Abstract
Purpose
To evaluate the impact of instillation angle and nozzle tip geometry on cross-contamination risk of multidose ocular solution bottles.
Methods
Pseudomonas aeruginosa solution was passed exclusively on the outside of the nozzle to simulate contamination on the exterior of topical agents. Three drops were administered from angles of 90° and 45° from bottles with either a round or sharp tip geometry, and the cultures were examined for growth. Two-hundred sixteen cultures from nine lubricant eyedrop brands currently existing in the Brazilian market were assessed for bacterial growth.
Results
After seven days, bacterial contamination was detected in 53.7% of cultures when drops were administered at 90° and in 70.4% of cultures at 45°. Eyedrops collected from a rounded nozzle tip and an instillation angle of 90° transmitted bacteria in 69.4% of cases, whereas those administered from a sharp tip transmitted bacteria in only 22.2% of cases (P = 0.001). At an instillation angle of 45°, contamination was identified in 83.3% of bottles with a rounded tip geometry and in only eight of 18 bottles (44.4%) from those with a sharp nozzle geometry (P = 0.005).
Conclusions
Adjusting the instillation angle of eyedrop solutions to 90°, as well as using a nozzle geometry that prevents flow of the solution to the side of the bottle, significantly reduced contamination rates.
Translational Relevance
Standardizing drop bottles and adjusting delivery angle shows promise in reducing contamination rates and may critically impact the quality of care for patients requiring topical therapeutic agents.
Keywords: cross-contamination, drop instillation, eyedrops, eye drop standardization, ophthalmic solutions
Introduction
Contaminated eyedrops represent one of the leading sources of preventable ocular infection globally.1,2 Ophthalmic solutions are commonly contaminated with microorganisms on repeated use, a rate varying from 0.07% to nearly 70%.2–9 Although contamination of eyedrops does not often cause severe infection because of the addition of preservatives to eyedrop solutions that inactivate pathogens in the residual liquid, infections from gram-negative keratitis, conjunctivitis, and even endophthalmitis have been reported from bacterial growth.3,6,10–14 Such infections can lead to significant damage to the corneal epithelium, resulting in surface ulcers and even blindness.4,5
Although pathogen growth from eye drop bottles is mostly representative of the skin flora and the environment,2,7,9,15–19 harmful bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and gram-negative rods such as Pseudomonas aeruginosa have been found at lower frequencies in contaminated ocular solutions.4,7,9,11,20 Bacteria such as MRSA can survive on some surfaces for weeks,21 and P. aeruginosa, a bacterium responsible for about 10% of all nosocomial infections, can survive from six hours to 16 months in dry and inanimate surfaces in hospitals.22 Some species of virus such as Adenoviruses are unusually resistant to chemical or physical agents and adverse pH conditions, allowing prolonged survival outside the body from three to eight weeks at room temperature.23,24
Previous studies investigating microbial cross-contamination of eyedrop solutions have found significant correlations between vial contamination and duration of use,19,25,26 as well as between brands containing an antimicrobial agent versus those lacking preservatives.19 However, contamination of the outer surface of the nozzle of solution bottles is not well studied. Specifically, bottle tips often touch the eyelids and lashes of patients during instillation, and cross-infection from transmission through objects such as a tonometer, door handle, and eyedrop bottle that had previously contacted a contaminated eye are common routes of infection.27 Additionally, standardization of eyedrop bottles currently on the market is lacking, leading to significant variation in the drop volume of ocular solutions among different manufacturers.27–30 As a result, eyedrops vary widely in form when detached from the bottle at the time of application, leading to potential cross-contamination or reinfection of the same eye. When an eyedrop bottle is tilted to 45°, the geometry of the bottle tip conducts liquid through the outer face of the nozzle before detaching from the bottle due to the capillary phenomenon (Fig. 1),29 passing through a contaminated area before reaching the eye.
Figure 1.
