Abstract
Background
Curcumin derivative C086 (cur C086) is a potential chemotherapeutic agent for patients with osteosarcoma. In this study, the effects of cur C086 combined with cisplatin on the biological processes of osteosarcoma cells were investigated.
Material/Methods
In this study, expression of BMIL1 was detected by real-time quantitative reverse transcription polymerase chain reaction and Western blotting in MG-63 cells treated with cur C086+cisplatin. Functions of cur C086+cisplatin on proliferation ability, apoptosis response, and metastatic potential of MG-63 cells were determined by MTT, flow cytometry, Hoechst 33258 staining and Transwell assays, respectively. In additionally, expression of P16, E-cadherin, epidermal growth factor (EGFR), and Notch1 was measured by Western blotting.
Results
Expression of BMIL1 decreased significantly in MG-63 cells treated with cur C086 (20 μM)+cisplatin (1.28 nM). Treatment with cur C086+cisplatin considerably inhibited growth, migration, and invasion potential in MG-63 cells, whereas apoptosis was obviously upregulated. Moreover, cur C086+cisplatin suppressed BMIL1 expression or its potential downstream targets, P16, E-cadherin, EGFR, and Notch1.
Conclusions
The current results demonstrate that combined treatment with cur C086+cisplatin may be an effective form of chemotherapy for patients with osteosarcoma.
MeSH Keywords: Cisplatin, Curcumin, Osteosarcoma
Background
Osteosarcoma is a malignant bone tumor more common in children or teenagers than in adults [1]. It develops from mesenchymal cells, and is also the most common primary bone tumor, characterized by rapid cell proliferation, strong metastatic potential, and resistance to chemotherapy [2]. At present, surgical excision, radiotherapy or chemotherapy are the main therapeutic strategies for osteosarcoma [3]. Recently, treatment of osteosarcoma has been improved by performing radical surgery. In addition, neoadjuvant chemotherapy increases 5-year overall survival from 30% to about 70% to 80% [4–6]. Although the survival rate has risen over the past decades, it is unsatisfactory and the prognosis for osteosarcoma patients with metastatic lesions is still poo. Despite the fact that chemotherapies and targeted drugs are useful against osteosarcoma, they can cause serious side effects [7,8]. Therefore, the molecular mechanism of osteosarcoma needs to be further studied to find better drugs with fewer adverse effects.
An increasing amount of evidence indicates that natural plant ingredients from traditional Chinese herbal medicines, such as ginsenoside, paclitaxel, and curcumin, have therapeutic advantages and effects on various tumors [9]. Curcumin reportedly is a powerful anticancer agent and can be isolated from ginger, which is encountered on a daily basis [10,11]. Multiple studies have revealed that curcumin can inhibit proliferation of the cells of various cancers, such as those of the breast, lung, and stomach, as well as osteosarcoma in vitro and in vivo [12–14]. Currently, numerous studies have reported the potential mechanisms of curcumin against osteosarcoma [15]. Previous reports have shown that curcumin repressed proliferation of osteosarcoma cells and inhibited metastasis of tumor cells through critical signal pathways, for example Janus kinase 2/signal transducer and activator of transcription 3 and mitogen-activated protein kinase in vivo [16,17].
The multifactorial pathogenesis of osteosarcoma involves genetic and epigenetic alterations of tumor suppressor genes, oncogenes or growth factors in osteosarcoma development [18]. BMIL1 is a member of the polycomb group family of transcriptional regulators [19]. Studies have indicated that it acts as an oncogene in development and progression of cancer [20]. Accumulated evidence suggested that the degree of expression of BMIL1 is related to the occurrence, development, invasion, and prognosis of tumors, as well as other pathological indicators in osteosarcoma [21,22]. BMIL1 is highly expressed in osteosarcoma cells, and its overexpression promotes cell growth and chemotherapeutic resistance to cisplatin in osteosarcoma [23]. Cisplatin is a commonly used chemotherapeutic drug in patients with osteosarcoma, but it often causes drug resistance, so the effects of chemotherapy are not ideal [24,25]. However, the exact role played by BMIL1 in osteosarcoma treated with curcumin and cisplatin is not fully understood.
C086 [4-(4-hydroxy-3-methoxy-phenyl-methyl)] is a new structural analog of curcumin. Being artificially synthesized, it has better properties than natural curcumin, such as solubility and antitumor effects. [26]. In this study, we intended to investigate the effect of the curcumin derivative C086 combined with cisplatin on proliferation and invasion of the MG-63 cells, as well as on expression of BMIL1 to elucidate molecular mechanisms of cur C086 and cisplatin in osteosarcoma.
Material and Methods
Reagents
Cur C086, cisplatin, and 5-fluorouracil (5-FU) were purchased from Mclean Corp (Shanghai, China). For in vitro studies, the concentration of cur C086 dissolved in dimethyl sulfoxide (DMSO) was 100 μM and the concentration of cisplatin suspended in PBS was 1.28 nM for next experiments. While the concentration of 5-FU suspended in phosphate-buffered saline (PBS) was 2 μg/mL.
Cell culture
MG-63 cells from ATCC (Manassas, Virginia, United States) were cultured in Dulbecco’s Modified Eagle Medium (Gibco; Thermo Fisher Scientific, Inc., United States) containing 10% fetal bovine serum, 100 μg/mL streptomycin and 100 U/mL penicillin. These cells were cultured in six-well plates under humidified conditions with 5% CO2 at 37°C. MG-63 cells were grown as a monolayer and cultured until 80% confluence was reached for use in further experiments.
