Abstract
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression via recognition of cognate sequences and interference of transcriptional, translational or epigenetic processes. Bioinformatics tools developed for miRNA study include those for miRNA prediction and discovery, structure, analysis and target prediction. We manually curated 95 review papers and ∼1000 miRNA bioinformatics tools published since 2003. We classified and ranked them based on citation number or PageRank score, and then performed network analysis and text mining (TM) to study the miRNA tools development trends. Five key trends were observed: (1) miRNA identification and target prediction have been hot spots in the past decade; (2) manual curation and TM are the main methods for collecting miRNA knowledge from literature; (3) most early tools are well maintained and widely used; (4) classic machine learning methods retain their utility; however, novel ones have begun to emerge; (5) disease-associated miRNA tools are emerging. Our analysis yields significant insight into the past development and future directions of miRNA tools.
Keywords: miRNA, bioinformatics tools, text mining, bibliometric, ranking
Introduction
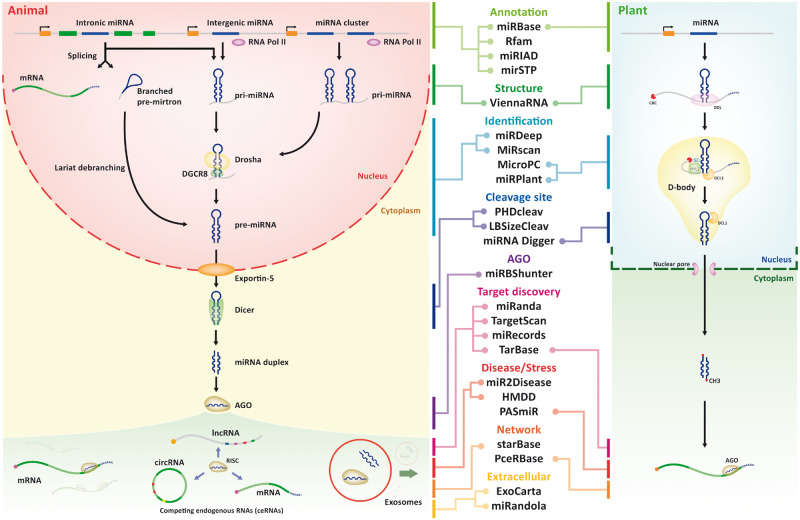
MicroRNA (miRNA) is a small ∼21–22 nt noncoding RNA, which is a known regulator of essential biological processes in animals and plants. The biogenesis of miRNA is shown in Figure 1. In animals, the miRNA gene is typically transcribed by RNA polymerase II as primary RNA, which is cleaved into hairpin-shaped precursor miRNA (pre-miRNA) by nuclear RNase III Drosha, and then exported to the cytosol by exportin-5 [1]. In cytosol, pre-miRNA is cleaved by Dicer into the miRNA duplex, of which one arm is loaded into Argonaute (AGO) protein in the RNA-induced silencing complex (RISC) and used as a guide sequence in binding with the protein-coding RNAs (mRNAs) [2]. Animal miRNAs bind to their target mRNAs imperfectly, and the process is dominated by the first eight nucleotides from miRNA 5′ end, which is called the seed region [3].
Figure 1.
miRNA biogenesis of animal/plant and bioinformatics tools associated within each process. The canonical and non-canonical miRNA biogenesis pathways of animal/plant are shown in the side panels. Examples of bioinformatics tools cataloged by biogenesis process are listed in the middle.
miRNA biogenesis is dynamic and has great diversity. One type of miRNA is called mirtron (or intronic miRNA), which arises from spliced-out introns in a Drosha-independent manner [4]. miRNA cluster is a group of miRNAs, which are adjacent to one another in the genome and transcribed as a single polycistronic unit [5]. Unlike in animals, the two-step process of plant miRNA biogenesis occurs in the nucleus [6]. Most plant miRNAs are transcribed by the DNA-dependent RNA polymerase II and generate pri-miRNA [7, 8]. After a forkhead-associated domain-containing protein encoded by Dawdle (DDL) acts to stabilize the molecule, the pri-miRNA transcript is processed to generate pre-miRNA by the nuclear RNase Dicer-like 1 (DCL1) and its associated RNA-binding proteins (RBPs) Serrate (SE) and Hyponastic Leaves 1 (HYL1) [9, 10]. The pre-miRNAs are then exported to the cytoplasm after methylation and incorporated into the Argonaute 1 (AGO1) to bind to mRNA and inhibit the expression of target mRNAs [11, 12]. In contrast with animals, plant miRNAs bind to their targets with extensive complementarity (with a maximum of five mismatches) [3].
miRNA functions in posttranscriptional regulation of target gene expression [1]. One miRNA could simultaneously target several genes located within the same cellular signaling pathway [13–15]. Recent studies have shifted our understanding of how miRNAs interact with their targets, which include not only mRNAs but also long noncoding RNAs (lncRNAs), pseudogenes and circular RNAs (circRNAs) [16]. Competing endogenous RNA (ceRNA) regulates other RNA transcripts by competing for shared miRNAs [17]. With the ability to interact with multiple target genes, miRNAs have been proven to influence many important biological processes such as cell growth, tissue differentiation, cell proliferation, embryonic development and apoptosis [18]. Dysregulated miRNA plays critical roles in the progression of various diseases, such as aging, cardiovascular disease and cancer [18]. In animals, miRNAs can be packaged into exosomes or microvesicles and secreted into the extracellular environment, including various biological fluids, and can therefore perform long distance cell–cell communication [19]. Circulating miRNAs could also act as potential biomarkers for the diagnosis and prognosis of various cancers as well as other known diseases and syndromes [20, 21].
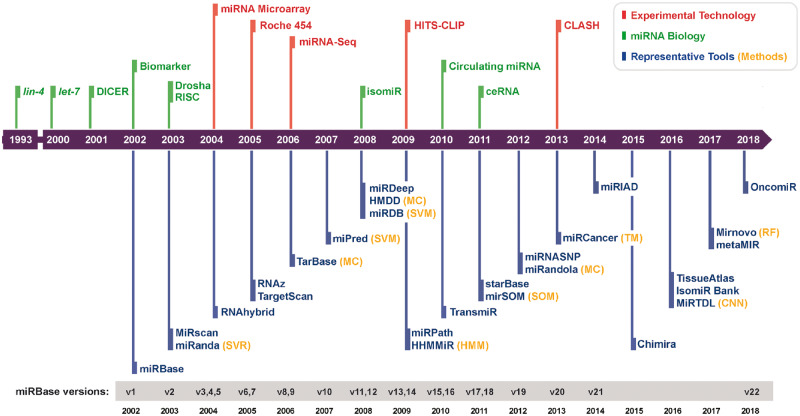
Since the discovery of the first miRNA lin-4 in 1993, 48 885 mature miRNAs in 271 species have been identified and deposited into the gold standard central repository miRBase [22]. Figure 2 shows a time line of the accumulation of miRNA biology knowledge, experimental technique progress and advances in bioinformatics tools that have led to several fundamental discoveries. We previously curated about 1000 miRNA bioinformatics tools to build a comprehensive database called miRToolsGallery [45]. In miRToolsGallery, tools are classified into categories such as miRNA sequence and annotation, miRNA target gene prediction, novel miRNA discovery and miRNA expression profiles [45]. miRToolsGallery contains comprehensive information about the tools, such as the implementation technology and method, date of publication and the number of citations. In this review, we mine the details in miRToolsGallery and miRNA tools review papers to obtain an overview of the range of miRNA bioinformatics tools and identify key trends in their development over time.
Figure 2.
Historical time line of miRNA research. The development of experimental and computational aspects of miRNA is illustrated. Red, green, orange and blue marks the event concerning experimental technology, miRNA biology, computational technology and representative tools, respectively. On the bottom panel, versions of miRBase are listed. Symbols and abbreviations follow. miRNA biology: lin-4, the first miRNA to be discovered [23]; let-7, the first human miRNA to be discovered [24]; DICER, Dicer was found to be required for miRNA maturation [25, 26]; Biomarker, miRNAs dysregulated in tumor tissue and could be potential biomarker [27]; Drosha, Drosha was identified as the initiator of the miRNA maturation process [28]; RISC, the mechanism of the miRNA into the RISC complex was characterized [29, 30]; isomiR, as a new term defined [31]; Circulating miRNA, the presence of miRNAs in 12 human body fluids was examined [19]; and ceRNA, hypothesis of ceRNA [17]. Experimental technology: miRNA microarray, early microarray application to profile miRNA [32, 33]; Roche 454, the first commercially successful second-generation sequencing system developed by 454 Life Sciences [34]; miRNA-Seq, early NGS application to profile miRNA [33, 35]; High-throughput sequencing of RNA isolated by crosslinking immunoprecipitation (HITS-CLIP), identified interaction sites between miRNA and target mRNA by sequencing AGO protein–RNA complexes [36]; CLASH, identified miRNA–target RNA duplexes associated with AGO [37]. Methods: Random forest (RF) [38]; SVM [39]; Support Vector Regression (SVR) [40]; TM [41]; Manually Curated (MC); Hidden Markov Model (HMM) [42]; SOM [43]; Convolutional Neural Networks (CNN) [44]. The representative tools are described in the main text.
Tools related to miRNA biogenesis and function
Many bioinformatics tools have been developed for each process of miRNA biogenesis and to help biologists investigate questions in miRNA biology. Annotation tools are among the most important in the field. A platform for miRNA data storage is required for each miRNA sequence, pre-miRNA secondary structure, miRNA gene loci and other miRNA annotation information. Widely used annotation associated miRNA tools are listed. miRBase [22] is the main portal for miRNA storage and acts as a repository, which collects all known miRNA sequences and annotations for all species. Rfam [46] is a uniform system for RNA annotation and contains miRNA family information. miRIAD [47] was designed to host information about intragenic miRNAs and their host genes, while mirtronPred [48] predicts mirtrons from intronic sequences. mirSTP [49] is a program for identifying miRNA transcription start sites (TSSs). MetaMirClust [50] provides comprehensive information about miRNA clusters and their conservation.
Structure tools are also important. The secondary and tertiary structure of miRNA is important for recognition by specific binding proteins or for interaction with other RNAs. Structural features and free energy are key features of a machine learning method for predicting miRNA molecules. Representative miRNA structure prediction tools are ViennaRNA and RNAstructure. ViennaRNA [51] software package contains many tools to predict and compare RNA structure. RNAstructure [52] is a complete package and includes algorithms for RNA secondary structure prediction and analysis.
Identification tools are also important. As novel miRNA identification is a complex but essential process, various tools have been designed for this purpose. MiRscan [53] is an early user-friendly tool for identification of conserved miRNAs in nematodes. miRNAFold [54] is a fast ab initio method for miRNA prediction at a large scale in the genome. There are several tools that identify miRNAs based on next-generation sequencing (NGS) data, such as miRDeep [55] and miRanalyzer [56].
