Abstract
Background
Long non-coding RNAs (lncRNAs) play key roles in the development and progression of diseases, including sepsis. Therefore, this study aimed to clarify the role and underlying molecular mechanisms of lncRNA NEAT1 in sepsis.
Material/Methods
We used real-time quantitative polymerase chain reaction (RT-qPCR) to analyze the expression of lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1), let-7b-5p, and tumor necrosis factor receptor-associated factor 6 (TRAF6). Western blot assay was used to measure the protein expression levels. After treatment with lipopolysaccharide (LPS), the biological behaviors of human renal tubular epithelial cells (HK-2), such as proliferation and apoptosis, were determined using 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazol-3-ium bromide (MTT) and flow cytometry assays, respectively. The interaction relationship among NEAT1, TRAF6, and let-7b-5p was analyzed by the bioinformatics starBase database and dual-luciferase reporter assay.
Results
lncRNA NEAT1 was expressed at higher levels in kidney tissues from sepsis patients than in healthy kidney tissues. Interestingly, LPS induced high expression of lncRNA NEAT1 in HK-2 cells in a time- and dose-dependent manner. Furthermore, silencing of NEAT1 weakened LPS-induced apoptosis, inflammation, and inhibition of proliferation, which was overturned by silencing of let-7b-5p. In addition, overexpression of TRAF6 abolished the overexpression of let-7b-5p-induced effects on apoptosis, inflammation, and growth of HK-2 cells exposed to LPS. In summary, NEAT1 regulated TRAF6 expression by sponging let-7b-5p in HK-2 cells, which promotes LPS-induced injury and inflammation in HK-2 cells.
Conclusions
Our data show that the lower expression of NEAT1 impeded sepsis development and LPS-induced injury inflammation by targeting let-7b-5p/TRAF6 axis, and NEAT1 may be a target for treatment of sepsis patients.
MeSH Keywords: RNA, Long Noncoding; Sepsis; TNF Receptor-Associated Factor 6
Background
Sepsis is a systemic inflammatory response syndrome induced by the dysfunctional response to infection, resulting in injury, inflammation, and organ dysfunction [1]. Kidney injury and dysfunction are common complications in sepsis patients, leading to high mortality rates [2]. In addition, podocytes are an important part of the glomerular filtration barrier. Lipopolysaccharide (LPS) was reported to induce podocyte injury and then promoted the release of inflammatory factors [3].
Previous studies found that long non-coding RNAs (lncRNAs) were involved in sepsis. Exploring the regulatory mechanism of these lncRNAs in sepsis may help to understand the pathogenesis and development of sepsis [4,5]. For instance, lncRNA metastasis-associated lung adenocarcinoma transcript 1 plays a key role in LPS-induced kidney injury by regulating the nuclear factor kappa-B (NFκB) pathway [6]. Importantly, NEAT1 is upregulated in sepsis-induced myocardial injury tissues of mice [7]. However, the underlying mechanism of NEAT1 in sepsis has not been defined.
There have been extensive studies of let-7b-5p in the context of cancer, suggesting that let-7b-5p is a tumor-suppressor miRNA and potential diagnostic biomarker [8,9]. Moreover, Yu et al. revealed that let-7b-5p was upregulated in neonatal leukocytes with LPS stimulation derived from cord blood of infants [10]. Furthermore, the cellular expression level of let-7b-5p is a specific diagnostic target in sepsis [11]. These reports show that let-7b-5p may be involved in inflammation of kidney diseases.
Importantly, Giriwono et al. recently found that geranylgeraniol prevented excessive activation of NFκB in numerous diseases by inhibiting tumor necrosis factor receptor-associated factor 6 (TRAF6) expression [12]. Consistently, Liu et al. reported that TRAF6 is involved in inflammatory arthritis by mediating NFκB activation [13]. Moreover, Fang et al. and Yang et al. reported that an in vitro sepsis model was successfully constructed in HK-2 cells exposed to LPS [14,15]. Hence, in the present study, an in vitro sepsis cell model was established by treatment with LPS. We first measured the expression level of NEAT1 in sepsis-kidney tissues and the cell model in vitro. Functionally, NEAT1 knockdown weakened LPS-induced apoptosis, inflammation, and inhibition of proliferation by regulating let-7b-5p. Our bioinformatics analysis discovered that TRAF6 is a potential target of let-7b-5p. This study investigated whether lncRNA NEAT1 regulates sepsis-induced inflammation and apoptosis via the let-7b-5p/TRAF6 axis.
Material and Methods
Patient specimens
This study was conducted with approval by the Human Ethics Committee of Jingzhou Central Hospital, the Second Clinical Medical College of Yangtze University. Kidney tissues were collected from 30 sepsis patients and 30 healthy volunteers at Jingzhou Central Hospital, the Second Clinical Medical College of Yangtze University. The inclusion criteria were: all patients with clear evidence of infection or highly suspected infection accompanied by at least 2 signs of SIRS and complete and reliable clinical data. The exclusion criteria were: drugs previously taken had renal toxicity; previous history of glomerulonephritis, nephrotic syndrome, end-stage nephropathy, renal cancer, or renal transplantation; and a lactating or pregnant woman. None of the patients or volunteers had received any preoperative treatments. Moreover, written informed consent was signed from each participant. All kidney tissues were promptly snap-frozen and then transferred to a −80°C freezer for long-term preservation. In addition, venous blood was collected and stored at −80°C after centrifugation to assess renal function indicators – blood urea nitrogen (BUN) and serum creatinine – with a Urea Nitrogen Colorimetric Detection Kit (Invitrogen, Carlsbad, CA, USA) and Creatinine Urinary Detection Kit (Invitrogen).