QRT-PCR
Total RNA was isolated with an RNA separation Kit (cat. no: Z3382, Promega, United States) and quantified with a spectrophotometer. Total RNA (400 ng) was used to synthesize cDNA using a PrimeScript reverse transcription polymerase chain reaction (RT-PCR) kit, according to reagent instructions (cat. no: RR055B, Takara, Japan). A SYBR Green I fluorescent dye (cat. no: RR420L, Takara, Japan) was used and quantitative PCR was performed on the ABI Prism PCR system. The experimental steps were as follows: 5 min at 95°C, followed by 36 cycles at 95°C for 35 s and 55°C for 45 s. All primers were from Invitrogen Company (Thermo Fisher Scientific, Inc., United States). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as control. The primers sequences used were as follows:
-
BMIL1 forward primers 5′-TCATCCTTCTGCTGATGCTG-3′ and
reverse primers 5′-GCATCACAGTCATTGCTGCT-3′;
-
GAPDH, forward primers 5′-GGTGAAGGTCGGAGTCAACG-3′ and
reverse primers 5′-CAAAGTTGTCATGGATGAC C-3′.
Fold change in BMIL1 mRNA expression was determined using the 2−ΔΔCt method. All samples were analyzed in triplicate.
Cell proliferation assay
Growth of MG-63 cells incubated with different concentrations of cur C086 for various durations was analyzed with MTT assays (cat. no: 88417, Sigma-Aldrich, United States). In brief, MG-63 cells were cultured in 96-well plates (Corning, NY, USA) at a density of 5×103 cells/well. The cells were treated with cur C086 (0, 10, 20, 40 and 80 μM) for 48 h, or cur C086 (20 μM) for 0, 12, 24, 48, and 72 h. MTT dye was added to each well for at least 4 h. The reaction was stopped by adding DMSO and absorbance at 570 nm was measured at each time point using an enzyme immunoassay analyzer. All samples were analyzed in triplicate.
Sensitivity analysis of cur C086
To assess the effect of cur C086 on the sensitivity of MG-63 cells to chemotherapeutic drugs, a single-drug intervention and an in vitro cell intervention experiment were designed. The MG-63 cells were treated with cisplatin (1.28 nM), 5-FU (2 μg/mL), cur C086 (20 μM), cisplatin (1.28 nM)+5-FU (2 μg/mL) or cur C086 (20 μM)+5-FU (2 μg/mL) for 48 h. The results were assessed through an MTT assay. All samples were evaluated in triplicate.
Synergy of cur C086 and cisplatin detected by MTT
The collaborative experiment was divided into 7 groups: control, cisplatin (1.28 nM), 5-FU (2 μg/mL), cur C086 (20 μM), cisplatin (1.28 nM)+5-FU (2 μg/mL), cur C086 (20 μM)+5-FU (2 μg/mL), and cur C086 (20 μM)+cisplatin (1.28 nM) group. The cell culture system was the same as the previous cell proliferation experiment. The calculation formula for the coefficient of drug interaction (CDI) was: CDI=AB/(A×B). In vitro experiments were calculated based on the number of viable cells (absorbance value). When CDI <1, the two drugs were considered to have a synergistic effect; when CDI <0.7, the synergistic effect was deemed significant.
Apoptosis assays
Cisplatin (1.28 nM), cur C086 (20 μM), and cur C086 (20 μM)+cisplatin (1.28 nM) were used to treat MG-63 cells for 48 h. The MG-63 (1×105) cells were collected and washed with PBS twice. Subsequently, the cells were stained with Hoechst 33258 reagents (cat. no: C1017, Beyotime Biotechnology, Shanghai, China) and observed under two-photon fluorescence microscopy (LaVision BioTec, Germany). At the same time, the treated cells (1.5×105) were collected and washed with PBS twice. Annexin-V/PI (5 μL) (cat. no: 556547, BD Biosciences, United States) was added into the treated cells resuspended in PBS for 15 min in the dark. Apoptosis was detected through flow cytometry (BD Biosciences, United States). All samples were analyzed in triplicate.
Migration and invasion assay
Nigration and invasion abilities assays were used with a 12-well Transwell chamber (8-mm pore size; Corning Incorporated) coated with Matrigel (200 mL per well, thickness: 3 mm; BD Biosciences) in triplicate. MG-63 cells were incubated with cisplatin (1.28 nM), cur C086 (4 μM), and cur C086 (20 μM)+cisplatin (1.28 nM) for 48 h. The treated cells (1×105) were resuspended in a medium without serum, added into the upper chamber and cultured for 48 h, then in a medium with serum added to the lower chamber. Following incubation, non-migrating or non-invading cells in the upper chamber were erased with a cotton swab. Cells attached to the underside of the filter membrane were stained with crystal violet and 5 random fields were taken from the microscope (Olympus, Tokyo, Japan). Then the number of migration and invasion cells dyed purple in each field under microscopy was counted. All samples were investigated in triplicate.