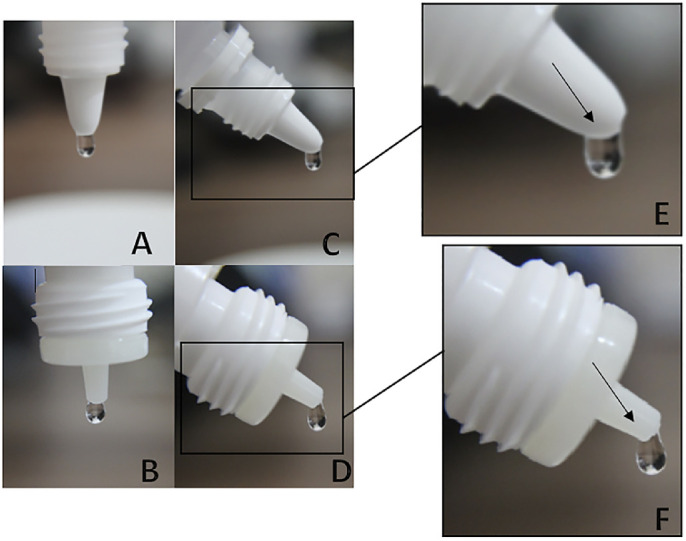
Detail of the formation of drops according to two types of eye drops bottles when inverted to 90° (A and B) and 45° (C and D). In a higher magnification, the geometry of the nozzle allows the eyedrop to contact a wider area of the outer face (E) or a narrow one (F).
Despite the higher risk of cross-infection from residual drops released from this region of solution bottles, no study to date has evaluated the impact of altering instillation angle on eyedrop contamination. The purpose of this study was to investigate the cross-contamination risk of multidose eyedrop bottles contaminated on the exterior nozzle and to subsequently assess the impact of adjusting the angle of administration. Optimizing eyedrop instillation angle bears significant implications for clinical practice, yielding the potential to reduce severe corneal surface infections and, in some cases, avoidable blindness.
Methods
The following experimental study was developed in the laboratory of Microbiology of the Department of Ophthalmology and Visual Sciences−Federal University of São Paulo, approved by the Research and Ethics Committee [Protocol no. 3417060816].
Nine existing brands of both preservative-containing and preservative-free lubricant eye drops in the Brazilian market were evaluated: Lacrifilm (carmellose sodium; Genom, São Paolo, Brazil); Hyabak (0.15% sodium hyaluronate; Théa, Oakville, Ontario, Canada); Optive (carboxymethylce-glycern-poly80; Allergan, Dublin, Ireland); SystaneUL (0.3% propylene glycol; Alcon, Geneva, Switzerland); Hylo Comod (0.1% sodium hyaluronate; Pfizer, New York, NY, USA); Lacribell (dextran 70 0.1%; hypromellose 0.3%; Latinofarma, São Paolo, Brazil); Mirugell (polyethylene glycol 400; propylene glycol; Latinofarma); Adaptis (carboxymethylcellulose; Legrand, Limoges, France); and Lacrilax (carmellose sodium; Cosmed, Rome, Italy). Drop vials were divided into two groups based on nozzle geometry: in Group 1, the geometry was characterized by a rounded shape in which the drop formed flows through the side and contacts the outer face of the nozzle when tilted; in Group 2, the geometry was characterized by a sharper edge, approximating a 90° angle from the lateral and tip orifices, in which the drop formed contacts only the tip of the nozzle without spreading to the external surface before detaching from the bottle.
Six sealed bottles from each of the nine lubricant eyedrop brands were randomly selected and opened for the collection of control samples. Three drops were released from each vial and directly inoculated on agar-chocolate culture plates (BMX PVX Choc. Polyvitex; bioMérieux, Marcy-l'Étoile, France) with the bottles first inverted to 90° and then inverted to 45°. An individual plate was used for each vial at each instillation position. Subsequently, a sterile cotton swab was soaked in an aqueous solution of Pseudomonas aeruginosa (ATCC 27853) at a concentration of 0.5 McFarland (∼1.5 × 108 bacteria), and passed exclusively on the outside of the nozzle to simulate contamination on the exterior of the eye drop vials. Attention was taken to uniformly soak the cotton swab to the line at which the cotton ends, passing the swab in a circular motion around the nozzle tip. Eyedrop samples were collected by first inverting each bottle to 90° and then to 45°, inoculating eyedrops on individual agar-chocolate culture plates at each instillation position.