Cell lines and cell culture
Human renal tubular epithelial cells (HK-2) were obtained from the American Type Culture Collection (Rockville, MD, USA). Dulbecco’s modified Eagle’s medium (Wisent, Shanghai, China) containing 10% (v/v) fetal bovine serum (FBS; Hyclone, South Logan, UT, USA) and 1% penicillin/streptomycin (GIBCO BRL, Grand Island, NY, USA) was used to culture HK-2 cells in a humidified atmosphere containing 5% CO2 at 37°C. To establish an LPS (L4516-1MG; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) stimulation for cell culture, HK-2 cells were incubated with increasing concentrations (0 mg, 0.1 mg, 1.0 mg, 10 mg, and 20 mg) of LPS for 24 h or treated with 1.0 mg LPS for 0 h, 6 h, 12 h, 18 h, and 24 h).
Real-time quantitative polymerase chain reaction (RT-qPCR)
The extraction of total RNA from tissues and cells was implemented using Trizol reagent (Takara, Dalian, China) in accordance with the operation manual. Reverse transcription of lncRNA/mRNA and miRNA were conducted using a SuperScript Reverse Transcriptase Kit (Vazyme, Nanjing, China) and Poly (A) Tailing Kit (Thermo Fisher Scientific, Waltham, MA, USA), respectively. Quantification of RNA was performed using SYBR Fast qPCR Mix (Thermo Fisher Scientific) in the Thermal Cycler CFX6 System (Bio-Rad, Hercules, CA, USA). The internal expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and endogenous small nuclear RNA U6 was used for the normalization of detected RNA using the 2−ΔΔCt method, and the PCR program was: at 95°C for 10 min, followed by 95°C for 15 s and 60°C for 1 min for 40 cycles.
The sequences of primers were:
-
NEAT1 (F, 5′-CTTCCTCCCTTTAACTTATCCATTCAC-3′;
R, 5′-CTCTTCCTCCACCATTACCAACAATAC-3′);
-
let-7b-5p (F, 5′-GCCGAGTGAGGTAGTAGGTT-3′;
R, 5′-CTCAACTGGTGTCGTGGA-3′);
-
TRAF6 (F, 5′-ATGCGGCCATAGGTTCTGC-3′;
R, 5′-TCCTCAAGATGTCTCAGTTCCAT-3′);
-
GAPDH (F, 5′-TCCCATCACCATCTTCCAGG-3′;
R, 5′-GATGACCCTTTTGGCTCCC-3′);
-
U6 (F, 5′-AACGCTTCACGAATTTGCGT-3′;
R, 5′-CTCGCTTCGGCAGCACA-3′).
Western blot assay
Briefly, the cells and tissues were lysed in ice-cold radio-immunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific) supplemented with 1% proteinase/phosphatase inhibitors, and then incubated on ice for 30 min. After that, the lysate was centrifuged for 10 min (12 000×g, 4°C), and then supernatant was collected for denaturation at 98°C for 5 min. Next, total protein was fractionated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis and then electrophoretically transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). After being blocked with 5% skim milk solution, the membranes were incubated with appropriate primary antibodies overnight at 4°C. After being washed, membranes were reacted with Goat polyclonal Secondary Antibody to Rabbit IgG-H&L (ab150077; 1: 2000 dilution; Abcam, Cambridge, MA, USA) for 2 h. Finally, the Western blot protein bands were visualized with the ECL Western Blotting Detection Kit (Solarbio, Beijing, China). The antibodies used were: GAPDH (ab181602; 1: 2000 dilution; Abcam), tumor necrosis factor-α (TNF-α; ab6671; 1: 1000 dilution; Abcam), interleukin 6 (IL-6; ab6672; 1: 1000 dilution; Abcam), interleukin 1β (IL-1β; ab2105; 1: 1000 dilution; Abcam, Cambridge, MA, USA), and TRAF6 (ab33915; 1: 1000 dilution; Abcam).
Transfection assay
Specific small interfering RNA (siRNA) against NEAT1 (si-NEAT1) and siRNA scrambled control (si-NC), let-7b-5p mimic (let-7b-5p) and its negative control (let-NC), and let-7b-5p inhibitor (anti-let-7b-5p) and its negative control (anti-let-NC) were generated by RiboBio (Guangzhou, China). TRAF6-overexpression vector (TRAF6), NEAT1-overexpression vector (NEAT1), and their negative control (pcDNA) were purchased from Genomeditech (Shanghai, China). All transient transfections were conducted with lipofectamine 3000 reagent (Invitrogen) in compliance with the producer’s directions. At 48 h after transfection, cells were collected for the next analysis.