Western blot analysis
Protein lysates of MG-63 cells treated with cisplatin (1.28 nM), cur C086 (20 μM) and cur C086 (20 μM)+cisplatin (1.28 nM) were lysed with a protein lysate buffer (Sigma-Aldrich; Merck KGaA) and the total protein concentration was quantified with a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Ten micrograms of each sample protein was resuspended in a loading buffer and electrophoresed on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Fractionated proteins were transferred onto polyvinylidene difluoride membranes (Invitrogen; Thermo Fisher Scientific, Inc.). The following primary antibodies were used: Anti-P16 (cat. no: sc-81866, Santa Cruz Biotechnology, United States), anti-E-cadherin (cat. no: sc-8426, Santa Cruz Biotechnology, United States), anti-BMIL1 (cat. no: sc-390443, Santa Cruz Biotechnology, United States), anti-Notch1 (cat. no: sc-376403, Santa Cruz Biotechnology, United States), anti-epidermal growth factor-2 (EGFR2) (cat. no: sc-373746, Santa Cruz Biotechnology, United States) and anti-β-tubulin (cat. no: 2148S, Cell Signaling Technology, United States). Membranes were incubated with the goat anti-Rabbit immunoglobulin G (H+L) secondary antibody, HRP (cat. no: 31460, Thermo Fisher Scientific, United States) at room temperature for 1 h. β-tubulin was adopted as an internal control. Protein bands were detected by the Odyssey Scanning System (LI-COR Biosciences, United States). ImageJ software (National Institutes of Health, United States) was used for densitometric analysis. All samples were analyzed in triplicate.
Statistical analysis
All experimental data analyses were performed in triplicate and all results were presented as the mean±SEM. Student’s t-test (two-tailed), one-way analysis of variance (twotailed) and Tukey’s post-hoc tests were used in the statistical analysis (SPSS 16; SPSS, Inc., Chicago, Illinois, United States). Differences in this study were considered statistically significant at P<0.05, P<0.01 and P <0.001.
Results
Cur C086 downregulates BMIL1 expression
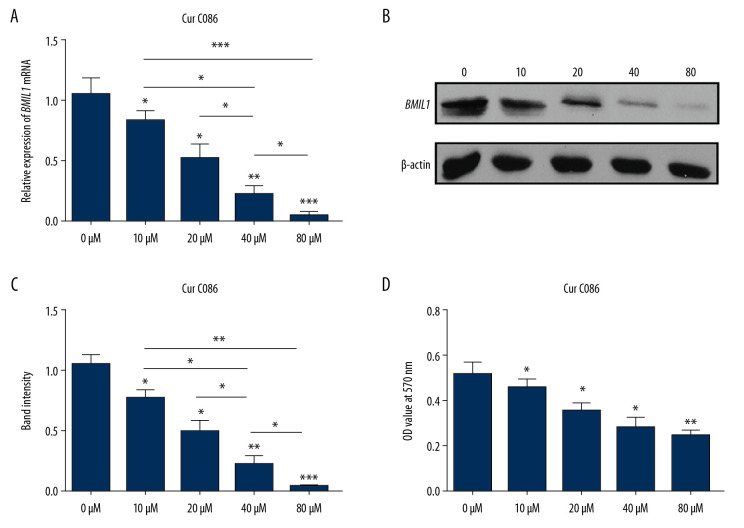
First, we analyzed expression of BMIL1 from mRNA and protein levels in MG-63 cells exposed to cur C086 at different concentrations for 48 h (Figure 1A–1C). The inhibitory effects of cur C086 were also measured via an MTT assay (Figure 1D). The results demonstrated that BMIL1 expression was significantly downregulated by cur C086, and that cur C086 inhibited proliferation of MG-63 cells in a dose-dependent manner. The IC50 values of cur C086 and cisplatin weree 20 μM and 1.28 nM, respectively.
Figure 1.
Expression of BMIL1 was inhibited by curcumin C086 in MG-63 cells. Levels of expression of BMIL1 mRNA (A) and protein (B, C) were downregulated by treatment with different concentrations of curcumin C086. (D) Curcumin C086 suppressed proliferation of MG-63 cells, as analyzed in MTT experiments. All data are presented as the mean±SEM. * P<0.05, ** P<0.01 and *** P<0.001.
Confirmation of most suitable drug concentration of cur C086
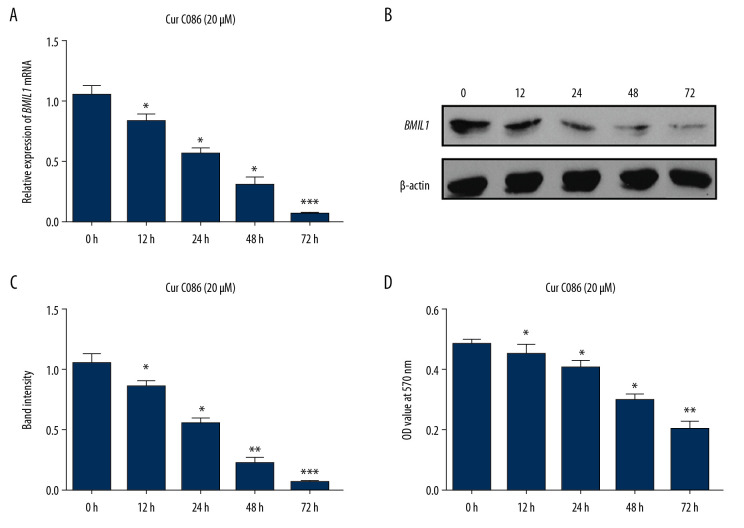
Expression of BMIL1 in MG-63 cells exposed to 20 μM cur C086 for different durations (Figure 2A, 2B) and effects of cur C086 on proliferation of MG-63 cells (Figure 2C) were measured. The results revealed that BMIL1 expression and MG-63 cell proliferation were affected in a time-dependent manner.