Collection of samples was performed using sterile conditions under laminar flow. Cultures were incubated in a microaerophile environment at 35°C for seven days and evaluated for growth daily. Positive bacterial growth was recorded according to the presence of colonies on the main inoculation site (Supplementary Figures S1 and S2).
Data were analyzed using Stata Statistical Software 14.2 (StataCorp LLC, College Station, TX, USA). A McNemar test was performed to assess the impact of instillation angle on contamination rates on adjusting instillation angle. The two-tailed Fisher's exact test was performed to assess the exact probability of the nozzle tip geometry on contamination rates. Statistical significance was determined at a threshold of P<0.05.
Results
Two-hundred sixteen cultures from nine lubricant eyedrop brands were analyzed for bacterial growth at instillation angles of 90° and 45°, respectively (Table 1).
Table 1.
Bacterial Growth upon Drop Bottle Inversion of 90° and 45°, Before and After Contamination of the Nozzles
| Bottle Position | |||||
|---|---|---|---|---|---|
| Before Contamination (Control) | After Contamination (Experimental Group) | ||||
| Brand Of Eye Drops*,† | Group No. | 90° | 45° | 90° | 45° |
| 1.1 | − | − | + | − | |
| 1.2 | − | − | − | − | |
| 1.3 | − | − | + | + | |
| 1.4 | 1 | − | − | − | + |
| 1.5 | − | − | + | + | |
| 1.6 | − | − | − | − | |
| 2.1 | − | − | − | + | |
| 2.2 | − | − | + | + | |
| 2.3 | 1 | − | − | + | + |
| 2.4 | − | − | − | + | |
| 2.5 | − | − | + | + | |
| 2.6 | − | − | − | + | |
| 3.1 | − | − | − | − | |
| 3.2 | 1 | − | − | + | + |
| 3.3 | − | − | − | − | |
| 3.4 | − | − | + | + | |
| 3.5 | − | − | − | + | |
| 3.6 | − | − | + | + | |
| 4.1 | − | − | + | + | |
| 4.2 | − | − | − | + | |
| 4.3 | 1 | − | − | + | + |
| 4.4 | − | − | + | + | |
| 4.5 | − | − | + | + | |
| 4.6 | − | − | − | − | |
| 5.1 | − | − | + | + | |
| 5.2 | − | − | + | + | |
| 5.3 | 1 | − | − | + | + |
| 5.4 | − | − | + | + | |
| 5.5 | − | − | + | + | |
| 5.6 | − | − | + | + | |
| 6.1 | − | − | + | + | |
| 6.2 | − | − | + | + | |
| 6.3 | − | − | + | + | |
| 6.4 | 1 | − | − | + | + |
| 6.5 | − | − | + | + | |
| 6.6 | − | − | + | + | |
| 7.1 | − | − | + | + | |
| 7.2 | − | − | − | + | |
| 7.3 | 2 | − | − | + | + |
| 7.4 | − | − | − | − | |
| 7.5 | − | − | − | + | |
| 7.6 | − | − | − | − | |
| 8.1 | − | − | − | − | |
| 8.2 | − | − | − | − | |
| 8.3 | 2 | − | − | − | − |
| 8.4 | − | − | − | − | |
| 8.5 | − | − | − | − | |
| 8.6 | − | − | − | + | |
| 9.1 | − | − | + | − | |
| 9.2 | − | − | − | − | |
| 9.3 | 2 | − | − | − | − |
| 9.4 | − | − | − | + | |
| 9.5 | − | − | − | + | |
| 9.6 | − | − | + | + | |
Brand of eyedrop used: (1) Lacrifilm (carmellose sodium), (2) Optive (carboxymethylce-glycern-poly80), (3) Lacribell (dextran 70 0.1%; hypromellose 0.3%), (4) Adaptis (carboxymethylcellulose), (5) Lacrilax (carmellose sodium), (6) Mirugell (polyethylene glycol 400; propylene glycol), (7) Hyabak (0.15% sodium hyaluronate), (8) Systane (0.3% propylene glycol), (9) Hylo Comod (0.1% sodium hyaluronate).