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2H-tetrazol-3-ium bromide (MTT) assay
HK-2 cells were seeded into each well of a 96-well plate. After incubation for indicated times, HK-2 cells were treated with 20 μL of MTT (Sigma-Aldrich, St. Louis, MO, USA) and further cultured for 4 h. After discarding the supernatant, 150 μL of dimethyl sulfoxide (DMSO) was used to dissolve formazan crystals and the oscillation reaction lasted for 10 min. The cell proliferation curves of HK-2 cells were plotted using absorbance at wavelength of 490 nm in each well on a microplate reader (Bio-Rad, Hercules, CA, USA).
Cell apoptosis assay
The Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection Kit (TransGen Biotech, Beijing, China) was applied to assess cell apoptosis. Briefly, cells were harvested and rinsed with cold phosphate-buffered saline to remove the residual medium. The single-cell suspension (1×106/mL) was incubated with 5 μL of Annexin V-labeled FITC and 5 μL of propidium iodide (PI) in the dark. Finally, the apoptotic rate was measured using an Attune NxT flow cytometer (Thermo Fisher Scientific). Cell Quest software was used for data analysis. Apoptosis rate=(Annexin V+PI+ cell numbers+Annexin V+PI− cell numbers)/106×100%).
Dual-luciferase reporter assay
We predicted the let-7b-5p binding sites withNEAT1 or 3′untranslated region (UTR) of TRAF6 using the bioinformatics database starBase (http://starbase.sysu.edu.cn/). The wild-type and mutant fragments in NEAT1 or 3′ UTR of TRAF6 associated with the let-7b-5p binding site were designed and inserted into pGL3 vectors (Realgene, Nanjing, China) to generate matched luciferase reporters. Subsequently, 10 ng of the indicated luciferase reporter and 96 nM of let-7b-5p mimic or let-NC were co-transfected into HK-2 cells. Luciferase activity was measured at 24 h after transfection using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) following the producer’s instructions. Firefly luciferase activity was normalized to Renilla luciferase activity.
Statistical analysis
All data are described as mean±standard deviation from at least 3 independent experiments, and statistical analyses were conducted using GraphPad Prism 7 (GraphPad, La Jolla, CA, USA). Statistical analysis of differences between groups was performed using the t test or one-way analysis of variance, and statistical differences were regarded as significant at P<0.05. The relationship among NEAT1, let-7b-5p, and TRAF6 was analyzed using Pearson’s correlation analysis.
Results
NEAT1 was upregulated in kidney tissues of patients with sepsis
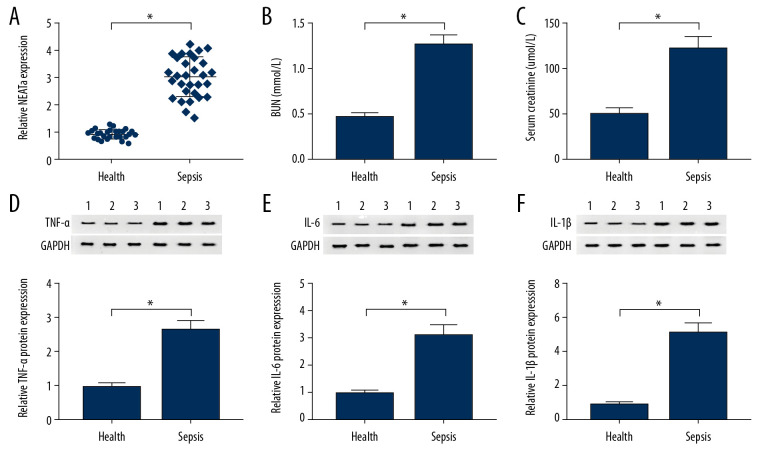
By using RT-qPCR analysis, we found that kidney tissues of patients with sepsis had higher NEAT1 levels than in healthy kidney tissues (Figure 1A). Then, we assessed levels of BUN and serum creatinine in sepsis patients, finding that they were both higher in serum from sepsis patients when compared with the control group (Figure 1B, 1C). Furthermore, the results of Western blot analysis indicated that TNF-α, IL-6, and IL-1β levels were higher in kidney tissues of patients with sepsis than in healthy kidney tissues (Figure 1D–1F). Therefore, our data suggest that NEAT1 plays important roles in sepsis.
Figure 1.
The expression level of NEAT1 in sepsis patients and healthy volunteers. (A) The relative expression level of NEAT1 in kidney tissues from sepsis patients and healthy kidney tissues was assessed with RT-qPCR assay. (B, C) BUN and serum creatinine were analyzed in kidney tissues from sepsis patients. (D–F) Western blot analysis was used to detect TNF-α, IL-6, and IL-1β levels in sepsis patients and healthy kidney tissues. * P<0.05.