Figure 2.
Expression of BMIL1 was downregulated in MG-63 cells incubated with curcumin C086 (20 μM) for 0, 12, 24, 48, and 72 h. Expression of BMIL1 mRNA (A) and protein (B) were repressed by curcumin C086. (C, D) Proliferation of MG-63 cells was inhibited by curcumin C086 (20 μM), as analyzed by MTT assay. Data are expressed as the mean±SEM. * P<0.05, ** P<0.01 and *** P<0.001.
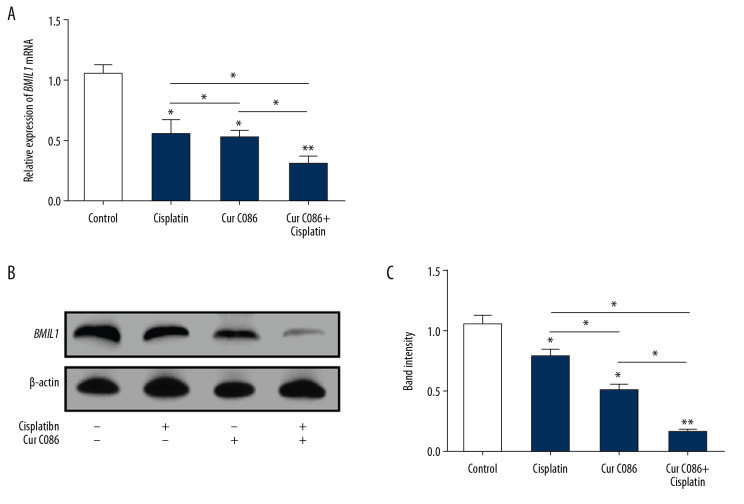
Effects of cur C086+cisplatin on BMIL1 expression
To determine whether BMIL1 expression was significantly affected by cur C086+cisplatin in MG-63 cells, quantitative RT-PCR and Western blotting were performed (Figure 3). The results proved that BMIL1 expression was obviously decreased following treatment with cur C086+cisplatin compared with the control, cur C086, and cisplatin groups.
Figure 3.
Expression of BMIL1 was analyzed in MG-63 cells treated with curcumin C086 and cisplatin. Expression of BMIL1 mRNA (A) and protein (B, C) was inhibited by curcumin C086 (20 μM)+cisplatin (1.28 nM). Data are expressed as the mean±SEM. * P<0.05 and ** P<0.01.
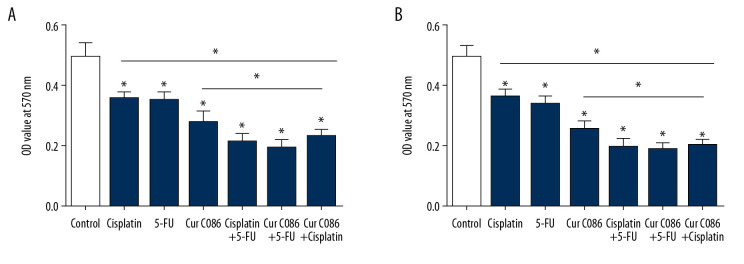
Cur C086 increased chemosensitivity of MG-63 cells
The sensitivity of MG-63 cells to cur C086 combined with chemotherapeutic drugs was studied in vitro (Figure 4). The results indicated that cell absorbance was significantly lower in the combined treatment groups after 48 h compared with the single-intervention groups. These results suggest that cur C086 intervention may enhance the chemosensitivity of MG-63 cells to cisplatin or 5-FU.
Figure 4.
Curcumin C086 had synergic anticancer effects when combined with cisplatin and 5-FU. Proliferation of MG-63 cells was inhibited following treatment with curcumin C086 (20 μM) and cisplatin or 5-FU for (A) 24 or (B) 48 h. Data are expressed as the mean±SEM. * P<0.05.
Synergistic effect of cur C086 and cisplatin on MG-63 cells
MG-63 cells were treated with cur C086 (20 μM) and cisplatin (1.28 nM) for 24 and 48 hours. MTT results showed that cur C086 and cisplatin increased the killing effect on MG-63 cells. Compared to the control group, the inhibition rates in the cur C086+cisplatin group were 42.29±3.5% and 46.32±4.1%, respectively. The CDI values of two different time groups (24 and 48 h) were 0.68 and 0.64, indicating that cur C086 and cisplatin were remarkably synergistic in inhibiting MG-63 cells.