Cleavage site, binding and target discovery tools also play an important role. As the mechanism of Dicer cleavage site selection is still not fully understood, tools like LBSizeCleav [57] and PHDcleav [58] train a Support Vector Machine (SVM) model to predict these sites in pre-miRNAs. The AGO protein family is an essential component of the RISC and plays a central role in miRNA targeting. Therefore, many tools were developed to detect miRNA-binding sites from AGO-CLIP-Seq (e.g. AGO-PAR-CLIP and AGO-HITS-CLIP) data, such as miRBShunter [59], Antar [60] and miRTar2GO [61]. For studying miRNA function, many tools are designed to predict or collect miRNA targets, such as miRNA target prediction tools like miRanda [62], TargetScan [63], PicTar [64], RNAhybrid [65] and PITA [66], and experimentally validated miRNA target databases like miRecords [67] and miRTarBase [68].
Phenotyping, networking and extracellular miRNA tools add diversity to the field. Linking miRNA to phenotype is another method for the annotation of miRNA function, so several tools aim to collect phenotype associated miRNAs for animals and plants. HMDD [69] collects the manually curated human disease-related miRNAs, and PASmiR [70] contains the specific miRNAs for plant stress. The existence of ceRNA and miRNA sponge makes miRNA linked to other noncoding RNAs (ncRNAs) (lncRNA, circRNA etc.), which contain miRNA response elements (MREs) [71, 72]. miRNA interaction network analysis is a popular research direction, and many databases record specific types of interactions (e.g. miRNA-lncRNA, miRNA-circRNA and miRNA-mRNA). Frequently used miRNA interaction databases are starBase [73] and PceRBase [74]. starBase integrates several data sets about miRNA interaction with other RNA and ceRNA network. PceRBase is specific for recording plant ceRNAs. Extracellular miRNAs are potential biomarkers for clinical application and are collected specifically in some databases such as miRandola [75] and ExoCarta [76].
Tools related to miRNA analysis workflow
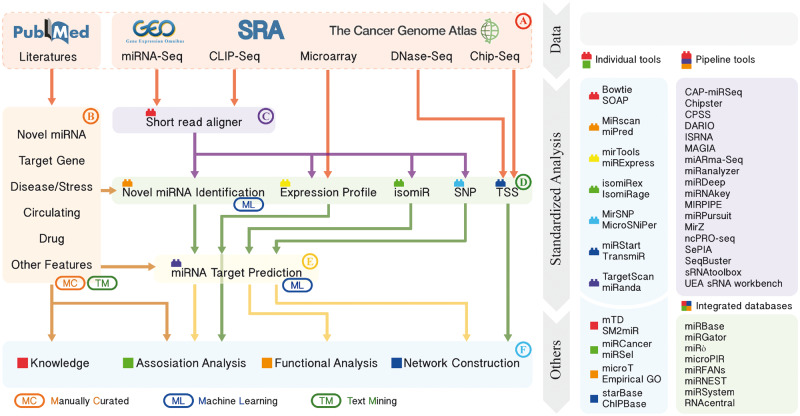
While no comprehensive tool exists for complete miRNA analysis, a robust analysis pipeline can be constructed from existing tools. A general miRNA bioinformatics analysis workflow is shown in Figure 3. As seen in Figure 3A, data sets for miRNA analysis can be downloaded from public databases and literature can be retrieved from PubMed. Expression data, including miRNA sequencing (miRNA-Seq) and miRNA microarray, can be downloaded from Gene Expression Omnibus (GEO) [77], Sequence Read Archive (SRA) [78], The Cancer Genome Atlas (TCGA) [79] and other biological data distribution centers. In Figure 3B, mining literature is one of the main activities in the bioinformatics field. Collecting and summarizing the results from previous work have profound significance, and databases constructed to host and organize this knowledge are required. In Figure 3C, recent technological advances in NGS have made it easier to capture the expression of miRNA. For miRNA NGS data analysis, the essential process is to align the short reads to the genome. Currently, many tools like Bowtie [80] and SOAP [81] can perform alignment efficiently, and short-read aligners are always wrapped in the pipeline tools.
Figure 3.
Standardized miRNA analysis workflow and examples of associated tools. The left panel with arrows shows the general bioinformatics miRNA analysis workflow, and the right panel shows the list of related tools. The tools are labeled with different colors and shapes corresponding to the same item on the left workflow. (A) Data sets download, (B) search of background knowledge, (C) read alignment, (D) identification and characterization of known and novel miRNAs, (E) target prediction and (F) downstream analysis.
In Figure 3D, novel miRNA identification can be curated from the literature, obtained from a genome via de novo prediction, or based on NGS data. A selected list of miRNA identification tools is shown in Table 1. For example, miPred [84] is a random forest (RF)-based miRNA predictor, which can distinguish between real and pseudo-miRNA precursors. miRDeep [55] is a pipeline tool supporting miRNA prediction and differential expression analysis based on miRNA-Seq data. Generation of miRNA expression profile is a key part of miRNA analysis. Identification of abnormally expressed miRNAs or co-expression of miRNAs may link miRNA to its function based on the experimental design. miRExpress [98] is implemented for generating miRNA expression profiles from miRNA-Seq data without the need for sequenced genomes. isomiRs (miRNA isoforms) refer to those sequences that have variations with respect to the canonical reference miRNA sequence [31]. isomiRex [99] is a Web-based tool for identification of miRNAs and isomiRs using NGS data. Single-nucleotide polymorphism (SNP) on miRNA or on miRNA target site could affect the interaction between them and further impact on the function of miRNA. MirSNP [100] is a database that collects SNPs in predicted miRNA target sites. To understand the transcriptional regulation of miRNAs, identifying their TSS and transcription factor binding site is required. microTSS [101] integrates H3K4me3 ChIP-Seq and DNase-Seq data to enable the characterization of tissue-specific promoters of miRNA, while miRStart [102] integrates cap-analysis gene expression with TSS-Seq and H3K4me3 ChIP-Seq data. TransmiR [103] is a database for storing TF–miRNA regulatory relationships.
Table 1.
Selected miRNA identification tools
| Tool name | Organism | Algorithm category | Publication span | Last update | Current version | Platform | Link | References |
|---|---|---|---|---|---|---|---|---|
| MiRscan | A | EC | 2003 | 2003 | – | WB | http://genes.mit.edu/mirscan/ | [53] |
| RNAz | A | SB, TS | 2005–10 | 2011 | v2.1 | WB, SA | https://www.tbi.univie.ac.at/software/RNAz/ | [82] |
| triplet–SVM | A, P | ML | 2005 | 2005 | – | SA | http://bioinfo.au.tsinghua.edu.cn/mirnasvm/ | [83] |
| MiPred | A, P | ML | 2007 | 2016 | v0.1 | WB | http://server.malab.cn/MiPred/ | [84] |
| miRDeep | A | NB, SB, ML | 2008–12 | 2016 | v2.0.0.8 | SA | https://www.mdc-berlin.de/8551903/en/ | [55] |
| CID-miRNA | A | ML | 2008 | 2008 | – | WB | https://github.com/alito/CID-miRNA | [85] |
| UEA sRNA workbench | A, P | NB | 2008–17 | 2018 | v4.5 | SA | http://srna-workbench.cmp.uea.ac.uk/mircat2/ | [86] |
| miRanalyzer | A, P | IA, NB | 2009–10 | 2012 | v0.3 | WB, SA | http://bioinfo2.ugr.es/miRanalyzer/standalone.html | [56] |
| MicroPC | P | EC | 2009 | 2009 | – | WB | http://www3a.biotec.or.th/micropc/ | [87] |
| HHMMiR | A, P | ML | 2009 | 2009 | v1.2 | SA | http://biodev.hgen.pitt.edu/kadriAPBC2009.html | [88] |
| MatureBayes | A | ML | 2010 | 2010 | – | WB, SA | http://mirna.imbb.forth.gr/MatureBayes.html | [89] |
| miRDeep-P | P | NB, SB, ML | 2011 | 2011 | v1.3 | SA | https://sourceforge.net/projects/mirdp | [90] |
| miRNAFold | A, P | SB, TS | 2012–16 | 2016 | – | WB, SA | https://evryrna.ibisc.univ-evry.fr/evryrna/mirnafold/mirnafold_home | [54] |
| miRDeep* | A, P | NB, IA, SB | 2013 | 2016 | v37 | SA | http://www.australianprostatecentre.org/research/software/mirdeep-star | [91] |
| miReader | A, P | NB, ML | 2013 | 2016 | – | SA | http://scbb.ihbt.res.in/2810-12/miReader.php | [92] |
| miRPlex | A, P | NB, ML | 2013 | 2013 | v0.1 | SA | https://www.uea.ac.uk/computing/mirplex | [93] |
| miRdentify | A | NB, TS | 2014 | 2014 | v1.0 | SA | http://www.ncrnalab.dk/#mirdentify/mirdentify.php | [94] |
| miRPlant | P | NB, IA | 2014 | 2017 | v5.1 | SA | https://sourceforge.net/projects/mirplant/ | [95] |
| deepSOM | A, P | ML | 2016 | 2016 | v0.19 | WB, SA | http://fich.unl.edu.ar/sinc/blog/web-demo/deepsom/ | [96] |
| Mirnovo | A, P | NB, ML | 2017 | 2018 | v1.0 | WB, SA | http://wwwdev.ebi.ac.uk/enright-dev/mirnovo/ | [97] |
Note: Algorithm category: Structure-based (SB), evolutionary conservation (EC), machine learning (ML), thermodynamic stability (TS), integrated approach (IA), NGS-based (NB); Organism: Animal (A), plant (P); Platform: Stand-alone (SA), Web-based (WB).
miRNA target prediction occupies the core position in the entire workflow, and it is the key step to reveal the miRNA function and links miRNA to other RNAs (mRNA, lncRNA and circRNA) as seen in Figure 3E. A list of representative miRNA target prediction tools is shown in Table 2. TargetScan [63], for example, is a Web server that predicts target genes of miRNA by searching for conserved sites that match the seed region of each miRNA. mirSOM [111] is a miRNA target prediction tool based on self-organizing map (SOM). DIANA-TarBase [105] is a manually curated experimentally validated miRNA targets database. Context-MMIA [118] collects miRNA targets based on text mining (TM).
Table 2.