Knockdown of NEAT1 inhibited LPS-induced effects on proliferation, apoptosis, and inflammation of HK-2 cells
The expression level of NEAT1 was dramatically upregulated in HK-2 cells exposed to LPS in time- and dose-dependent manners (Figure 2A, 2B). HK-2 cells were incubated with 1 mg/L of LPS for 24 h for subsequence experiments. The results of RT-qPCR assay showed a notable increase of NEAT1 in HK-2 cells exposed to LPS, which was reversed by silencing of NEAT1 (Figure 2C). Importantly, silencing of NEAT1 mitigated the inhibition effect on cell proliferation caused by LPS (Figure 2D). LPS induced cell apoptosis of HK-2 cells, whereas this reaction was abated by knockdown of NEAT1 (Figure 2E). We also observed that knockdown of NEAT1 could alleviate LPS-induced inflammation of HK-2 cells by decreasing TNF-α, IL-6, and IL-1β expression (Figure 2F). These results revealed that downregulation of NEAT1 weakens LPS-induced apoptosis and inflammation, as well as inhibition of proliferation in HK-2 cells.
Figure 2.
Effects of NEAT1 silencing on proliferation, apoptosis, and inflammation of LPS-treated HK-2 cells. (A) NEAT1 level was assessed in HK-2 cells treated with different concentrations LPS (0, 0.1, 1.0, 10, and 20 mg/L) for 24 h by RT-qPCR assay. (B) NEAT1 level was detected in HK-2 cells stimulated with 1.0 mg/L LPS at various time points (0, 6, 12, 18, and 24 h) by RT-qPCR assay. (C–F) HK-2 cells were separated into 4 groups: control, LPS, LPS+si-NC, and LPS+si-NEAT1 groups. (C) The relative expression level of NEAT1 was quantified with RT-qPCR analysis in HK-2 cells. (D) The growth curves of HK-2 cells were drawn using MTT assay. (E) Flow cytometry was used to measure the apoptosis rate of HK-2 cells. (F) The protein expression levels of TNF-α, IL-6, and IL-1β were assessed with Western blot analysis. * P<0.05.
Let-7b-5p was a target of NEAT1 and let-7b-5p was decreased in HK-2 cells exposed to LPS
The binding sites of let-7b-5p with WT-NEAT1 or MUT-NEAT1 are indicated in Figure 3A. Next, the analysis results of dual-luciferase reporter assay indicated that let-7b-5p decreased the luciferase activity of WT-NEAT1 in HK-2 cells, but had no effects on the luciferase activity of MUT-NEAT1 in comparison with the let-NC group (Figure 3B). Additionally, let-7b-5p was lower in kidney tissues in sepsis patients compared to healthy kidney tissues (Figure 3C). Interestingly, let-7b-5p was inhibited by LPS in time- and dose-dependent manners (Figure 3D, 3E). Knockdown of NEAT1 significantly increased let-7b-5p expression in HK-2 cells treated with LPS, while overexpression of NEAT1 aggravated LPS-induced let-7b-5p underexpression (Figure 3F). Collectively, our findings show that let-7b-5p is downregulated in HK-2 cells exposed to LPS and is a target of NEAT1.
Figure 3.
Let-7b-5p was a direct target of NEAT1. (A) The complementary sequence between let-7b-5p and NEAT1 along with mutant sites were shown. (B) The luciferase activity was analyzed by dual-luciferase reporter assay. (C) RT-qPCR assay was used to test the expression level of let-7b-5p in kidney tissues in sepsis patients and healthy kidney tissues. (D, E) Let-7b-5p expression was analyzed by RT-qPCR assay in HK-2 cells. (F) RT-qPCR analysis was used to analyze let-7b-5p expression in HK-2 cells treated with LPS+si-NC, LPS+si-NEAT1, LPS+pcDNA, or LPS+NEAT1. * P<0.05.
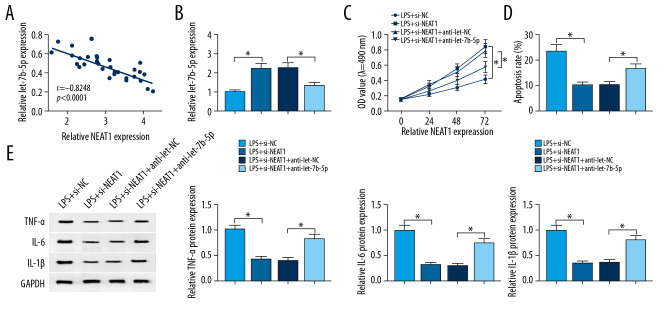
Silencing of NEAT1 attenuated LPS-induced apoptosis, inflammation, and inhibition of proliferation by regulating let-7b-5p
As presented in Figure 4A, the expression of NEAT1 was negatively correlated with let-7b-5p in kidney tissues of sepsis patients. The upregulation of let-7b-5p could be seen in HK-2 cells treated with LPS and si-NEAT1, but the promotion effect was reversed by anti-let-7b-5p (Figure 4B). When compared with HK-2 cells treated with LPS, proliferation was enhanced, but apoptosis was repressed in HK-2 cells treated with LPS and si-NEAT1, which was reversed by silencing of let-7b-5p (Figure 4C, 4D). si-NEAT1 obviously weakened inflammation in HK-2 cells exposed to LPS by decreasing TNF-α, IL4, and IL-1β expression, which was reversed by knockdown of let-7b-5p (Figure 4E). All data suggested that NEAT1 regulates LPS-induced apoptosis, inflammation, and suppression of proliferation by targeting let-7b-5p expression.