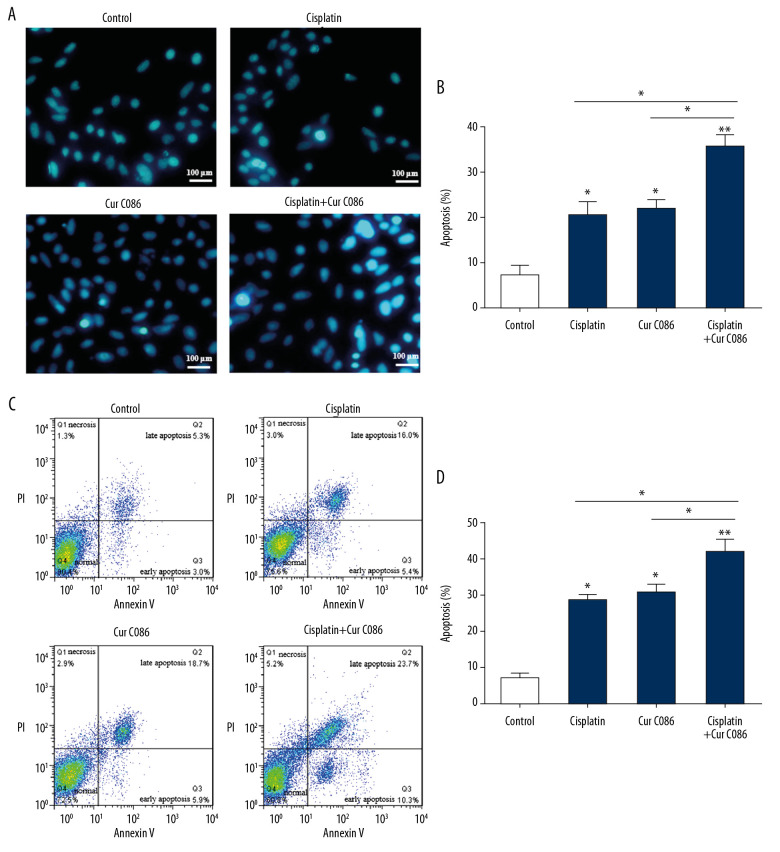
Cur C086 induced apoptosis in MG-63 cells
To determine the effect of cur C086 on MG-63 cell apoptosis, cells were treated with cisplatin (1.28 nM), cur C086 (20 μM), and cur C086 (20 μM)+cisplatin (1.28 nM) for 48 h and analyzed using flow cytometry (Figure 5A, 5B) and Hoechst 33258 reagents (Figure 5C, 5D). Annexin V-FITC/PI double staining was used to detect cells in different phases of apoptosis. The results indicated that apoptosis of MG-63 cells increased considerably after cur C086+cisplatin treatment compared with the other groups.
Figure 5.
Treatment of MG-63 cells with curcumin C086+cisplatin significantly promoted apoptosis. Curcumin C086 (20 μM)+cisplatin increased apoptosis of MG-63 cells analyzed by flow cytometry (A, B) and Hoechst 33258 reagents (C, D). All images were acquired under an inverted microscope (200× magnification). Data are expressed as the mean±SEM. * P<0.05 and ** P<0.01.
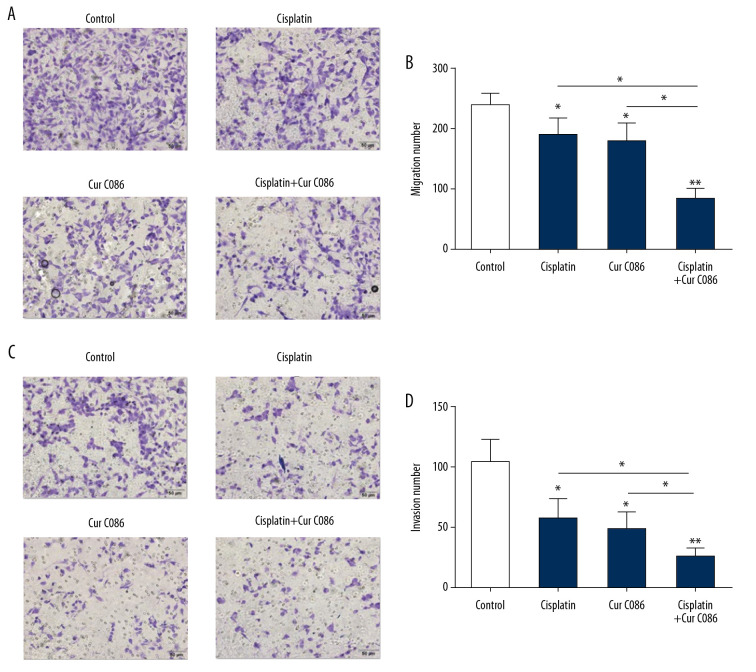
Cur C086 suppressed migration and invasion of MG-63 cells
Cisplatin (1.28 nM), cur C086 (20 μM), and cur C086 (20 μM)+cisplatin were used to treat MG-63 cells for 48 h, and Transwell assays were conducted. The assays revealed that the migration abilities of MG-63 cells decreased significantly in the cur C086+cisplatin group compared to in the control cell group (Figure 6A, 6B). Similarly, the invasive abilities of MG-63 cells treated with cur C086+cisplatin obviously declined after 48 h of incubation (Figure 6C, 6D).
Figure 6.
Migration and invasion abilities of MG-63 cells were inhibited by treatment with curcumin C086+cisplatin. (A, B) The effects of curcumin C086 (20 μM)+cisplatin on MG-63 cell migration potential were measured by Transwell assay. (C, D) The invasiveness of MG-63 cells incubated with curcumin C086 (20 μM)+cisplatin was evaluated through Transwell assay. Images were obtained under an inverted microscope with 200× magnification. Data are expressed as the mean±SEM. * P<0.05 and ** P<0.01.