Selected miRNA target prediction tools
| Tool name | Organism | Algorithm category | Publication span | Last update | Current version | Platform | Link | References |
|---|---|---|---|---|---|---|---|---|
| miRanda | A | SM, CH, ML, CM | 2003–10 | 2010 | v3.3a | WB, SA | http://34.236.212.39/microrna/home.do | [62] |
| RNAhybrid | A | SM, CH | 2004–06 | 2006 | v2.1.2 | WB, SA | https://bibiserv.cebitec.uni-bielefeld.de/rnahybrid | [65] |
| TargetScan | A | SM, EC, CM | 2005–15 | 2018 | v7.2 | WB, SA | http://www.targetscan.org | [63] |
| PicTar | A | CM, EC | 2005–06 | 2007 | – | WB | http://pictar.mdc-berlin.de/ | [64] |
| TargetFinder | P | CM | 2005–10 | 2015 | v1.7 | SA | https://github.com/carringtonlab/TargetFinder | [104] |
| TarBase | A, P | MC, IA | 2006–17 | 2017 | v8 | WB | http://carolina.imis.athena-innovation.gr/diana_tools/web/index.php? r=tarbasev8 | [105] |
| RNA22 | A | CM, CH | 2006–12 | 2015 | v2.0 | WB | https://cm.jefferson.edu/rna22/ | [106] |
| GenMiR++ | A, P | EX, ML | 2007 | 2007 | – | SA | http://www.psi.toronto.edu/genmir/ | [107] |
| PolymiRTS | A | IA, SM, PE | 2007–14 | 2014 | v3.0 | WB | http://compbio.uthsc.edu/miRSNP/ | [108] |
| miRDB | A | ML | 2008–16 | 2016 | v5.0 | WB | http://www.mirdb.org | [109] |
| miRGator | A | IA, EX | 2008–13 | 2013 | v3.0 | WB | http://mirgator.kobic.re.kr/ | [110] |
| miRecords | A | MC | 2009 | 2013 | v4 | WB | http://c1.accurascience.com/miRecords/ | [67] |
| mirSOM | A | ML, SM, CM | 2011 | 2011 | – | WB | https://bioinformatics.uef.fi/mirsom/ | [111] |
| miRWalk | A | IA, TM | 2011–15 | 2018 | v3.0 | WB | http://mirwalk.umm.uni-heidelberg.de/ | [112] |
| mirDIP | A | IA | 2011–17 | 2018 | v4.1 | WB | http://ophid.utoronto.ca/mirDIP/ | [113] |
| miRTarBase | A, P | MC | 2011–18 | 2017 | v7.0 | WB | http://mirtarbase.mbc.nctu.edu.tw | [114] |
| psRNATarget | P | SM, CM | 2011–18 | 2018 | v2 | WB | http://plantgrn.noble.org/psRNATarget/ | [115] |
| miRTarCLIP | A | IB | 2013 | 2013 | v1.0.1 | WB, SA | http://mirtarclip.mbc.nctu.edu.tw/ | [116] |
| MiRTDL | A | ML | 2016 | 2016 | – | WB, SA | http://nclab.hit.edu.cn/CCRM/ | [117] |
| miRBShunter | A | IB | 2017 | 2017 | v0.2 | SA | https://github.com/TrabucchiLab/miRBShunter | [59] |
| miRTar2GO | A | IB, CH, SM | 2017 | 2017 | – | WB | http://www.mirtar2go.org | [61] |
Note: Algorithm category: Seed matching (SM), complement matching (CM), compensatory hybridization (CH), evolutionary conservation (EC), machine learning (ML), Immunoprecipitation-Methods based (IB), expression correlation (EX), text mining (TM), manually curated (MC), integrated approach (IA), polymorphism effects (PE); Organism: Animal (A), plant (P); Platform: Stand-alone (SA), Web-based (WB).
Finally, a variety of tools can assist downstream analysis as seen in Figure 3F. Many databases are based on manually curated knowledge. miR2Disease [119] records associations between miRNA and disease, miRandola [75] collects extracellular miRNAs and SM2miR [120] contains associations between drugs and miRNAs. Many databases focus on a specific purpose, such as miRCancer [121], ChIPBase [122], while others integrate various miRNA data such as miRGator [110], miRNEST [123], miRSystem [124] and RNAcentral [125]. Selected resources to deal with different aspects of miRNA related research are shown in Table 3.
Table 3.
Selected other functional miRNA tools
| Tool name | Brief introduction | Organism | Publication span | Last update | Current version | Platform | Link | References |
|---|---|---|---|---|---|---|---|---|
| ViennaRNA | RNA secondary structure predictor | A, P | 2003–15 | 2018 | v2.4.7 | WB, SA | http://rna.tbi.univie.ac.at/ | [51] |
| miRBase | Archive of miRNA sequences and annotations | A, P | 2004–14 | 2018 | v22 | WB | http://www.mirbase.org/ | [22] |
| HMDD | Human miRNA disease database | A | 2008–14 | 2014 | v2.0 | WB | http://210.73.221.6/hmdd | [69] |
| Bowtie | Short-read aligner | A, P | 2009 | 2017 | v1.2.2 | SA | http://bowtie-bio.source forge.net | [80] |
| mirPath | miRNA pathway analysis | A | 2009–15 | 2015 | v3.0 | WB | http://snf-515788.vm.okeanos.grnet.gr/ | [126] |
| miR2Disease | Human miRNA disease database | A | 2009 | 2008 | - | WB | http://www.mir2disease.org/ | [119] |
| ExoCarta | Exosome miRNA database | A | 2009–16 | 2015 | - | WB | http://www.exocarta.org/ | [76] |
| SeqBuster | Pipeline for the analysis of miRNA-Seq data set | A | 2010–16 | 2016 | v1.2.1 | SA | https://pypi.org/project/seqcluster/ | [127] |
| TransmiR | A database of transcription factor-miRNA regulations | A, P | 2010 | 2013 | v1.2 | WB | http://www.cuilab.cn/transmir | [103] |
| dbDEMC | A database of differentially expressed miRNAs in cancers | A | 2010–17 | 2017 | v2.0 | WB | http://www.picb.ac.cn/dbDEMC | [128] |
| starBase | Pan-Cancer ceRNA database | A | 2011–14 | 2013 | v2.0 | WB | http://starbase.sysu.edu.cn/ | [73] |
| miTALOS | miRNA pathway analysis | A | 2011–16 | 2016 | v2 | WB | http://mips.helmholtz-muenchen.de/mitalos | [129] |
| miRStart | A database of miRNA TSSs | A | 2011 | 2011 | - | WB | http://mirstart.mbc.nctu.edu.tw/ | [102] |
| miRandola | A database of circulating miRNA | A | 2012–17 | 2017 | v2017 | WB | http://mirandola.iit.cnr.it/ | [75] |
| miRNEST | Integrative resource of miRNA-associated data | A, P | 2012–14 | 2014 | v2.0 | WB | http://rhesus.amu.edu.pl/mirnest/copy/ | [123] |
| miR_editing | Scripts for detecting editing sites in miRNA-Seq data set | A | 2012–13 | 2013 | - | SA | http://www.tau.ac.il/∼elieis/miR_editing | [130] |
| ChIPBase | A database of transcription factor–miRNA regulations | A | 2013–17 | 2016 | v2.3.4 | WB | http://rna.sysu.edu.cn/chipbase/ | [122] |
| SM2miR | A database of the association between miRNA and small molecules | A | 2013 | 2015 | - | WB | http://210.46.85.180:8080/sm2mir/index.jsp | [120] |
| YM500 | Database for miRNA-Seq in human cancer research | A | 2013–17 | 2017 | v3 | WB | http://driverdb.tms.cmu.edu.tw/ym500v3/ | [150] |
| isomiRex | Web platform for isomiR identification | A, P | 2013 | 2013 | - | WB | http://bioinfo1.uni-plovdiv.bg/isomiRex/ | [99] |
| PHDcleav | Dicer cleavage sites predictor | A | 2013 | 2013 | - | WB | http://crdd.osdd.net/raghava/phdcleav/ | [58] |
| PASmiR | A database for miRNA molecular regulation in plant abiotic stress | P | 2013 | 2015 | - | WB | http://pcsb.ahau.edu.cn:8080/PASmiR | [70] |
| microTSS | miRNA TSS identification scripts | A | 2014 | 2014 | v1.0 | SA | http://www.microrna.gr/microTSS/ | [101] |
| Chimira | Web platform for miRNA modifications detection | A, P | 2015 | 2017 | v1.5 | WB | http://wwwdev.ebi.ac.uk/enright-dev/chimira/ | [161] |
| MirGeneDB | Curated miRNA gene database | A | 2015 | 2018 | v2.0 | WB | http://mirgenedb.org/ | [159] |
| DREAM | Web platform for detecting RNA editing association with miRNAs | A | 2015 | 2015 | - | WB | http://www.cs.tau.ac.il/∼mirnaed/ | [160] |
| IsomiR Bank | A database for tracking isomiRs | A, P | 2016 | 2016 | - | WB | http://mcg.ustc.edu.cn/bsc/isomir/ | [151] |
| TissueAtlas | Tissue specificity miRNA database | A | 2016 | 2016 | - | WB | https://ccb-web.cs.uni-saarland.de/tissueatlas/ | [152] |
| miRNAme Converter | miRNA ID converter | A, P | 2016 | 2018 | v1.8.0 | WB, SA | http://163.172.134.150/miRNAmeConverter-shiny | [149] |
| mirSTP | miRNA TSS tracking program | A | 2017 | 2017 | - | SA | http://bioinfo.vanderbilt.edu/mirSTP/ | [49] |
| ParSel | Web platform for predicting survival associated miRNA | A | 2017 | 2017 | - | SA | https://github.com/debsin/ParSel | [153] |
| isomiR2 Function | Integrated workflow for identifying isomiRs in plants | P | 2017 | 2017 | - | SA | https://github.com/347033139/isomiR2Function | [162] |
| PceRBase | Plant ceRNA database | P | 2017 | 2016 | - | WB | http://bis.zju.edu.cn/pcernadb | [74] |
| miRsig | Pan-cancer miRNA-miRNA interaction database | A | 2017 | 2017 | - | WB | http://bnet.egr.vcu.edu/miRsig/ | [154] |
| OncomiR | Web platform for exploring pan-cancer miRNA dysregulation | A | 2017 | 2017 | - | WB | http://www.oncomir.org/ | [155] |
Note: Organism: Animal (A), plant (P); Platform: Stand-alone (SA), Web-based (WB).
An increasing number of databases and methods for predicting diagnostic and prognostic miRNA biomarkers of disease are being developed. Collecting biomarker features (association with disease, secretion characteristics, etc.) and constructing a network of related miRNAs are popular research strategies. Integrated databases collect data from different sources and integrate various types of data sets, with a user-friendly query interface and data visualization function. Pipeline tools aggregate basic or classic tools and involve several additional downstream steps to perform a particular complex miRNA analysis. There are variant tools with different purposes, forms and implementation technologies.