Figure 4.
Knockdown of NEAT1-mediated effects on proliferation, apoptosis, and inflammation in LPS-induced HK-2 cells was abolished by silencing of let-7b-5p. (A) Relationship between the expression levels of NEAT1 and let-7b-5p was analyzed by Pearson’s correlation analysis. (B–E) HK-2 cells were exposed to LPS+si-NC, LPS+si-NEAT1, LPS+si-NEAT1+anti-let-NC, or LPS+si-NEAT1+anti-let-7b-5p. (B) RT-qPCR was enforced to measure expression level of let-7b-5p in HK-2 cells. (C) MTT assay was performed for examining the cell viability of HK-2 cells. (D) The apoptosis rate of HK-2 cells was assessed by performing flow cytometry assay. (E) The protein expressions of TNF-α, IL-6, and IL-1β in HK-2 cells were examined by Western blot assay. * P<0.05.
TRAF6 is a target gene of let-7b-5p and is associated with sepsis
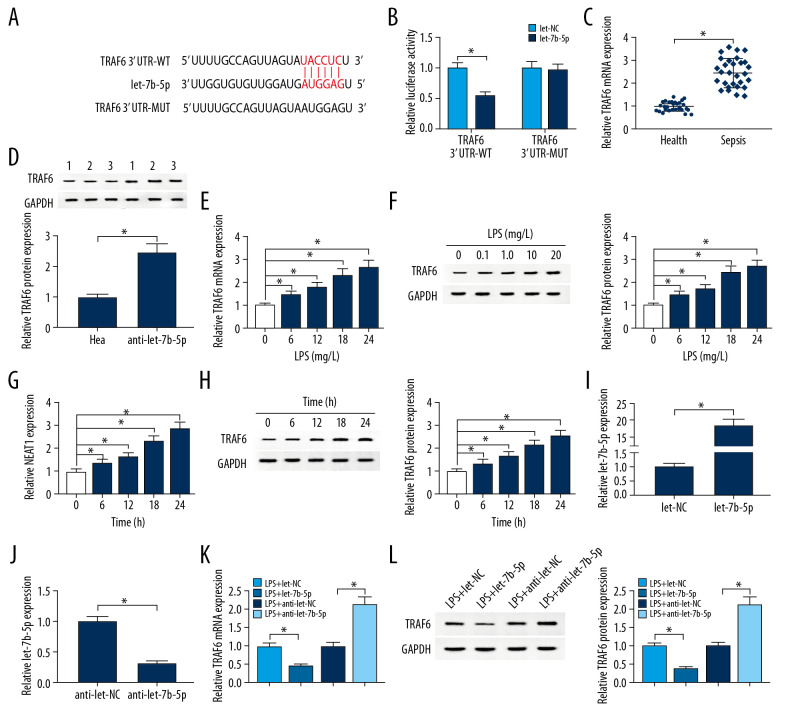
Bioinformatics analysis in starBase database showed that let-7b-5p has a complementary sequence to 3′UTR of TRAF6 (Figure 5A). Dual-luciferase reporter assay revealed that let-7b-5p remarkably decreased the luciferase activity in HK-2 cells transfected with the reporter vector TRAF6 3′UTR-WT, but not in HK-2 cells transfected with TRAF6 3′UTR-WT, suggesting let-7b-5p targets 3′UTR of TRAF6 m RNA (Figure 5B). Moreover, TRAF6 levels in kidney tissues of sepsis patients were notably higher than in healthy kidney tissues (Figure 5C, 5D). More importantly, LPS induced overexpression of TRAF6 in time- and dose-dependent manners, including mRNA and protein (Figure 5E–5H). We also observed that the expression of let-7b-5p was increased in HK-2 cells transfected with let-7b-5p compared with the let-NC group, while let-7b-5p was decreased in HK-2 cells transfected with anti-let-7b-5p (Figure 5I, 5J). After treatment with LPS (1 mg/L, 24 h), TRAF6 was downregulated in HK-2 cells transfected with let-7b-5p compared to those transfected with let-NC, and anti-let-7b-5p had the opposite results (Figure 5K, 5L). Synthetically, let-7b-5p negatively regulated the expression of TRAF6, which plays important roles in sepsis.
Figure 5.
Let-7b-5p negatively regulated TRAF6 expression. (A) Binding region between let-7b-5p and TRAF6 as well as mutated nucleotides ofTRAF6 3′UTR were shown. (B) Dual-luciferase reporter assay was used to show the luciferase activity of TRAF6 3′UTR-WT and TRAF6 3′UTR-MUT in HK-2 cells. (C, D) The expression levels of TRAF6 in kidney tissues from sepsis patients and healthy kidney tissues were evaluated by RT-qPCR and Western blot assay, respectively. (E–H) LPS elevated TRAF6 expression in HK-2 cells. (I, J) Transfection efficiency of let-7b-5p and anti-let-7b-5p was checked by RT-qPCR assay, with let-NC and anti-let-NC as controls. (K, L) The expression level of TRAF6 was analyzed with RT-qPCR and Western blot assay in HK-2 cells treated with LPS+let-NC, LPS+let-7b-5p, LPS+anti-let-NC, or LPS+anti-let-7b-5p. * P<0.05.