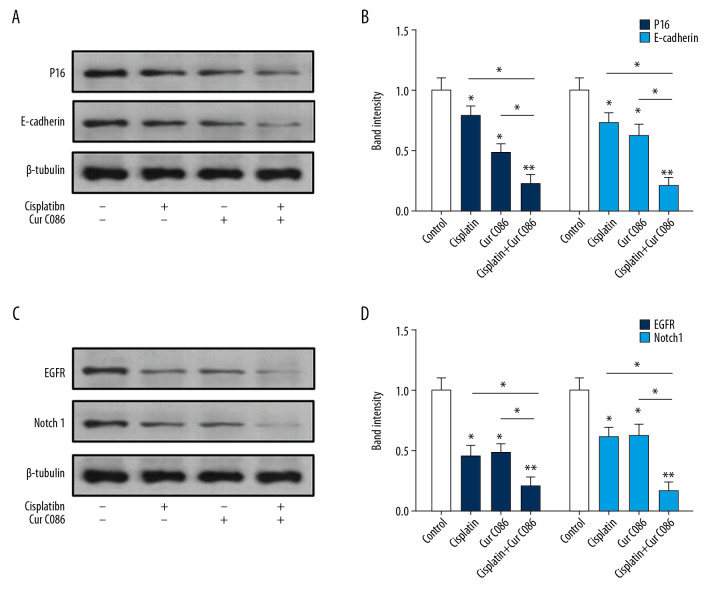
Cur C086 inhibited expression of P16, E-cadherin, EGFR, and Notch1 in MG-63 cells
Expression of P16, E-cadherin, EGFR, and Notch1 in osteosarcoma cells treated with C086 and cisplatin was investigated. Experimental results suggested that P16 and E-cadherin proteins were markedly downregulated in the cur C086+cisplatin treatment group in comparison with the single-treatment groups (Figure 7A, 7B). Furthermore, EGFR and Notch1 inhibition was observed in MG-63 cells treated with cur C086+cisplatin (Figure 7C, 7D).
Figure 7.
Levels of expression of P16, E-cadherin, Notch1 and EGFR in MG-63 cells were analyzed by Western blotting. (A, B) P16 and E-cadherin proteins were decreased in MG-63 cells treated with cisplatin, curcumin C086, and curcumin C086+cisplatin. (C, D) Meanwhile, Notch1 and EGFR proteins were downregulated in MG-63 cells treated with cisplatin, curcumin C086, and curcumin C086+cisplatin. Data are expressed as the mean±SEM. * P<0.05 and ** P<0.01.
Discussion
Curcumin and curcumin derivatives exert significant inhibitory effects on the growth and metastasis of osteosarcoma cell lines, and they induce apoptosis in these cancerous cells [27]. Cisplatin is a platinum complex with broad-spectrum antitumor activity. At high doses, it inhibits synthesis of DNA, and at lower doses, it inhibits synthesis of RNA and protein in treatment of osteosarcoma [28,29]. In this study, the influence of curcumin C086 combined with cisplatin on osteosarcoma was evaluated.
BMIL1 is a significant oncogene that regulates development and metastasis of cancers. Neoplastic cells exhibit abnormally high expression of BMIL1, which has been related to malignant biological characteristics of tumor tissues and poor patient prognosis [30,31]. BMIL1 has been considered as a molecular marker in various cancers and is an important therapeutic target that has the potential for clinical transformation [32,33]. In this study, BMIL1 expression in MG-63 cells was clearly inhibited by treatment with cur C086 or cisplatin. Expression of BMIL1 was downregulated by cur C086 doses as low as 20 μM over different periods of time (12, 24, 48, and 72 h). In addition, proliferation of MG-63 cells was hindered by cur C086 (20 μM), and combined treatment with cur C086+cisplatin significantly downregulated BMIL1 compared with other groups.
Epithelial to mesenchymal transition (EMT) may increase the motility of cells and enable them to gain invasive properties, a process that is mediated by repression of E-cadherin [34,35]. EMT is a critical step in invasion and metastasis of cancers, and could be used as an indicator of cancer progression or prognosis, or a treatment target [36]. Invasion and metastasis of tumor cells are the main causes of death in patients with cancers including osteosarcoma. Interestingly, we discovered that cur C086 and cisplatin inhibited the migration and invasion potential of MG-63 cells and increased apoptosis of them.
A recent report showed that BMIL1 silencing resulted in inhibition of the invasion and migration potential of lung cancer cells [37]. P16 and E-cadherin, being downstream proteins regulated by BMIL1, are undoubtedly key mediators in epithelial–mesenchymal transition and tumor invasion [38]. In this study, expression of P16 and E-cadherin was downregulated in MG-63 cells treated with cur C086 or cisplatin, and the migration and invasion abilities of the treated MG-63 cells were decreased. These events may indicate that cur C086 could suppress migration and invasion of osteosarcoma cells by hindering EMT.