Trends from miRNA bioinformatics tool publications
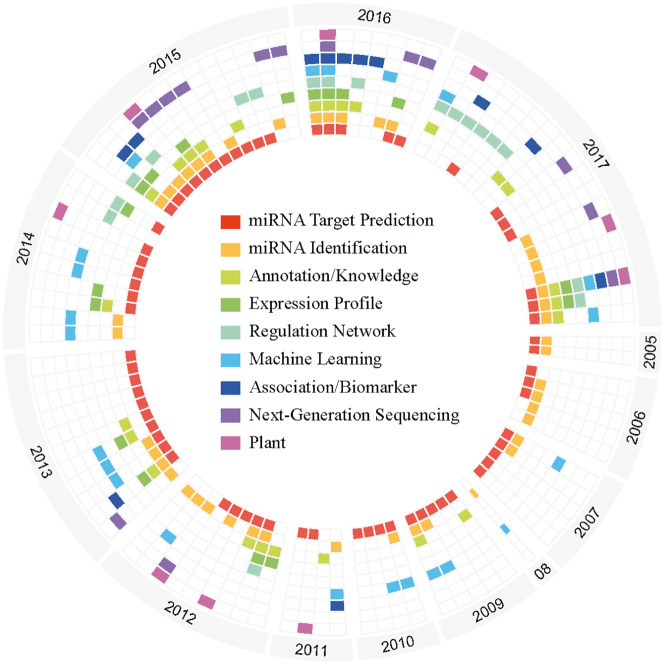
We obtained 95 miRNA bioinformatics tools associated review papers from PubMed, which can be classified into the following main topics: miRNA identification, miRNA target prediction, miRNA-regulated network, expression profile, features (disease or stress, biomarker) association, NGS tools, tools based on machine learning algorithms and tools specific for plants. The statistics of review topics by year since 2005 is shown in Figure 4. As the figure shows, most review papers concern miRNA identification and miRNA target prediction. miRNA identification tools show the technological migration from non-NGS to NGS-based analysis [131]. With improvements in laboratory and computational techniques, the tools of miRNA target prediction have evolved accordingly. AGO-CLIP-Seq, AGO-RIP-Seq and AGO-HITS-CLIP using tools can tell us the binding site of miRNAs [132]. Many reviews discuss the application of machine learning techniques for miRNA analysis, and deep learning has already been applied to target prediction [133]. More miRNA-associated disease databases or tools are emerging, especially for cancer research, implemented based on expression profiles, TM or manually curated [134]. The full review paper list is available in Supplementary Table S1.
Figure 4.
Circular graphic of miRNA identification and miRNA target prediction mentioned in reviews since 2005. Each sector contains the reviews published in each year. Each column represents a review paper, and each block with a different color indicates the specific topics in the review paper.
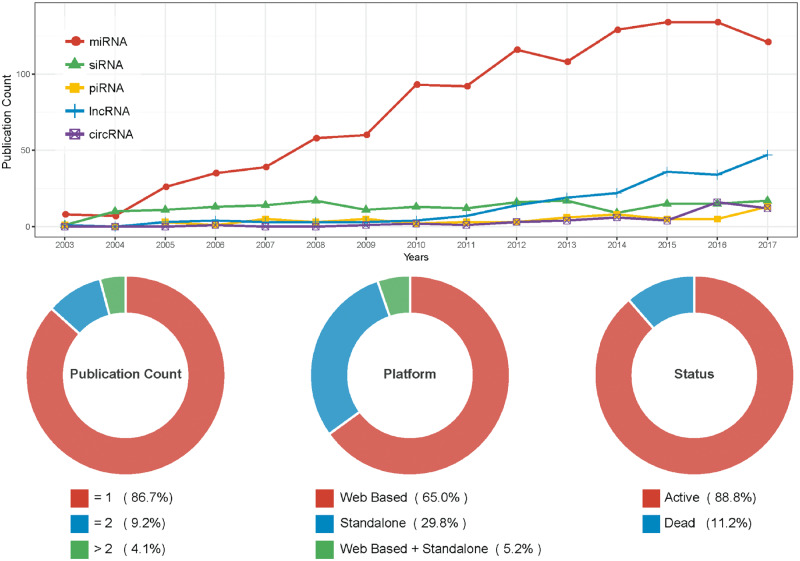
The number of miRNA tool papers has risen rapidly from 2003 to 2017 (shown in Figure 5 line chart, red line) and the amount of miRNA tools is larger than other ncRNA tools. Based on basic statistics from miRToolsGallery, 66.6% of papers were published in seven journals [45]. A substantial fraction (13.3%) of tools have published an updated version (Figure 5) suggesting many tools are updated regularly and maintained well. Web-based tools are the most popular type of miRNA tool, and the databases or Web services often integrate multilevel omics data or multiple basic tools.
Figure 5.
Statistic of miRNA tools. Line chart: The number of publications of ncRNA-related bioinformatics tools by year since 2003. Different colors represent different ncRNAs, including miRNA, siRNA, piRNA, lncRNA and circRNA. miRNA statistic data were extracted from miRToolsGallery [45], and other ncRNAs were collected with the same method as described in miRToolsGallery. Donut chart: the number of publications per tool, platforms of tools and status of tools are presented as percentages.
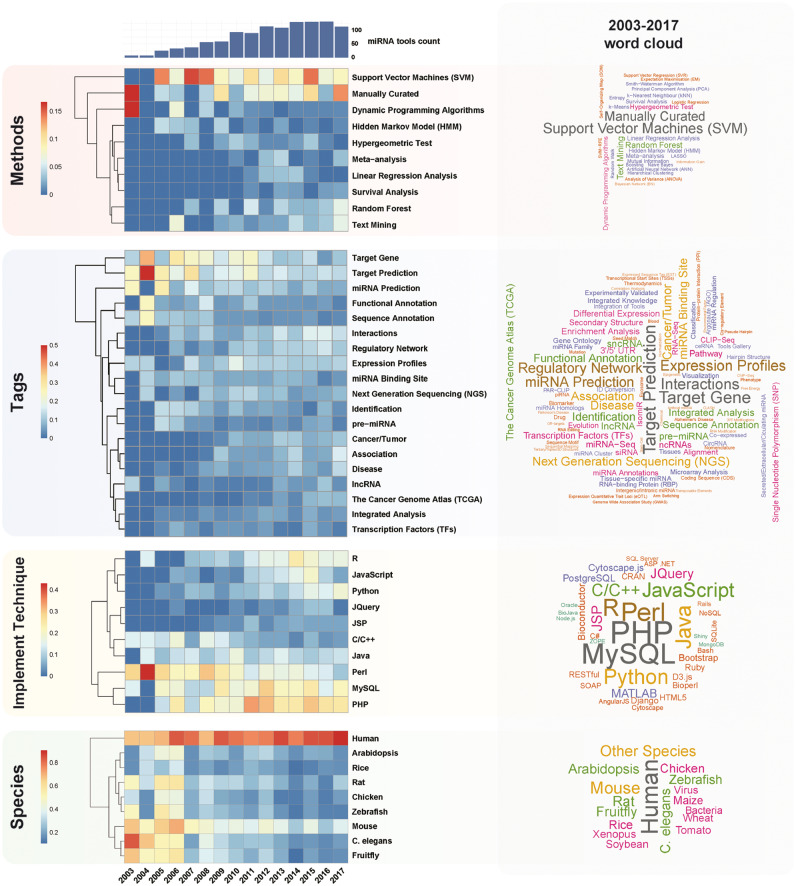
Based on TM and tag statistics from miRToolsGallery (Figures 6 and 7), we observed several notable trends. First, we found that topics from 2003 to 2017 changed. Through the tag usage rate across years in four different sub-catalogs (‘Implementation technology’, ‘Species’, ‘Methods’ and ‘Tags’), we found ‘SVM’ and ‘Random forest’ were the most widely used machine learning methods applied in the miRNA field. Representative tools are miRDB [109], SVMicrO [137], miPred [84] and MiRmat [138]. ‘Manually curated’ is another label to mark a database, and it occupied the second position after ‘SVM’ based on the word cloud in Figure 6. Representative tools were DIANA-TarBase [105], miRecords [67], miR2Disease [119], HMDD [69] and TransmiR [103].
Figure 6.
Tags statistic of miRNA tools based on miRToolsGallery. Heat map for top tags in each catalog, the values of which equal the term frequency divided by miRNA tool count in each year. The bar chart shows the miRNA tool count in each year. The word cloud shows the tag usage in each tag catalogs based on all data from 2003 to 2017. The full tags statistic table is available in Supplementary Table S3.
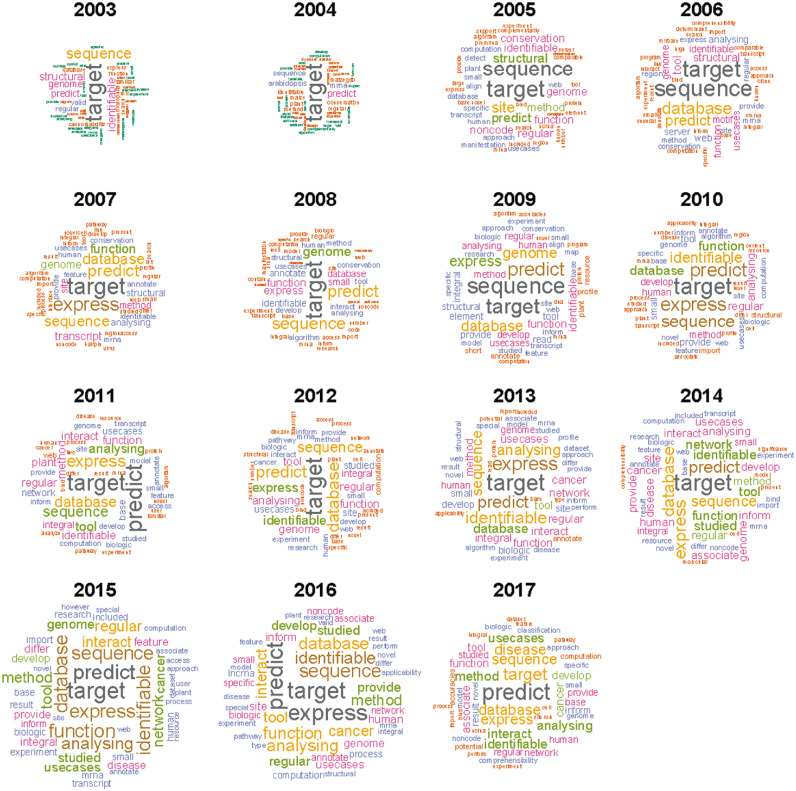
Figure 7.
Word cloud based on literature in each year. The word cloud was generated based on the publication’s abstract and title. TM was performed by a CRAN R package ‘tm’ [135], and figure was drawn by the ‘wordcloud’ package [136]. ‘miRNA’ is the universal keyword in all the texts, so the ‘miRNA’ was removed from the word cloud.