Let-7b-5p regulated proliferation, apoptosis, and inflammation of HK-2 cells treated with LPS by affecting TRAF6 expression
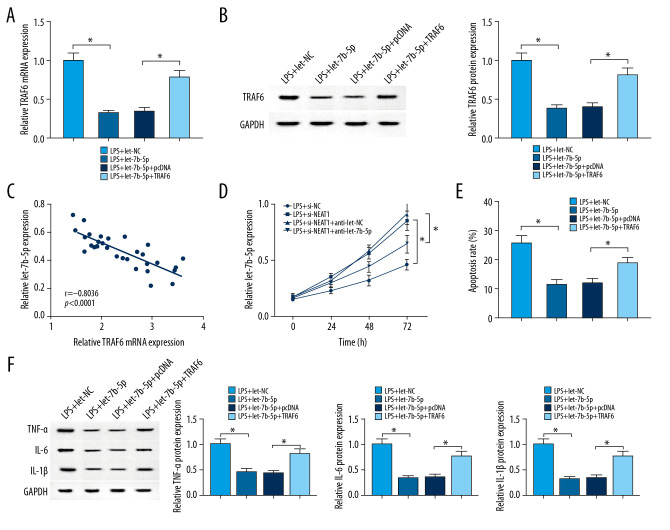
After LPS treatment, TRAF6 was significantly downregulated in HK-2 cells transfected with let-7b-5p compared with the let-NC group, which was rescued by transfection with TRAF6 (Figure 6A, 6B). As indicated in Figure 6C, results of Pearson correlation analysis showed that let-7b-5p was negatively correlated with TRAF6. Furthermore, overexpression of let-7b-5p enhanced proliferation of HK-2 cells treated with LPS, which was weakened by overexpression of TRAF6 (Figure 6D). We performed flow cytometry analysis in HK-2 cells, showing that upregulation of let-7b-5p partly decreased the cell apoptosis, which was promoted by LPS, while the reintroduction of TRAF6 counteracted this effect (Figure 6E). Consistently, transfection of let-7b-5p into HK-2 cells canceled the LPS-induced inflammation by decreasing TNF-α, IL-6, and IL-1β expression, which was attenuated by overexpression of TRAF6 (Figure 6F). In summary, the let-7b-5p/TRAF6 axis regulates proliferation, apoptosis, and inflammation in HK-2 cells exposed to LPS.
Figure 6.
Overexpression of TRAF6 reversed let-7b-5p-induced the effect on proliferation, apoptosis, and inflammation in LPS-induced HK-2 cells. (A–F) HK-2 cells were treated with LPS+let-NC, LPS+let-7b-5p, LPS+let-7b-5p+pcDNA, or LPS+let-7b-5p+TRAF6. (A, B) The level of TRAF6 in HK-2 cells was measured by using RT-qPCR and Western blot assays. (C) Pearson’s correlation analysis revealed a negative correlation between the levels of TRAF6 mRNA and let-7b-5p. (D) Cell proliferation capability was examined in HK-2 with MTT analysis. (E) The apoptosis of HK-2 cells was performed using flow cytometry assay. (F) The expression levels of TNF-α, IL-6, and IL-1β were shown in HK-2 cells with Western blot analysis. * P<0.05.
NEAT1 regulated TRAF6 expression by sponging let-7b-5p
As shown in Figure 7A, NEAT1 was positively correlated with let-7b-5p expression. Results of RT-qPCR and Western blot assays showed that TRAF6 level was significantly lower in HK-2 cells treated with LPS+si-NEAT1 than in the LPS+si-NC group, but it was rescued by knockdown of let-7b-5p (Figure 7B, 7C). These data suggest that NEAT1 binds to let-7b-5p, thereby promoting TRAF6 expression.
Figure 7.
NEAT1 regulated let-7b-5p/TRAF6 axis inHK-2 cells. (A) The relationship between NEAT1 and TRAF6 was shown by Pearson’s correlation analysis. (B, C) The expression level ofTRAF6 was estimated with RT-qPCR and Western blot assays in HK-2 cells treated with LPS+si-NC, LPS+si-NEAT1, LPS+si-NEAT1+anti-let-NC, or LPS+si-NEAT1+anti-let-7b-5p. * P<0.05.
Discussion
Currently, the unknown function of most lncRNAs in organ injury caused sepsis is a major challenge for the application of lncRNAs in the diagnosis and treatment of sepsis. The important finding of the present study is that lncRNA NEAT1 was increased in kidney tissue of sepsis patients, and could serve as an underlying therapeutic target in sepsis-triggered kidney injury by boosting apoptosis and inflammation by regulating the let-7b-5p/TRAF6 axis.
