsu Y, Shimoda K, Miyahara M, Kobayashi M. Increases in immunoglobulin and complement in patients with esophageal or gastric cancer. Surg Today. 1992;22:537–542. doi: 10.1007/BF00308900. [DOI] [PubMed] [Google Scholar]
- 25.Niculescu F, Rus HG, Retegan M, Vlaicu R. Persistent complement activation on tumor cells in breast cancer. Am J Pathol. 1992;140:1039–1043. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Qiao QL, Chen XX, Liu P, Qiu JX, Zhao H, Zhao JX, Liu YC, Wan YL. Improved outcomes after complete resection of underlying tumors for patients with paraneoplastic pemphigus: a single-center experience of 22 cases. J Cancer Res Clin Oncol. 2011;137:229–234. doi: 10.1007/s00432-010-0874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 28.Nousari HC, Deterding R, Wojtczack H, Aho S, Uitto J, Hashimoto T, Anhalt GJ. The mechanism of respiratory failure in paraneoplastic pemphigus. N Engl Med J. 1999;340:1406–10. doi: 10.1056/NEJM199905063401805. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi M, Shimatsu Y, Kazama T, Kimura K, Otsuka T, Hashimoto T. Paraneoplastic pemphigus associated with bronchiolitis obliterans. Chest. 2000;117:603–607. doi: 10.1378/chest.117.2.603. [DOI] [PubMed] [Google Scholar]
- 30.Nikolskaia OV, Nousari CH, Anhalt GJ. Paraneoplastic pemphigus in association with Castleman’s disease. Br J Dcrmatol. 2003;149:1143–1151. doi: 10.1111/j.1365-2133.2003.05659.x. [DOI] [PubMed] [Google Scholar]
- 31.Siegel MJ, Bhalla S, Gutierrez FR, Hildebolt C, Sweet S. Post-lung transplantation bronchiolitis obliterans syndrome: usefulness of expiratory thin-section CT for Diagnosis. Radiology. 2001;220:455–462. doi: 10.1148/radiology.220.2.r01au19455. [DOI] [PubMed] [Google Scholar]
- 32.Lau DM, Siegel MJ, Hildebolt CF, Cohen AH. Bronchiolitis obliterans syndrome: thin-section CT diagnosis of obstructive changes in infants and young children after lung transplantation. Radiology. 1998;208:783–8. doi: 10.1148/radiology.208.3.9722860. [DOI] [PubMed] [Google Scholar]
- 33.Hansell DM, Rubens MB, Padley SP, Wells AU. Obliterative bronchiolitis: individual CT signs of small airways disease and functional correlation. Radiology. 1997;203:721–726. doi: 10.1148/radiology.203.3.9169694. [DOI] [PubMed] [Google Scholar]
- 34.Worthy SA, Park CS, Kim JS, Müller NL. Bronchiolitis obliterans after lung transplantation: high-resolution CT findings in 15 patients. AJR Am J Roentgenol. 1997;169:673–677. doi: 10.2214/ajr.169.3.9275875. [DOI] [PubMed] [Google Scholar]
- 35.Leung AN, Fisher K, Valentine V, Girgis RE, Berry GJ, Robbins RC, Theodore J. Bronchiolitis obliterans after lung transplantation: detection using expiratory HRCT. Chest. 1998;113:365–370. doi: 10.1378/chest.113.2.365. [DOI] [PubMed] [Google Scholar]