Although neural network is not new for the miRNA field, deep learning, as a new form, began to be applied to miRNA prediction in tools such as MiRTDL [117], iDeep [139] and deepTarget [140]. In bioinformatics tools developing techniques, PHP and MySQL are the most frequently used, and Perl is the dominant programing language in this field; however, R and Python are emerging. Web-based tools occupied a dominant proportion of all the tools, as they have the advantage of ease-of-use and do not require programming skills [45]. Before 2010, target prediction was a popular research direction, while after 2011, integration of previous works (e.g. target prediction), miRNA–target interaction network analysis and NGS data analysis became popular. With a deeper understanding of miRNA biochemistry and function, new sequencing technology (NGS) development, novel experimental results, publication of computational algorithms/techniques and novel miRNA bioinformatics tools have sprung up. During the first years, miRNA tools were developed equally for various species, but recently, tools for human research have been dominating the field. As shown in Figure 7, word clouds based on the abstract and title of papers published each year illustrate that ‘target’ is in the keywords across all the years. As miRNA target prediction is beneficial to analyze miRNA function, there are many target prediction tools, experimentally validated target databases and miRNA–target gene interaction analysis tools, which form the basis for other studies.
We can divide the development of miRNA analysis tools into three stages: Stage 1 (2003–05), Stage 2 (2006–09) and Stage 3 (2010–17). In Stage 1, functions of miRNA tools are usually specific and mainly focus on miRNA sequence annotation, miRNA target prediction and miRNA identification. Since 1993, biological and bioinformatics approaches have discovered thousands of miRNAs in animals, plants and other species and deposited these into miRBase [22]. Many bioinformatics tools have been developed to identify miRNA. Based on the column ‘Algorithm Category’ in Table 1, we can see the major bioinformatics techniques behind the tools: structure based, evolutionary conservation, machine learning, thermodynamic stability, integrated approach and NGS-based [141, 142]. In this stage, the sequence conservation, thermodynamic analysis and structure prediction were applied to discover novel miRNA in tools such as MiRscan [53] and RNAz [82]. miRNAs function by interacting with target genes [1]. Based on the column ‘Algorithm Category’ in Table 2, we can see the major bioinformatics techniques behind the tools: seed matching, complement matching, compensatory hybridization, evolutionary conservation, machine learning, immunoprecipitation-Methods-based, text mining, expression correlation, manually curated, integrated approach and polymorphism effects [141, 143–145]. Seed matching, complement matching and evolutionary conservation were used to predict the miRNA targets in tools such as TargetScan [63]. Many tools created in Stage 1 are updated regularly and still frequently used, such as miRBase [22] and TargetScan [63]. Those tools are considered classic based on citation, usage and longevity, and they have a profound impact on subsequent tools.
In Stage 2, miRNA expression profile-related tools, such as miRGator [110], miRNAMap [146] and miRExpress [98] appeared, benefiting from the development of miRNA expression techniques (Microarray and NGS). Predicting miRNA target via paired mRNA/miRNA expression data made great progress at this stage, such as GenMiR++ [107]. As the technology developed, more ways were available to study miRNA and more problems needed to be solved with the help of bioinformatics tools.
In Stage 3, an increasing number of integrated tools emerged, such as mirDIP [113], miRSystem [124], Chipster [147], miRDeep* [91] and Tools4miRs [148]. With the generation of big data, integrated tools (also known as meta-server) and knowledge (manually curated database), miRNA bioinformatics tools appeared to be entering a new phase. New knowledge of miRNA, isomiR database, arm switching phenomena and miRNA modification tools came out. Through time, miRBase updated the nomenclatures of miRNA and provided handy and useful ID conversion tools that were in high demand, such as miRNAmeConverter [149] and miRBase Tracker [156]. At the same time, many types of minority but useful tools were developed. Impressively, a smart phone application (APP) was developed to view arm switching based on miRNA-Seq data called RNA-Seq Viewer [157]. Advanced immunoprecipitation methods combined with NGS technology gave new insight into the interaction between RBPs and miRNA, provided more evidence for miRNA target prediction with tools such as starBase [73] and SimiRa [158], and more training data for ML enhanced tools like iDeep [139]. Meanwhile, novel global run-on sequencing techniques and precision run-on sequencing provided a new means to identify active miRNA TSSs and have been incorporated in mirSTP [49]. miRBase [22], as the standard central repository for miRNA sequences and annotation, has been challenged recently by miRCarta, a superset of miRBase, and mirGeneDB [159], a uniform system for the annotation and nomenclature of miRNA genes. Meanwhile, miRNA modification (or RNA editing) and isomiR analysis are frequently considered in recent miRNA-Seq analysis tools. For example, DREAM [160] and miR_editing [130] are tools for detecting RNA editing association from miRNA-Seq dataset. Chimira [161] can analyze small RNA sequencing data and miRNA modifications. isomiR2Function [162] is a pipeline for analysis of plant miRNA variants. isomiR-Benchmark [163] is an isomiR identification tool. QuickMIRSeq [164] is a pipeline for accurate quantification of known miRNAs and isomiRs.
Trends from popularity analysis
miRNA tools popularity is measured by PageRank algorithm and total citation count. A total of 1254 miRNA tool-related papers with rank criterions are listed in Supplementary Table S2. When ranking the papers by total citation count (citation scope is total literature from PubMed), we found that short-read alignment tools BWA [165] and Bowtie [80] occupied the first and second position, followed by miRNA target prediction tools TargetScan [63] and PicTar [64], and the comprehensive miRNA registry database miRBase [22]. When ranking the papers by PageRank algorithm (citation network only limited to all the miRNA tool papers), the order was different. Three types of tools occupied the top 10 positions: the sequence and annotation databases Rfam [46] and miRBase [22]; target prediction tools TargetScan [63], MiRscan [53], miRanda [62] and miRNA–Target Gene Prediction at EMBL [166]; RNA structure prediction tool suite ViennaRNA [51] in the 10th place. A miRNA tool list is shown in Supplementary Table S3.
As Figure 5 shows, the number of publications in the field has leveled off, suggesting that the field appears to be mature, which could be supported by the highly cited papers, of which the first two were published in 2009 (BWA and Bowtie). In addition, it is notable that updated publications of those major databases were highly cited, suggesting the necessity and utility of follow-up publications. As an example, the miRBase publication was in the top 20 cited list five times from 2004 to 2014. Another example is the target prediction tool TargetScan [63], which was the most highly cited, and its updated version was the second most highly cited publication in the list. Notably, there is a significant overlap between bioinformatics tools that achieved top rankings based on either total citations or PageRank algorithm. Many early tools are top ranked, which not only explains their popularity but also indicates that classic tools are well maintained and still widely used. For example, many old target prediction tools or miRNA identification tools are still integrated together as a source of new tools, such as mirDIP [113] and mirMeta [167]. mirDIP integrates miRNA targets from about 30 different miRNA target databases, such as TargetScan [63], miRDB [109] and PITA [66]. mirMeta constructs an artificial neural network to predict miRNAs based on the predicted results of the following software tools: MiPred [84], MiReNA [168], miRPara [169], ProMiR [170] and triplet-SVM [83].
Trends from network analysis
Network analysis of miRNA tool citation relations provides another perspective of the field. The evolving miRNA paper citation network is drawn in Supplementary Figure S1, in which a node represents a specific paper and if two nodes have citation relationship, they will be connected by an edge. As the figure shows, the networks grow through time and stays tightly connected, suggesting coherence among bioinformatics tools and subsequent papers based on previous work or available resources.
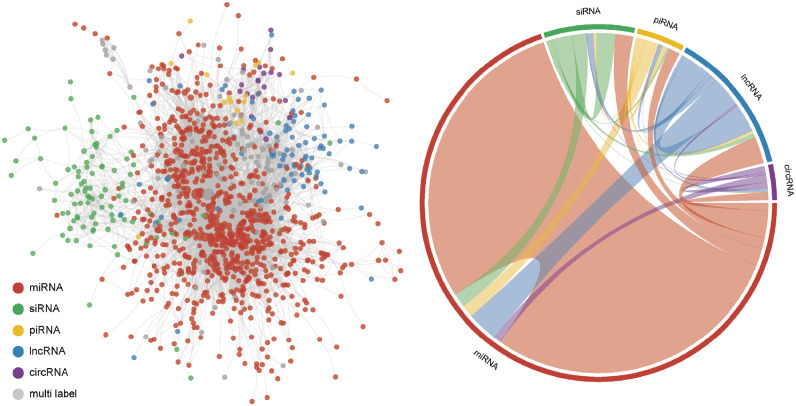
Similarly, we built an ncRNA tools network in Figure 8. Here, we chose miRNA, small interfering RNA (siRNA), Piwi-interacting RNA (piRNA), lncRNA and circRNA, and marked them with different colors. miRNA tools constitute the majority of the network, which is divided into five different color partitions. siRNA tools are located separately from the other parts of the network and are centered around a different hub. Each type of ncRNA tool gets the most internal citations and closely interacts with miRNA tools. As miRNA can play an important role in ceRNA network and be the target of miRNA sponges, miRNA is a central part of other ncRNA studies. Recently, circRNA emerged as a new entity. Notably, most circRNA tools, except identification tools, are integrated with miRNA research, for circRNA contains the MREs and can act as a miRNA sponge. With the help of other ncRNA tools, more ceRNA tools have been developed.
Figure 8.
ncRNA tools interaction network. The left network was based on the tool’s publication citation. The miRNA tools publications were extracted from miRToolsGallery. Other ncRNA tools were retrieved from PubMed with the same criterion like miRToolsGallery. Gray nodes represented the tools that can be applied by up to two different ncRNA analyses. The right chord diagram represents the interaction strength of each different ncRNA tool. Different sectors represent different ncRNA tools, and the link represents the citation number from source to target (e.g. the red link means miRNA tools cited by other ncRNA tools.). The network was generated by a CRAN R package ‘igraph’ [171] and was drawn with a force-directed layout.
Conclusions
Since 2002, miRNA research tools have evolved along with the development of experimental methods (Figure 2). After the introduction of NGS technologies, the number of novel miRNA sequences submitted to miRBase has exploded [22]. However, new miRNA features, like isomiRs [31] and miRNA SNPs [172], have been observed from the sequencing data, which has led research to new directions. miRNA target prediction, as a computational way to predict miRNA function, is probably the most important part in the miRNA study. Target prediction algorithms have evolved from seed matching (SM) combined with thermodynamic stability [144], via requirement of co-expression with target genes [107] to methods using miRNA-binding site knowledge from AGO IP experiments [36]. Machine learning, most recently deep learning, is widely used [133, 141]. Single methods are combined to integrated platforms to improve the plausibility of the predictions [113]. The algorithms are improved continuously by adding novel knowledge, for example, the recent finding that alternative polyadenylation of target genes may mediate miRNA regulation [173, 174] will probably be integrated into forthcoming target prediction tools.
The involvement of miRNAs in several human diseases makes them potential diagnostic biomarkers [20, 21], and therefore, miRNA tools for disease research are emerging [134, 175]. Novel miRNA biomarkers are explored from manually curated information, by TM from literature and from predicted miRNA–target relations in expression data. Increasingly, database records or methods to predict diagnostic and prognostic miRNA biomarkers of disease are being developed [176].
Although a number of pipeline tools [45, 141] have been developed to analyze portions of miRNA-related problems, there is still no ‘one-stop’ tool to integrate all steps. As miRNA-associated high-throughput sequencing data continue to grow at an exponential rate, the need for data integration is becoming critical. Demand for unified nomenclature of new miRNA knowledge, such as isomiRs, microRNA-offset RNA [177], loop-miRs [178], is increasing, and therefore, the current lack of uniform names complicates each step of data analysis and pipeline automation [22, 159, 179].