EGFR expression has been detected in osteosarcoma, and because its activity is associated with tumor proliferation, invasion, and metastasis, EGFR been a significant target in cancer therapy [39,40]. EGFR is overexpressed in osteosarcoma and the response to EGFR-targeted agents is inversely correlated with EMT [41]. Notch signaling plays a role in development and progression of osteosarcoma [42]. Wang et al. [43] found that curcumin inhibits hypoxia-induced proliferation and invasion of MG-63 cells through downregulation of Notch1. We found that expression of EGFR and Notch1 was downregulated in MG-63 cells treated with cur C086 or cisplatin, and especially with combined treatment with cur C086 and cisplatin.
Conclusions
Our findings demonstrate that combined treatment with cur C086 and cisplatin inhibits expression of BMIL1, downregulating signaling pathways associated with p16, E-cadherin, EGFR, and Notch-1, thereby inducing apoptosis in osteosarcoma cells and suppressing tumor cell proliferation. Combined treatment with cur C086, therefore, have the potential to cure osteosarcoma. However, further studies on the molecular mechanism of C086 and cisplatin, as well as clinical investigations, are required to verify our results.
Footnotes
Source of support: Departmental sources
References
- 1.Zhou W, Hao M, Du X, et al. Advances in targeted therapy for osteosarcoma. Discov Med. 2014;17:301–7. [PubMed] [Google Scholar]
- 2.Liu YJ, Li W, Chang F, et al. MicroRNA-505 is down-regulated in human osteosarcoma and regulates cell proliferation, migration and invasion. Oncol Rep. 2018;39:491–500. doi: 10.3892/or.2017.6142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Cortini M, Avnet S, Baldini N. Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett. 2017;405:90–99. doi: 10.1016/j.canlet.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 4.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: A report from the Children’s Cancer Group. J Clin Oncol. 1997;15:76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Vassiliou LV, Lalabekyan B, Jay A, et al. Head and neck sarcomas: A single institute series. Oral Oncol. 2017;65:16–22. doi: 10.1016/j.oraloncology.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Bishop MW, Janeway KA, Gorlick R. Future directions in the treatment of osteosarcoma. Curr Opin Pediatr. 2016;28:26–33. doi: 10.1097/MOP.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otoukesh B, Boddouhi B, Moghtadaei M, et al. Novel molecular insights and new therapeutic strategies in osteosarcoma. Cancer Cell Int. 2018;18:158. doi: 10.1186/s12935-018-0654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, Jiang H, Cao Y, et al. Drug target identification using network analysis: Taking active components in Sini decoction as an example. Sci Rep. 2016;6:24245. doi: 10.1038/srep24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Golonko A, Lewandowska H, Świsłocka R, et al. Curcumin as tyrosine kinase inhibitor in cancer treatment. Eur J Med Chem. 2019;181:111512. doi: 10.1016/j.ejmech.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Zhao S, Pi C, Ye Y, et al. Recent advances of analogues of curcumin for treatment of cancer. Eur J Med Chem. 2019;180:524–35. doi: 10.1016/j.ejmech.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y, Zhang J, Han J, et al. Curcumin inhibits tumor proliferation induced by neutrophil elastase through the upregulation of α1-antitrypsin in lung cancer. Mol Oncol. 2012;6:405–17. doi: 10.1016/j.molonc.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv ZD, Liu XP, Zhao WJ, et al. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int J Clin Exp Pathol. 2014;7:2818–24. [PMC free article] [PubMed] [Google Scholar]
- 14.Wilken R, Veena MS, Wang MB, et al. A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angulo P, Kaushik G, Subramaniam D, et al. Natural compounds targeting major cell signaling pathways: A novel paradigm for osteosarcoma therapy. J Hematol Oncol. 2017;10:10. doi: 10.1186/s13045-016-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Liu L, Wang Y, et al. Curcumin inhibits the proliferation and invasion of MG-63 cells through inactivation of the p-JAK2/p-STAT3 pathway. Onco Targets Ther. 2019;12:2011–21. doi: 10.2147/OTT.S172909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelleher FC, O’Sullivan H. Monocytes, macrophages, and osteoclasts in osteosarcoma. J Adolesc Young Adult Oncol. 2017;6:396–405. doi: 10.1089/jayao.2016.0078. [DOI] [PubMed] [Google Scholar]
- 18.Dang H, Wu W, Wang B, et al. CXCL5 plays a promoting role in osteosarcoma cell migration and invasion in autocrine- and paracrine-dependent manners. Oncol Res. 2017;25:177–86. doi: 10.3727/096504016X14732772150343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Bombard J, Cintron K, et al. BMI1 as a novel target for drug discovery in cancer. J Cell Biochem. 2011;112:2729–41. doi: 10.1002/jcb.23234. [DOI] [PubMed] [Google Scholar]