Preservative-containing: (1) Lacrifilm (carmellose sodium), (2) Optive (carboxymethylce-glycern-poly80), (3) Lacribell (dextran 70 0.1%; hypromellose 0.3%), (4) Adaptis (carboxymethylcellulose), (5) Lacrilax (carmellose sodium), (6) Mirugell (polyethylene glycol 400; propylene glycol), (8) Systane (0.3% propylene glycol); Preservative-free: (7) Hyabak (0.15% sodium hyaluronate), (9) Hylo Comod (0.1% sodium hyaluronate).
None of the 54 tested bottles indicated bacterial growth in the control group at instillation angles of 90° or 45°. In the experimental group, bacterial contamination was detected in two bottles at 90° alone, 27 bottles at both 90° and 45°, and 11 bottles at 45° alone. (P = 0.01). (Table 2)
Table 2.
Bacterial Growth After Contamination by Instillation Angle
| 45° | |||
|---|---|---|---|
| Instillation Angle | Positive | Negative | |
| 90° | Positive | 27 | 2 |
| Negative | 11 | 14 | |
P = 0.01 statistical significance at a threshold of P < 0.05 for McNemar's test.
Eyedrops administered from Group 1 ocular solutions (Lacrifilm [carmellose sodium], Optive [carboxymethylce-glycern-poly80], Lacribell [dextran 70 0.1%; hypromellose 0.3%], Adaptis [carboxymethylcellulose], Lacrilax [carmellose sodium], Mirugell [polyethylene glycol 400; propylene glycol]) at 90° transmitted bacteria in 25 of 36 bottles (69.4%), whereas those administered from Group 2 ocular solutions (Hyabak (0.15% sodium hyaluronate), Systane (0.3% propylene glycol), Hylo Comod (0.1% sodium hyaluronate)) transmitted bacteria in only four of 18 bottles (22.2%; P = 0.001). However, at an instillation angle of 45°, contamination was identified in 30 of 36 bottles (83.3%) from Group 1 ocular solutions and in only eight of 18 vials (44.4%) from Group 2 ocular solutions (P = 0.005; Table 3).
Table 3.
Bacterial Growth After Contamination by Instillation Angle and Bottle Geometry
| Instillation Angle | Positive | Negative | Total | % Contaminated | P Value |
|---|---|---|---|---|---|
| 90° [Total] | 29 | 25 | 54 | 53.7% | |
| 90° [Group 1 | Group 2] | 25 | 4 | 11 | 14 | 36 | 18 | 69.4% | 22.4% | * P = 0.001 |
| 45° [Total] | 38 | 16 | 54 | 70.4% | |
| 45° [Group 1 | Group 2] | 30 | 8 | 6 | 10 | 36 |18 | 83.3% | 44.4% | * P = 0.005 |
Indicates the two-tailed statistical significance at a threshold of P < 0.05 for a Fisher's exact test.
Interestingly, only a single bottle from Systane (0.3% propylene glycol) yielded bacterial growth at 45°, indicating that the sharper nozzle geometry from this class of ocular solution brands may induce the eyedrop to detach from the bottle with minimal contact with the outer surface (Fig. 2).
Figure 2.
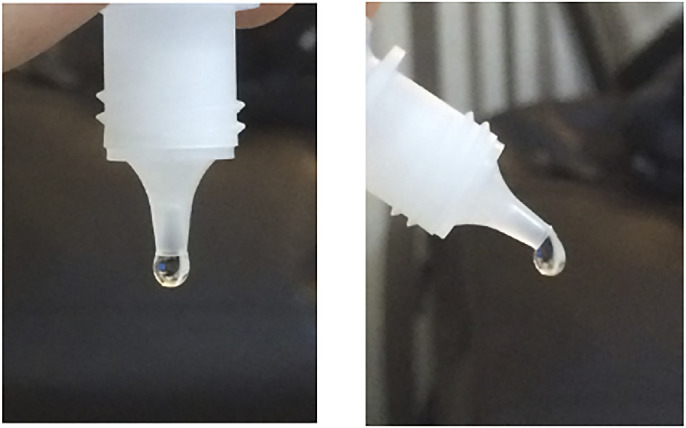
Systane eyedrop formation at 90° and 45°.