The most troubling aspect of the trends was the date of the most highly cited or PageRanked tools. As these were very old, it suggests stagnation in the field. However, many early tools are still well maintained and frequently wrapped in new tools. Predictably, future miRNA bioinformatics tools will contain the following characteristics: (1) aim for new miRNA knowledge, (2) analyze high-throughput miRNA technology data, (3) integrate multilevel omics data and (4) focus on human disease. Taken together, this review highlights trends in miRNA bioinformatics tools development, which may be beneficial to direct and improve future activity and efforts.
Key Points
miRNA identification and target prediction remain hot spots in the miRNA bioinformatics tools field, while recent advances in NGS technologies provide improved target prediction based on experimental validation.
Manual curation and TM are the main methods for collecting miRNA knowledge from literature. The collection goals are diverse and include experimentally validating targets, disease association, effects of drug action and biomarker discovery.
Most early tools are still well maintained and widely used. They are deservedly classic tools and wrapped in new single tools or pipeline tools.
Classic machine learning methods, such as SVM, are still popularly used in the miRNA field, while novel and advanced deep learning methods are beginning to appear.
Supplementary Material
Acknowledgments
The authors wish to thank all the members of the Wong Laboratory who provided critical comments and feedback. TM was performed in part at the high-performance computing cluster (HPCC) that is supported by information and communication technology office (ICTO) of the University of Macau.
Funding
This work was supported by the Academy of Finland (project #62340); University of Macau Faculty of Health Sciences (grant number MYRG2016-00101-FHS); and the National Natural Science Foundation of China (grant numbers 61472158, 61572228 and 61602207).
Biographies
Liang Chen is a Post-doctoral Fellow of the Faculty of Health Sciences, University of Macau. His research interest is focused on machine learning and small noncoding RNA bioinformatics.
Liisa Heikkinen is a Post-doctoral Fellow and Visiting Scholar of the Faculty of Health Sciences, University of Macau. Her research interests lie in the field of transcriptomics and especially applications involving nematodes.
Changliang Wang is a PhD student of the Faculty of Health Sciences, University of Macau. His research interests include data integration analysis, database construction, ageing and cancer associated research.
Yang Yang is a PhD student of the Faculty of Health Sciences, University of Macau. His research interests are in discovering microRNA-offset RNA (moRNA) from NGS data sets and bioinformatics tool development.
Huiyan Sun is a PhD student of College of Computer Science and Technology, Jilin University. She is interested in developing and applying statistical and computational methods for mining cancer-omic data.
Garry Wong is a Full Professor of Faculty of Health Sciences, University of Macau. His laboratory focus on Parkinson’s disease, aging and environmental stress and development of bioinformatics tools to investigate these processes.
References
- 1. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116(2):281–97. [DOI] [PubMed] [Google Scholar]
- 2. Krol J, Loedige I, Filipowicz W.. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010;11(9):597–610. [DOI] [PubMed] [Google Scholar]
- 3. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136(2):215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okamura K, Hagen JW, Duan H, et al. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007;130(1):89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Altuvia Y, Landgraf P, Lithwick G, et al. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res 2005;33(8):2697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Achkar NP, Cambiagno DA, Manavella PA.. miRNA biogenesis: a dynamic pathway. Trends Plant Sci 2016;21(12):1034–44. [DOI] [PubMed] [Google Scholar]
- 7. Kim YJ, Zheng B, Yu Y, et al. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J 2011;30(5):814–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie Z, Allen E, Fahlgren N, et al. Expression of Arabidopsis MIRNA genes. Plant Physiol 2005;138(4):2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han M-H, Goud S, Song L, et al. The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc Natl Acad Sci USA 2004;101(4):1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rogers K, Chen X.. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 2013;25(7):2383–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou Y, Honda M, Zhu H, et al. Spatiotemporal sequestration of miR165/166 by Arabidopsis Argonaute10 promotes shoot apical meristem maintenance. Cell Rep 2015;10(11):1819–27. [DOI] [PubMed] [Google Scholar]
- 12. Yu B, Yang Z, Li J, et al. Methylation as a crucial step in plant microRNA biogenesis. Science 2005;307(5711):932–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lima RT, Busacca S, Almeida GM, et al. MicroRNA regulation of core apoptosis pathways in cancer. Eur J Cancer 2011;47(2):163–74. [DOI] [PubMed] [Google Scholar]
- 14. Rao X, Di Leva G, Li M, et al. MicroRNA-221/222 confers breast cancer fulvestrant resistance by regulating multiple signaling pathways. Oncogene 2011;30(9):1082–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Y, Huang J, Guo M, et al. MicroRNAs regulating signaling pathways: potential biomarkers in systemic sclerosis. Genomics Proteomics Bioinformatics 2015;13(4):234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tay Y, Rinn J, Pandolfi PP.. The multilayered complexity of ceRNA crosstalk and competition. Nature 2014;505(7483):344–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011;146(3):353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sayed D, Abdellatif M.. MicroRNAs in development and disease. Physiol Rev 2011;91(3):827–87. [DOI] [PubMed] [Google Scholar]
- 19. Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56(11):1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105(30):10513–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 2008;18(10):997–1006. [DOI] [PubMed] [Google Scholar]
- 22. Kozomara A, Griffiths-Jones S.. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res 2014;42(D1):D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee RC, Feinbaum RL, Ambros V.. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75(5):843–54. [DOI] [PubMed] [Google Scholar]
- 24. Pasquinelli AE, Reinhart BJ, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000;408(6808):86–9. [DOI] [PubMed] [Google Scholar]
- 25. Grishok A, Pasquinelli AE, Conte D, et al. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001;106(1):23–34. [DOI] [PubMed] [Google Scholar]
- 26. Hutvagner G, McLachlan J, Pasquinelli AE, et al. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001;293(5531):834–8. [DOI] [PubMed] [Google Scholar]
- 27. Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci USA 2002;99(24):15524–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee Y, Ahn C, Han J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003;425(6956):415–19. [DOI] [PubMed] [Google Scholar]
- 29. Hutvagner G, Zamore PD.. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002;297(5589):2056–60. [DOI] [PubMed] [Google Scholar]
- 30. Schwarz DS, Hutvagner G, Du T, et al. Asymmetry in the assembly of the RNAi enzyme complex. Cell 2003;115(2):199–208. [DOI] [PubMed] [Google Scholar]
- 31. Morin RD, O'Connor MD, Griffith M, et al. Application of massively parallel sequencing to microRNA profiling and discovery in human embryonic stem cells. Genome Res 2008;18(4):610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu CG, Calin GA, Meloon B, et al. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci USA 2004;101(26):9740–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aldridge S, Hadfield J.. Introduction to miRNA profiling technologies and cross-platform comparison. Methods Mol Biol 2012;822:19–31. [DOI] [PubMed] [Google Scholar]
- 34. Kulski JK. Next-generation sequencing—an overview of the history, tools, and “omic” applications In: Next Generation Sequencing-Advances, Applications and Challenges. London: Intech, 2016. [Google Scholar]
- 35. Ruby JG, Jan C, Player C, et al. Large-scale sequencing reveals 21U-RNAs and additional microRNAs and endogenous siRNAs in C. elegans. Cell 2006;127(6):1193–207. [DOI] [PubMed] [Google Scholar]
- 36. Chi SW, Zang JB, Mele A, et al. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature 2009;460(7254):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Helwak A, Kudla G, Dudnakova T, et al. Mapping the human miRNA interactome by CLASH reveals frequent noncanonical binding. Cell 2013;153(3):654–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ho TK. Random decision forests. In: Proceedings of the Third International Conference on Document Analysis and Recognition, 1995 IEEE, 1995, 278–82.