LPS stimulation leads to a severe inflammatory response, thus producing a large number of inflammatory factors such as TNF-α, and further inducing apoptosis of cells and renal injury [16]. Acute kidney injury can be diagnosed with BUN and serum creatinine [17]. Consistently, BUN and serum creatinine were higher in serum from sepsis patients than in healthy volunteers. We also found that lncRNA NEAT1 expression in surgically obtained septic kidney tissues was higher than that in surgically obtained healthy kidney tissues. Interestingly, our study also found that protein levels of TNF-α, IL-1β, and IL-6 were increased in LPS-induced HK-2 cells, while NEAT1 knockdown obviously decreased their levels.
It was reported that lncRNAs are important regulators in the development of many biological events [18]. In addition, lncRNAs are involved in the occurrence and development of many diseases, including kidney diseases [19,20]. Zhang et al. [21] examined the expression level of NEAT1 in liver tissue of with sepsis-induced liver injury and verified that NEAT1 was overexpressed, and silencing of NEAT1 mitigated LPS-induced high expression of TNF-α, IL-6, and IL-1β in vitro, which was similar to our results. Analogously, lncRNA NEAT1 was strongly associated with the LPS-induced mesangial cell injury by regulating inflammatory response by targeting miR-204 [22].
Moreover, lncRNA NEAT1 interacted with let-7b-5p and negatively regulated let-7b-5p expression. It has been reported that several miRNAs play important roles in sepsis-induced kidney injury and are potential biomarkers for sepsis [23,24]. In addition, cell apoptosis is regarded as a key part of septic acute kidney injury [25]. Recently, let-7b-5p expression was found to be significantly downregulated in the LPS-induced sepsis model, while let-7b-5p was highly expressed under LPS stimulation in neonatal leukocytes [10]. Furthermore, overexpression of let-7b-5p relieved LPS-derived injury and inflammation in HK-2 cells. Let-7b-5p overexpression and NEAT1 silencing had similar results on sepsis, and further study revealed that downregulation of NEAT1 weakened LPS-induced apoptosis, inflammation, and suppression of proliferation by regulating let-7b-5p.
In this study, let-7b-5p and TRAF6 were inversely expressed in the sepsis cell model. TRAF6 has crucial regulatory functions in inflammatory response and is a vital adapter molecule in the NFκB pathway [26]. NFκB is a common translation factor involved in expression of many genes, such as TNF-α, IL-1β, and IL-6 [27]. Wang et al. reported that miR-125b impeded NFκB activation by targeting TRAF6 in myocardium injury [28]. In the present study, the suppression effects on inflammatory response and TNF-α, IL-1β and IL-6 expression in HK-2 cells exposed to LPS induced by let-7b-5p overexpression were abolished by upregulation of TRAF6. We also proved that TRAF6 was connected with the regulation of the HK-2 cell apoptosis caused by treatment with LPS. Given the interaction between TRAF6 and let-7b-5p, we confirmed in functional experiments that let-7b-5p can restrain LPS-induced HK-2 cell apoptosis and repression of proliferation via TRAF6.
NEAT1 was upregulated in kidney tissue of sepsis patients and in LPS-induced HK-2 cells. Functionally, NEAT1 triggered HK-2 cell apoptosis and aggravated inflammatory response via targeting the let-7b-5p/TRAF6 signaling pathway, which provides new understanding and diagnostic markers for sepsis-induced kidney injury.
Conclusions
In summary, NEAT1 was overexpressed in sepsis-induced kidney injury tissues and LPS-induced HK-2 cells. Mechanistically, NEAT1 increased apoptosis and inflammatory but impeded proliferation by regulating the let-7b-5p/TRAF6 pathway in HK-2 cells, which may be a new therapeutic target for sepsis-induced kidney injury.
Abbreviations
- NEAT1
nuclear paraspeckle assembly transcript 1
- TRAF6
tumor necrosis factor receptor-associated factor 6
- LPS
lipopolysaccharide
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Bettaieb A, Koike S, Chahed S, et al. Podocyte-specific soluble epoxide hydrolase deficiency in mice attenuates acute kidney injury. FEBS J. 2017;284:1970–86. doi: 10.1111/febs.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gómez H, Kellum JA, Ronco C. Metabolic reprogramming and tolerance during sepsis-induced AKI. Nat Rev Nephrol. 2017;13:143–51. doi: 10.1038/nrneph.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheng X, Zuo X, Liu X, et al. Crosstalk between TLR4 and Notch1 signaling in the IgA nephropathy during inflammatory response. Int Urol Nephrol. 2018;50:779–85. doi: 10.1007/s11255-017-1760-2. [DOI] [PubMed] [Google Scholar]
- 4.Huang S, Qian K, Zhu Y, et al. Diagnostic value of the lncRNA NEAT1 in peripheral blood mononuclear cells of patients with sepsis. Dis Markers. 2017;2017 doi: 10.1155/2017/7962836. 7962836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang W, Lan X, Li X, et al. Long non-coding RNA PVT1 promote LPS-induced septic acute kidney injury by regulating TNFα and JNK/NF-κB pathways in HK-2 cells. Int Immunopharmacol. 2017;47:134–40. doi: 10.1016/j.intimp.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y, Guo F, Zhu T, et al. Mechanism of long non-coding RNA MALAT1 in lipopolysaccharide-induced acute kidney injury is mediated by the miR-146a/NF-κB signaling pathway. Int J Mol Med. 2018;41:446–54. doi: 10.3892/ijmm.2017.3232. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Liu G, Xian H, et al. LncRNA NEAT1 alleviates sepsis-induced myocardial injury by regulating the TLR2/NF-κB signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:4898–907. doi: 10.26355/eurrev_201906_18078. [DOI] [PubMed] [Google Scholar]