Discussion
Topically-applied agents represent the first-line of therapy for many patients4 but constitute a common source of infection when contaminated on the exterior upon repeated use. However, few studies have investigated cross-infection stemming solely from the tip of ocular solution bottles, and no study has assessed the impact of instillation angle on likelihood of contamination. Our study found that upon contamination exclusively on the exterior of ocular solution bottles, cross-infection occurred in more than 50% of tested bottles. Rates of contamination were significantly lower when drops were administered at an angle of 90° (53.7%) versus at an angle of 45° (70.4%); only two bottles tested positive at 90° and negative at 45°; 11 bottles tested negative for 90° and positive for 45°. Intriguingly, rounded nozzle tip geometry appeared to exacerbate contamination probability, increasing the contamination rate to 83.3% at a 45° instillation angle. Possible explanations for this phenomenon involve the idea that rounded bottle tips favor draining of the solution to a wider external orifice, as well as the possibility of solution reflux upon instillation.
Despite the decrease in contamination risk that both a perpendicular instillation angle and a sharper tip geometry afford, the presence of microbial cross-infection was still surprisingly high at an average of over two in 10 bottles. These results suggest that if the exterior of the multi-dose eyedrops tips were contaminated, they would no longer meet pharmaceutical standards for sterility, even though the product itself is sterile inside the bottle.31 In evaluating the use of topically-applied agents for diagnostic and prophylactic purposes, these findings suggest the importance of considering alternatives to multidose vials, which are typically used on several patients in inpatient and outpatient settings.10 The use of ophthalmic strips or single-dose vials offer a putative solution to cross-contamination resulting from multi-dose agents. However, Somner et al.32 has shown that the costs and plastic waste of this infection risk–free practice is likely high, and accepting a certain threshold of contamination risk would result in large savings in resource-strained settings.
A reasonable method of reducing infection risk, while balancing the high costs and environmental impact of single-dose vials, may involve implementing standardization of topically-applied agents currently on the market. Our study indicates that simple adjustments to nozzle tip geometry may ameliorate the inherent contamination risk of multi-dose droppers. An alternative method of minimizing cross-contamination involves cleaning the nozzle of multidose vials with 70% isopropyl alcohol immediately before or after instillation. A prior pilot study revealed that contaminated bottles cleaned with a 70% alcohol pad and re-tested for bacterial growth lacked contamination upon treatment with alcohol (unpublished data, 2015). Additionally, factors such as drop size and solution viscosity may impact cross-contamination; our current study simulated real-world conditions in an effort to increase external validity by manually releasing drops that inevitably contain variation in size, but further studies may investigate the impact of systematically controlling these parameters.
Lubricant eye drops are widely prescribed by ophthalmologists, and they were selected for use in this study over diagnostic or dilation drops due to the variety of bottle types and availability on the market. Additionally, Pseudomonas aeruginosa is known to be a hostile pathogen, that, when introduced into the cornea, acts with extreme virulence and may even require enucleation;33 our study was limited to the use of this bacterium due to its laboratory availability and for its status as a frequent isolate of conjunctivitis among Gram negative bacteria.34 Nonetheless, further research may involve other bacterial strains responsible for other strains of conjunctivitis, keratitis, endophthalmitis, blepharitis, or orbital cellulitis,34 as well as other types of diagnostic or prophylactic ocular solutions.