- 39. Cortes C, Vapnik V.. Support-vector networks. Mach Learn 1995;20(3):273–97. [Google Scholar]
- 40. Drucker H, Burges CJ, Kaufman L, et al. Support vector regression machines. In: Advances in Neural Information Processing Systems. Cambridge, MA: MIT Press, 1997, 155–161. [Google Scholar]
- 41. Cohen KB, Hunter L.. Getting started in text mining. PLoS Comput Biol 2008;4(1):e20.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yoon BJ. Hidden Markov models and their applications in biological sequence analysis. Curr Genomics 2009;10(6):402–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kohonen T. Self-organized formation of topologically correct feature maps. Biol Cybernet 1982;43(1):59–69. [Google Scholar]
- 44. LeCun Y, Bottou L, Bengio Y, et al. Gradient-based learning applied to document recognition. Proc IEEE 1998;86(11):2278–324. [Google Scholar]
- 45. Chen L, Heikkinen L, Wang C, et al. miRToolsGallery: a tag-based and rankable microRNA bioinformatics resources database portal. Database 2018;2018:bay004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nawrocki EP, Burge SW, Bateman A, et al. Rfam 12.0: updates to the RNA families database. Nucleic Acids Res 2015;43:D130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hinske LC, Franca GS, Torres HA, et al. miRIAD-integrating microRNA inter- and intragenic data. Database 2014;2014:bau099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joshi PK, Gupta D, Nandal UK, et al. Identification of mirtrons in rice using MirtronPred: a tool for predicting plant mirtrons. Genomics 2012;99(6):370–5. [DOI] [PubMed] [Google Scholar]
- 49. Liu Q, Wang J, Zhao Y, et al. Identification of active miRNA promoters from nuclear run-on RNA sequencing. Nucleic Acids Res 2017;45(13):e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chan WC, Lin WC.. MetaMirClust: discovery and exploration of evolutionarily conserved miRNA clusters. Methods Mol Biol 2016;1375:75–89. [DOI] [PubMed] [Google Scholar]
- 51. Lorenz R, Bernhart SH, Honer Zu Siederdissen C, et al. ViennaRNA package 2.0. Algorithms Mol Biol 2011;6:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bellaousov S, Reuter JS, Seetin MG, et al. RNAstructure: web servers for RNA secondary structure prediction and analysis. Nucleic Acids Res 2013;41:W471–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lim LP, Lau NC, Weinstein EG, et al. The microRNAs of Caenorhabditis elegans. Genes Dev 2003;17(8):991–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tav C, Tempel S, Poligny L, et al. miRNAFold: a web server for fast miRNA precursor prediction in genomes. Nucleic Acids Res 2016;44(W1):W181–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Friedlander MR, Mackowiak SD, Li N, et al. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 2012;40(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hackenberg M, Rodriguez-Ezpeleta N, Aransay AM.. miRanalyzer: an update on the detection and analysis of microRNAs in high-throughput sequencing experiments. Nucleic Acids Res 2011;39:W132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bao Y, Hayashida M, Akutsu T.. LBSizeCleav: improved support vector machine (SVM)-based prediction of Dicer cleavage sites using loop/bulge length. BMC Bioinformatics 2016;17(1):487.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ahmed F, Kaundal R, Raghava GP.. PHDcleav: a SVM based method for predicting human Dicer cleavage sites using sequence and secondary structure of miRNA precursors. BMC Bioinformatics 2013;14(Suppl 14):S9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bottini S, Hamouda-Tekaya N, Tanasa B, et al. From benchmarking HITS-CLIP peak detection programs to a new method for identification of miRNA-binding sites from Ago2-CLIP data. Nucleic Acids Res 2017;45:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wen J, Parker BJ, Jacobsen A, et al. MicroRNA transfection and AGO-bound CLIP-seq data sets reveal distinct determinants of miRNA action. RNA 2011;17(5):820–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahadi A, Sablok G, Hutvagner G.. miRTar2GO: a novel rule-based model learning method for cell line specific microRNA target prediction that integrates Ago2 CLIP-Seq and validated microRNA-target interaction data. Nucleic Acids Res 2017;45(6):e42.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Betel D, Koppal A, Agius P, et al. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol 2010;11(8):R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Agarwal V, Bell GW, Nam JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. Elife 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lall S, Grun D, Krek A, et al. A genome-wide map of conserved microRNA targets in C. elegans. Curr Biol 2006;16(5):460–71. [DOI] [PubMed] [Google Scholar]
- 65. Kruger J, Rehmsmeier M.. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res 2006;34:W451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kertesz M, Iovino N, Unnerstall U, et al. The role of site accessibility in microRNA target recognition. Nat Genet 2007;39(10):1278–84. [DOI] [PubMed] [Google Scholar]
- 67. Xiao F, Zuo Z, Cai G, et al. miRecords: an integrated resource for microRNA-target interactions. Nucleic Acids Res 2009;37:D105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chou CH, Chang NW, Shrestha S, et al. miRTarBase 2016: updates to the experimentally validated miRNA-target interactions database. Nucleic Acids Res 2016;44(D1):D239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li Y, Qiu C, Tu J, et al. HMDD v2.0: a database for experimentally supported human microRNA and disease associations. Nucleic Acids Res 2014;42(D1):D1070–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang S, Yue Y, Sheng L, et al. PASmiR: a literature-curated database for miRNA molecular regulation in plant response to abiotic stress. BMC Plant Biol 2013;13(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013;495(7441):333–8. [DOI] [PubMed] [Google Scholar]
- 72. Cesana M, Cacchiarelli D, Legnini I, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell 2011;147(2):358–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 2014;42(D1):D92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yuan C, Meng X, Li X, et al. PceRBase: a database of plant competing endogenous RNA. Nucleic Acids Res 2017;45(D1):D1009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Russo F, Di Bella S, Vannini F, et al. miRandola 2017: a curated knowledge base of non-invasive biomarkers. Nucleic Acids Res 2018;46(D1):D354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Keerthikumar S, Chisanga D, Ariyaratne D, et al. ExoCarta: a web-based compendium of exosomal cargo. J Mol Biol 2016;428(4):688–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barrett T, Wilhite SE, Ledoux P, et al. NCBI GEO: archive for functional genomics data sets–update. Nucleic Acids Res 2013;41:D991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Leinonen R, Sugawara H, Shumway M.. The sequence read archive. Nucleic Acids Res 2011;39:D19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Weinstein JN, Collisson EA, Mills GB, et al. The cancer genome atlas pan-cancer analysis project. Nat Genet 2013;45(10):1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Langmead B, Trapnell C, Pop M, et al. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li R, Yu C, Li Y, et al. SOAP2: an improved ultrafast tool for short read alignment. Bioinformatics 2009;25(15):1966–7. [DOI] [PubMed] [Google Scholar]
- 82. Gruber AR, Findeiss S, Washietl S, et al. RNAz 2.0: improved noncoding RNA detection. Pac Symp Biocomput 2010;69–79. [PubMed] [Google Scholar]
- 83. Xue C, Li F, He T, et al. Classification of real and pseudo microRNA precursors using local structure-sequence features and support vector machine. BMC Bioinformatics 2005;6:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jiang P, Wu H, Wang W, et al. MiPred: classification of real and pseudo microRNA precursors using random forest prediction model with combined features. Nucleic Acids Res 2007;35:W339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tyagi S, Vaz C, Gupta V, et al. CID-miRNA: a web server for prediction of novel miRNA precursors in human genome. Biochem Biophys Res Commun 2008;372(4):831–4. [DOI] [PubMed] [Google Scholar]
- 86. Paicu C, Mohorianu I, Stocks M, et al. miRCat2: accurate prediction of plant and animal microRNAs from next-generation sequencing datasets. Bioinformatics 2017;33(16):2446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mhuantong W, Wichadakul D.. MicroPC (microPC): a comprehensive resource for predicting and comparing plant microRNAs. BMC Genomics 2009;10:366.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kadri S, Hinman V, Benos PV.. HHMMiR: efficient de novo prediction of microRNAs using hierarchical hidden Markov models. BMC Bioinformatics 2009;10(Suppl 1):S35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gkirtzou K, Tsamardinos I, Tsakalides P, et al. MatureBayes: a probabilistic algorithm for identifying the mature miRNA within novel precursors. PLoS One 2010;5(8):e11843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yang X, Li L.. miRDeep-P: a computational tool for analyzing the microRNA transcriptome in plants. Bioinformatics 2011;27(18):2614–5. [DOI] [PubMed] [Google Scholar]
- 91. An J, Lai J, Lehman ML, et al. miRDeep*: an integrated application tool for miRNA identification from RNA sequencing data. Nucleic Acids Res 2013;41(2):727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jha A, Shankar R.. miReader: discovering Novel miRNAs in species without sequenced genome. PLoS One 2013;8(6):e66857.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mapleson D, Moxon S, Dalmay T, et al. MirPlex: a tool for identifying miRNAs in high-throughput sRNA datasets without a genome. J Exp Zool B Mol Dev Evol 2013;320(1):47–56. [DOI] [PubMed] [Google Scholar]
- 94. Hansen TB, Veno MT, Kjems J, et al. miRdentify: high stringency miRNA predictor identifies several novel animal miRNAs. Nucleic Acids Res 2014;42(16):e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. An J, Lai J, Sajjanhar A, et al. miRPlant: an integrated tool for identification of plant miRNA from RNA sequencing data. BMC Bioinformatics 2014;15:275.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Stegmayer G, Yones C, Kamenetzky L, et al. High class-imbalance in pre-miRNA prediction: a novel approach based on deepSOM. IEEE/ACM Trans Comput Biol Bioinform 2017;14(6):1316–26. [DOI] [PubMed] [Google Scholar]
- 97. Vitsios DM, Kentepozidou E, Quintais L, et al. Mirnovo: genome-free prediction of microRNAs from small RNA sequencing data and single-cells using decision forests. Nucleic Acids Res 2017;45(21):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wang WC, Lin FM, Chang WC, et al. miRExpress: analyzing high-throughput sequencing data for profiling microRNA expression. Bmc Bioinformatics 2009;10:328.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sablok G, Milev I, Minkov G, et al. isomiRex: web-based identification of microRNAs, isomiR variations and differential expression using next-generation sequencing datasets. FEBS Lett 2013;587(16):2629–34. [DOI] [PubMed] [Google Scholar]
- 100. Liu C, Zhang F, Li T, et al. MirSNP, a database of polymorphisms altering miRNA target sites, identifies miRNA-related SNPs in GWAS SNPs and eQTLs. BMC Genomics 2012;13(1):661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Georgakilas G, Vlachos IS, Paraskevopoulou MD, et al. microTSS: accurate microRNA transcription start site identification reveals a significant number of divergent pri-miRNAs. Nat Commun 2014;5(1):5700. [DOI] [PubMed] [Google Scholar]
- 102. Chien CH, Sun YM, Chang WC, et al. Identifying transcriptional start sites of human microRNAs based on high-throughput sequencing data. Nucleic Acids Res 2011;39(21):9345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang J, Lu M, Qiu C, et al. TransmiR: a transcription factor-microRNA regulation database. Nucleic Acids Res 2010;38(Suppl 1):D119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Fahlgren N, Carrington JC.. miRNA target prediction in plants. Methods Mol Biol 2010;592:51–7. [DOI] [PubMed] [Google Scholar]
- 105. Karagkouni D, Paraskevopoulou MD, Chatzopoulos S, et al. DIANA-TarBase v8: a decade-long collection of experimentally supported miRNA-gene interactions. Nucleic Acids Res 2018;46(D1):D239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Loher P, Rigoutsos I.. Interactive exploration of RNA22 microRNA target predictions. Bioinformatics 2012;28(24):3322–3. [DOI] [PubMed] [Google Scholar]
- 107. Huang JC, Babak T, Corson TW, et al. Using expression profiling data to identify human microRNA targets. Nat Methods 2007;4(12):1045–9. [DOI] [PubMed] [Google Scholar]
- 108. Bhattacharya A, Ziebarth JD, Cui Y.. PolymiRTS Database 3.0: linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucleic Acids Res 2014;42(D1):D86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wang X. Improving microRNA target prediction by modeling with unambiguously identified microRNA-target pairs from CLIP-ligation studies. Bioinformatics 2016;32(9):1316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cho S, Jang I, Jun Y, et al. MiRGator v3.0: a microRNA portal for deep sequencing, expression profiling and mRNA targeting. Nucleic Acids Res 2012;41(D1):D252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Heikkinen L, Kolehmainen M, Wong G.. Prediction of microRNA targets in Caenorhabditis elegans using a self-organizing map. Bioinformatics 2011;27(9):1247–54. [DOI] [PubMed] [Google Scholar]
- 112. Dweep H, Gretz N.. miRWalk2.0: a comprehensive atlas of microRNA-target interactions. Nat Methods 2015;12(8):697.. [DOI] [PubMed] [Google Scholar]
- 113. Tokar T, Pastrello C, Rossos AEM, et al. mirDIP 4.1-integrative database of human microRNA target predictions. Nucleic Acids Res 2018;46(D1):D360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Chou CH, Shrestha S, Yang CD, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res 2018;46:D296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dai X, Zhao PX.. psRNATarget: a plant small RNA target analysis server. Nucleic Acids Res 2011;39:W155–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chou CH, Lin FM, Chou MT, et al. A computational approach for identifying microRNA-target interactions using high-throughput CLIP and PAR-CLIP sequencing. BMC Genomics 2013;14 (Suppl 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Shuang C, Maozu G, Chunyu W, et al. MiRTDL: a deep learning approach for miRNA target prediction. IEEE/ACM Trans Comput Biol Bioinform 2016;13(6):1161–9. [DOI] [PubMed] [Google Scholar]
- 118. Oh M, Rhee S, Moon JH, et al. Literature-based condition-specific miRNA-mRNA target prediction. PLoS One 2017;12(3):e0174999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jiang Q, Wang Y, Hao Y, et al. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res 2009;37:D98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liu X, Wang S, Meng F, et al. SM2miR: a database of the experimentally validated small molecules' effects on microRNA expression. Bioinformatics 2013;29(3):409–11. [DOI] [PubMed] [Google Scholar]
- 121. Xie B, Ding Q, Han H, et al. miRCancer: a microRNA-cancer association database constructed by text mining on literature. Bioinformatics 2013;29(5):638–44. [DOI] [PubMed] [Google Scholar]
- 122. Zhou KR, Liu S, Sun WJ, et al. ChIPBase v2.0: decoding transcriptional regulatory networks of non-coding RNAs and protein-coding genes from ChIP-seq data. Nucleic Acids Res 2017;45(D1):D43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Szczesniak MW, Makalowska I.. miRNEST 2.0: a database of plant and animal microRNAs. Nucleic Acids Res 2014;42:D74–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lu TP, Lee CY, Tsai MH, et al. miRSystem: an integrated system for characterizing enriched functions and pathways of microRNA targets. PLoS One 2012;7(8):e42390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. RNAcentral: a comprehensive database of non-coding RNA sequences. Nucleic Acids Res 2017;45(D1):D128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Vlachos IS, Zagganas K, Paraskevopoulou MD, et al. DIANA-miRPath v3.0: deciphering microRNA function with experimental support. Nucleic Acids Res 2015;43(W1):W460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Pantano L, Friedlander MR, Escaramis G, et al. Specific small-RNA signatures in the amygdala at premotor and motor stages of Parkinson's disease revealed by deep sequencing analysis. Bioinformatics 2016;32(5):673–81. [DOI] [PubMed] [Google Scholar]
- 128. Yang Z, Wu L, Wang A, et al. dbDEMC 2.0: updated database of differentially expressed miRNAs in human cancers. Nucleic Acids Res 2017;45(D1):D812–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Preusse M, Theis FJ, Mueller NS.. miTALOS v2: analyzing tissue specific microRNA function. PLoS One 2016;11(3):e0151771.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Alon S, Eisenberg E.. Identifying RNA editing sites in miRNAs by deep sequencing. Methods Mol Biol 2013;1038:159–70. [DOI] [PubMed] [Google Scholar]
- 131. Bortolomeazzi M, Gaffo E, Bortoluzzi S.. A survey of software tools for microRNA discovery and characterization using RNA-seq. Brief Bioinform 2017, doi: 10.1093/bib/bbx148. [DOI] [PubMed] [Google Scholar]
- 132. Bottini S, Pratella D, Grandjean V, et al. Recent computational developments on CLIP-seq data analysis and microRNA targeting implications. Brief Bioinform 2017, doi: 10.1093/bib/bbx063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Abbas Q, Raza SM, Biyabani AA, et al. A review of computational methods for finding non-coding RNA genes. Genes 2016;7(12):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen X, Xie D, Zhao Q, et al. MicroRNAs and complex diseases: from experimental results to computational models. Brief Bioinform 2017, doi: 10.1093/bib/bbx130. [DOI] [PubMed] [Google Scholar]
- 135. Meyer D, Hornik K, Feinerer I.. Text mining infrastructure in R. J Stat Softw 2008;25(5):1–54. [Google Scholar]
- 136. https://cran.r-project.org/web/packages/wordcloud/index.html.