- 8.Zheng S, Liu Q, Ma R, et al. Let-7b-5p inhibits proliferation and motility in squamous cell carcinoma cells through negative modulation of KIAA1377. Cell Biol Int. 2019;43:63–41. doi: 10.1002/cbin.11136. [DOI] [PubMed] [Google Scholar]
- 9.Xi X, Chu Y, Liu N, et al. Joint bioinformatics analysis of underlying potential functions of hsa-let-7b-5p and core genes in human glioma. J Transl Med. 2019;17:129. doi: 10.1186/s12967-019-1882-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H-R, Hsu T-Y, Huang H-C, et al. Comparison of the functional microRNA expression in immune cell subsets of neonates and adults. Front Immunol. 2016;7:615. doi: 10.3389/fimmu.2016.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reithmair M, Buschmann D, Märte M, et al. Cellular and extracellular mi RNA s are blood-compartment-specific diagnostic targets in sepsis. J Cell Mol Med. 2017;21:2403–11. doi: 10.1111/jcmm.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giriwono PE, Shirakawa H, Ohsaki Y, et al. Geranylgeraniol suppresses the expression of IRAK1 and TRAF6 to inhibit NFκB activation in lipopolysaccharide-induced inflammatory responses in human macrophage-like cells. Int J Mol Sci. 2019;20:2320. doi: 10.3390/ijms20092320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K, Chen K, Zhang Q, et al. TRAF6 neddylation drives inflammatory arthritis by increasing NF-κB activation. Lab Invest. 2019;99:528–38. doi: 10.1038/s41374-018-0175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Hu J, Wang Z, et al. GAS5 promotes podocyte injury in sepsis by inhibiting PTEN expression. Eur Rev Med Pharmacol Sci. 2018;22:8423–30. doi: 10.26355/eurrev_201812_16541. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y, Li Y, Ding L, et al. Regulatory effect of lncRNA NKILA on autophagy induced by sepsis kidney injury. Eur Rev Med Pharmacol Sci. 2019;23:8011–17. doi: 10.26355/eurrev_201909_19017. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Moser AH, Shigenaga JK, et al. Downregulation of liver X receptor-α in mouse kidney and HK-2 proximal tubular cells by LPS and cytokines. J Lipid Res. 2005;46:2377–87. doi: 10.1194/jlr.M500134-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Manoeuvrier G, Bach-Ngohou K, Batard E, et al. Diagnostic performance of serum blood urea nitrogen to creatinine ratio for distinguishing prerenal from intrinsic acute kidney injury in the Emergency Department. BMC Nephrol. 2017;18:173. doi: 10.1186/s12882-017-0591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10:155. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 19.Wang P, Wu Y, Zhong X, et al. Prognostic significance of overexpressed long non-coding RNA TUG1 in patients with clear cell renal cell carcinoma. Eur Rev Med Pharmacol Sci. 2017;21:82–86. [PubMed] [Google Scholar]
- 20.Lorenzen JM, Thum T. Long noncoding RNAs in kidney and cardiovascular diseases. Nat Rev Nephrol. 2016;12:360. doi: 10.1038/nrneph.2016.51. [DOI] [PubMed] [Google Scholar]
- 21.Zhang C-C, Niu F. LncRNA NEAT1 promotes inflammatory response in sepsis-induced liver injury via the Let-7a/TLR4 axis. Int Immunopharmacol. 2019;75:105731. doi: 10.1016/j.intimp.2019.105731. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y, Qiu J, Chen B, et al. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-κB pathway. Int Immunopharmacol. 2018;59:252–60. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 23.Dumache R, Rogobete AF, Bedreag OH, et al. Use of miRNAs as biomarkers in sepsis. Anal Cell Pathol (Amst) 2015;2015 doi: 10.1155/2015/186716. 186716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giza DE, Fuentes-Mattei E, Bullock MD, et al. Cellular and viral microRNAs in sepsis: Mechanisms of action and clinical applications. Cell Death Differ. 2016;23:1906–18. doi: 10.1038/cdd.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S-Y, Lee Y-S, Choi H-M, et al. Distinct pathophysiologic mechanisms of septic acute kidney injury: role of immune suppression and renal tubular cell apoptosis in murine model of septic acute kidney injury. Crit Care Med. 2012;40:2997–3006. doi: 10.1097/CCM.0b013e31825b912d. [DOI] [PubMed] [Google Scholar]
- 26.El Gazzar M, Church A, Liu T, et al. MicroRNA-146a regulates both transcription silencing and translation disruption of TNF-α during TLR4-induced gene reprogramming. J Leukoc Biol. 2011;90:509–19. doi: 10.1189/jlb.0211074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Verma IM. NF-κB regulation in the immune system. Nat Rev Immunol. 2002;2:725. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Ha T, Zou J, et al. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–95. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]