- 20.Kang MK, Kim RH, Kim SJ, et al. Elevated BMI-1 expression is associated with dysplastic cell transformation during oral carcinogenesis and is required for cancer cell replication and survival. Br J Cancer. 2007;96:126–23. doi: 10.1038/sj.bjc.6603529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Luo B, Zhao M. BMI-1 targeting suppresses osteosarcoma aggressiveness through the NF-κB signaling pathway. Mol Med Rep. 2017;16:7949–58. doi: 10.3892/mmr.2017.7660. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Xie X, Ye Z, Yang D, et al. Effects of combined c-myc and Bmi-1 siRNAs on the growth and chemosensitivity of MG-63 osteosarcoma cells. Mol Med Rep. 2013;8:168–72. doi: 10.3892/mmr.2013.1484. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Min L, Chen D, et al. Overexpression of BMI-1 promotes cell growth and resistance to cisplatin treatment in osteosarcoma. PLoS One. 2011;6:e14648. doi: 10.1371/journal.pone.0014648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng C, Ding Q, Zhang Z, et al. PTBP1 modulates osteosarcoma chemoresistance to cisplatin by regulating the expression of the copper transporter SLC31A1. J Cell Mol Med. 2020;24(9):5274–89. doi: 10.1111/jcmm.15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fanelli M, Tavanti E, Patrizio MP, et al. Cisplatin resistance in osteosarcoma: In vitro validation of candidate DNA repair-related therapeutic targets and drugs for tailored treatments. Front Oncol. 2020;10:331. doi: 10.3389/fonc.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Liu Y, Chen Y. C086, a novel analog of curcumin, induces growth inhibition and down-regulation of NF-κB in colon cancer cells and xenograft tumors. Cancer Biol Ther. 2011;12:797–807. doi: 10.4161/cbt.12.9.17671. [DOI] [PubMed] [Google Scholar]
- 27.Maran A, Yaszemski MJ, Kohut A. Curcumin and osteosarcoma: Can invertible polymeric micelles help? Materials (Basel) 2016;9:E520. doi: 10.3390/ma9070520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang B, Zhang Y, Li R, et al. Oncolytic adenovirus Ad11 enhances the chemotherapy effect of cisplatin on osteosarcoma cells by inhibiting autophagy. Am J Transl Res. 2020;12:105–17. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Hou Z, Zhou Y, Li J, et al. Selective in vivo and in vitro activities of 3,3′-4-nitrobenzylidene-bis-4-hydroxycoumarin against methicillin-resistant Staphylococcus aureus by inhibition of DNA polymerase III. Sci Rep. 2015;5:13637. doi: 10.1038/srep13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benard A, Goossens-Beumer IJ, van Hoesel AQ, et al. Prognostic value of polycomb proteins EZH2, BMI1 and SUZ12 and histone modification H3K27me3 in colorectal cancer. PLoS One. 2014;9:e108265. doi: 10.1371/journal.pone.0108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Wang Y, Yuan C, et al. Oncogenic roles of BMI1 and its therapeutic inhibition by histone deacetylase inhibitor in tongue cancer. Lab Invest. 2014;94:1431–45. doi: 10.1038/labinvest.2014.123. [DOI] [PubMed] [Google Scholar]
- 32.Mayr C, Wagner A, Loeffelberger M, et al. The BMI1 inhibitor PTC-209 is a potential compound to halt cellular growth in biliary tract cancer cells. Oncotarget. 2016;7:745–58. doi: 10.18632/oncotarget.6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreso A, van Galen P, Pedley NM, et al. Self-renewal as a therapeutic target in human colorectal cancer. Nat Med. 2014;20:29–36. doi: 10.1038/nm.3418. [DOI] [PubMed] [Google Scholar]
- 34.Kong D, Li Y, Wang Z, et al. Cancer stem cells and epithelial-to-mesenchymal transition (EMT)-phenotypic cells: Are they cousins or twins? Cancers (Basel) 2011;3:716–29. doi: 10.3390/cancers30100716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brabletz T, Jung A, Reu S, et al. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci USA. 2001;98:10356–61. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang Z, Wu R, Xie W, et al. Curcumin reverses tobacco smoke-induced epithelial-mesenchymal transition by suppressing the MAPK pathway in the lungs of mice. Mol Med Rep. 2018;17:2019–25. doi: 10.3892/mmr.2017.8028. [DOI] [PubMed] [Google Scholar]
- 37.Meng X, Wang Y, Zheng X, et al. shRNA-mediated knockdown of Bmi-1 inhibit lung adenocarcinoma cell migration and metastasis. Lung Cancer. 2012;77:24–30. doi: 10.1016/j.lungcan.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Yang MH, Hsu DS, Wang HW, et al. BMI1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–92. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 39.Fard SS, Saliminejad K, Sotoudeh M, et al. The correlation between EGFR and androgen receptor pathways: A novel potential prognostic marker in gastric cancer. Anticancer Agents Med Chem. 2019;19:2097–107. doi: 10.2174/1871520619666190930142820. [DOI] [PubMed] [Google Scholar]
- 40.Benli Yavuz B, Koç M, Kozacıoğlu S, et al. Prognostic importance of PTEN, EGFR, HER-2, and IGF-1R in patients treated with postoperative chemoradiation. Turk J Med Sci. 2019;49:1025–32. doi: 10.3906/sag-1802-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, Wang D, Zhang L, et al. The TGFβ-miR-499a-SHKBP1 pathway induces resistance to EGFR inhibitors in osteosarcoma cancer stem cell-like cells. J Exp Clin Cancer Res. 2019;38:226. doi: 10.1186/s13046-019-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu L, Fan Z, Fang S, et al. Cisplatin selects for stem-like cells in osteosarcoma by activating notch signaling. Oncotarget. 2016;7:33055–68. doi: 10.18632/oncotarget.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Zhang K, Zhu Y, et al. Curcumin inhibits hypoxia-induced proliferation and invasion of MG-63 osteosarcoma cells via downregulating Notch1. Mol Med Rep. 2017;15:1747–52. doi: 10.3892/mmr.2017.6159. [DOI] [PubMed] [Google Scholar]