- 137. Liu H, Yue D, Chen Y, et al. Improving performance of mammalian microRNA target prediction. BMC Bioinformatics 2010;11:476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. He C, Li YX, Zhang G, et al. MiRmat: mature microRNA sequence prediction. PLoS One 2012;7(12):e51673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Pan X, Shen HB.. RNA-protein binding motifs mining with a new hybrid deep learning based cross-domain knowledge integration approach. BMC Bioinformatics 2017;18:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lee B, Baek J, Park S, et al. deepTarget: end-to-end learning framework for microRNA target prediction using deep recurrent neural networks In: Proceedings of the 7th ACM International Conference on Bioinformatics, Computational Biology, and Health Informatics. New York, NY: ACM, 2016, p. 434–42. [Google Scholar]
- 141. Akhtar MM, Micolucci L, Islam MS, et al. Bioinformatic tools for microRNA dissection. Nucleic Acids Res 2016;44(1):24–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zhang Y, Huang H, Zhang D, et al. A review on recent computational methods for predicting noncoding RNAs. Biomed Res Int 2017;2017:9139504.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Peterson SM, Thompson JA, Ufkin ML, et al. Common features of microRNA target prediction tools. Front Genet 2014;5:23.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Afonso-Grunz F, Muller S.. Principles of miRNA-mRNA interactions: beyond sequence complementarity. Cell Mol Life Sci 2015;72(16):3127–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Singh NK. miRNAs target databases: developmental methods and target identification techniques with functional annotations. Cell Mol Life Sci 2017;74(12):2239–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Hsu SD, Chu CH, Tsou AP, et al. miRNAMap 2.0: genomic maps of microRNAs in metazoan genomes. Nucleic Acids Res 2008;36:D165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kallio MA, Tuimala JT, Hupponen T, et al. Chipster: user-friendly analysis software for microarray and other high-throughput data. BMC Genomics 2011;12:507.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lukasik A, Wojcikowski M, Zielenkiewicz P.. Tools4miRs—one place to gather all the tools for miRNA analysis. Bioinformatics 2016;32(17):2722–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Haunsberger SJ, Connolly NM, Prehn JH.. miRNAmeConverter: an R/bioconductor package for translating mature miRNA names to different miRBase versions. Bioinformatics 2017;33:592–3. [DOI] [PubMed] [Google Scholar]
- 150. Chung IF, Chang SJ, Chen CY, et al. YM500v3: a database for small RNA sequencing in human cancer research. Nucleic Acids Res 2017;45(D1):D925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Zhang Y, Zang Q, Xu B, et al. IsomiR Bank: a research resource for tracking IsomiRs. Bioinformatics 2016;32(13):2069–71. [DOI] [PubMed] [Google Scholar]
- 152. Fehlmann T, Ludwig N, Backes C, et al. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol 2016;13(11):1084–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sinha D, Sengupta D, Bandyopadhyay S.. ParSel: parallel selection of micro-RNAs for survival classification in cancers. Mol Inform 2017;36(7):1600141.. [DOI] [PubMed] [Google Scholar]
- 154. Nalluri JJ, Barh D, Azevedo V, et al. miRsig: a consensus-based network inference methodology to identify pan-cancer miRNA-miRNA interaction signatures. Sci Rep 2017;7:39684.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Wong NW, Chen Y, Chen S, et al. OncomiR: an online resource for exploring pan-cancer microRNA dysregulation. Bioinformatics 2018;34(4):713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Van Peer G, Lefever S, Anckaert J, et al. miRBase Tracker: keeping track of microRNA annotation changes. Database 2014;2014:bau080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Pan CY, Kuo WT, Chiu CY, et al. Visual display of 5p-arm and 3p-arm miRNA expression with a mobile application. Biomed Res Int 2017;2017:6037168.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Preusse M, Marr C, Saunders S, et al. SimiRa: a tool to identify coregulation between microRNAs and RNA-binding proteins. RNA Biol 2015;12(9):998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fromm B, Billipp T, Peck LE, et al. A uniform system for the annotation of vertebrate microRNA genes and the evolution of the human microRNAome. Annu Rev Genet 2015;49:213–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Alon S, Erew M, Eisenberg E.. DREAM: a webserver for the identification of editing sites in mature miRNAs using deep sequencing data. Bioinformatics 2015;31(15):2568–70. [DOI] [PubMed] [Google Scholar]
- 161. Vitsios DM, Enright AJ.. Chimira: analysis of small RNA sequencing data and microRNA modifications. Bioinformatics 2015;31(20):3365–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Yang K, Sablok G, Qiao G, et al. isomiR2Function: an integrated workflow for identifying MicroRNA variants in plants. Front Plant Sci 2017;8:322.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Amsel D, Vilcinskas A, Billion A.. Evaluation of high-throughput isomiR identification tools: illuminating the early isomiRome of Tribolium castaneum. Bmc Bioinformatics 2017;18(1):359.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zhao S, Gordon W, Du S, et al. QuickMIRSeq: a pipeline for quick and accurate quantification of both known miRNAs and isomiRs by jointly processing multiple samples from microRNA sequencing. Bmc Bioinformatics 2017;18(1):180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Li H, Durbin R.. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Stark A, Brennecke J, Russell RB, et al. Identification of Drosophila MicroRNA targets. PLoS Biol 2003;1(3):E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Xue B, Lipps D, Devineni S.. Integrated strategy improves the prediction accuracy of miRNA in large dataset. Plos One 2016;11(12):e0168392.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Mathelier A, Carbone A.. MIReNA: finding microRNAs with high accuracy and no learning at genome scale and from deep sequencing data. Bioinformatics 2010;26(18):2226–34. [DOI] [PubMed] [Google Scholar]
- 169. Wu Y, Wei B, Liu H, et al. MiRPara: a SVM-based software tool for prediction of most probable microRNA coding regions in genome scale sequences. BMC Bioinformatics 2011;12:107.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Nam JW, Kim J, Kim SK, et al. ProMiR II: a web server for the probabilistic prediction of clustered, nonclustered, conserved and nonconserved microRNAs. Nucleic Acids Res 2006;34:W455–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Csardi G, Nepusz T.. The igraph software package for complex network research. InterJ Complex Syst 2006;1695:1–9. [Google Scholar]
- 172. Sun G, Yan J, Noltner K, et al. SNPs in human miRNA genes affect biogenesis and function. RNA 2009;15(9):1640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Boutet SC, Cheung TH, Quach NL, et al. Alternative polyadenylation mediates microRNA regulation of muscle stem cell function. Cell Stem Cell 2012;10(3):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Blazie SM, Geissel HC, Wilky H, et al. Alternative polyadenylation directs tissue-specific miRNA targeting in Caenorhabditis elegans somatic tissues. Genetics 2017;206(2):757–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Zeng X, Zhang X, Zou Q.. Integrative approaches for predicting microRNA function and prioritizing disease-related microRNA using biological interaction networks. Brief Bioinform 2016;17(2):193–203. [DOI] [PubMed] [Google Scholar]
- 176. Baldassarre A, Felli C, Prantera G, et al. Circulating microRNAs and bioinformatics tools to discover novel diagnostic biomarkers of pediatric diseases. Genes 2017;8(9):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Bortoluzzi S, Biasiolo M, Bisognin A.. MicroRNA-offset RNAs (moRNAs): by-product spectators or functional players? Trends Mol Med 2011;17(9):473–4. [DOI] [PubMed] [Google Scholar]
- 178. Winter J, Link S, Witzigmann D, et al. Loop-miRs: active microRNAs generated from single-stranded loop regions. Nucleic Acids Res 2013;41(10):5503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Backes C, Fehlmann T, Kern F, et al. miRCarta: a central repository for collecting miRNA candidates. Nucleic Acids Res 2018;46:D160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.