An additional consideration involves the use of preservative-containing versus preservative-free formulations. In our study, only Hyabak (0.15% sodium hyaluronate) and Hylo Comod (0.1% sodium hyaluronate) were preservative-free agents that instead contain either filtration (Hyabak bottle) or pump (Comod dosage) systems built within the bottle. Interestingly, although some multidose nonpreserved containers such as Hylo Comod (0.1% sodium hyaluronate) use a silver wire placed in the proximity of the outlet tip to protect from microbial contamination, this method was not able to avoid cross-contamination once the contamination happened after the drop was formed.35 However, the preservatives of the other studied eyedrop brands were unable to prevent bacterial growth in all cases in this experiment, with some preservative-containing brands exceeding the contamination rate of preservative-free solutions. For instance, sodium perborate converts to hydrogen peroxide upon exposure to air and subsequently breaks down into oxygen and water, inactivating the preservative after instillation. Further experimentation should investigate the efficacy of preservatives in reducing contamination stemming from the solution itself, as well as the threshold at which solution pH reduces drug efficacy.36
The risk of cross-contamination through eyedrops is high, even for preservative-containing ocular solutions. Our study reveals that altering the instillation angle to 90°, as well as using a nozzle tip geometry that prevents flow of the solution to the side of the bottle, reduced contamination rates from 83.3% to 22.4%. These results suggest that additional methods beyond instillation angle and nozzle tip geometry may be required to achieve 0% contamination; the use of non-return valve systems used simultaneously with silicone members to filter returning air offer one potential solution, but these systems are often costlier for patients.37 Encouraging patients to consider using plastic instillation aids that attach to eyedrop bottles to deliver topical agents may be a more feasible and cost-effective solution, reducing the challenges associated with achieving an optimal instillation angle and significantly reducing contamination rates with repeated use. Standardization of eyedrop bottles is vital to ensure that topical agents have minimal contact with the outside of the vial before reaching patients’ eyes. Further studies are needed to optimize bottle geometry, instillation angle, and solution pH to reduce cross-contamination risk and ensure higher quality of care for patients using topical agents on a daily regimen.
Supplementary Material
Acknowledgments
The authors thank Luiz Alberto S. Melo Jr, MD, for crucial advice concerning the statistical analyses.
Supported by CAPES (Coordination of Superior Level Staff Improvement).
Disclosure: A.X. da Costa, None; M.C.Z. Yu, None; D. de Freitas, None; P.C. Cristovam, None; L.C. LaMonica, None; V.R. dos Santos, None; J.A.P. Gomes, Allergan (C, E), MSD (C), Bausch & Lomb/Valeant (C, E), Mundipharma (C, E), EMS/Legrand (C), Shire (C), Alcon (E), Genom (E), Pfizer (E), Grin (E), Ofta Vision Health (E), FAPESP (R), Capes (R), Cnpq (R)
References
- 1. Hazlett L, Suvas S, McClellan S, Ekanayaka S. Challenges of corneal infections. Expert Rev Ophthalmol. 2016; 11: 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nentwich MM, Kollmann KH, Meshack J, et al.. Microbial contamination of multi-use ophthalmic solutions in Kenya. Br J Ophthalmol. 2007; 91: 1265–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teuchner B, Wagner J, Bechrakis NE, Orth-Höller D, Nagl M. Microbial contamination of glaucoma eyedrops used by patients compared with ocular medications used in the hospital. Medicine. 2015; 94: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geyer O, Bottone EJ, Podos SM, et al.. Microbial contamination of medications used to treat glaucoma. Br J Ophthalmol. 1995; 79: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mayo MS, Schlitzer RL, Ward MA, Wilson LA, Ahearn DG. Association of Pseudomonas and Serratia corneal ulcers with use of contaminated solutions. J Clin Microbiol. 1987; 25: 1398–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jokl DH, Wormser GP, Nichols NS, et al.. Bacterial contamination of ophthalmic solutions used in an extended care facility. Br J Ophthalmol. 2007; 91: 1308–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Porges Y, Rothkoff L, Glick J, et al.. Sterility of glaucoma medications among chronic users in the community. J Ocul Pharmacol Ther. 2004; 20: 123–128. [DOI] [PubMed] [Google Scholar]
- 8. Schein OD, Hibberd PL, Starck T, et al.. Microbial contamination of in-use ocular medications. Arch Ophthalmol. 1992; 110: 82–85. [DOI] [PubMed] [Google Scholar]
- 9. Fazeli MR, Nejad HB, Mehrgan H, et al.. Microbial contamination of preserved ophthalmic drops in outpatient departments: possibility of an extended period of use. DARU J Pharmaceut Sci. 2004; 12: 151–155. [Google Scholar]
- 10. Stevens JD, Matheson MM.. Survey of the contamination of eyedrops of hospital inpatients and recommendations for the changing of current practice in eyedrop dispensing. Br J Ophthalmol. 1992; 76: 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schein OD, Wasson PJ, Boruchoff SA, et al.. Microbial keratitis associated with contaminated ocular medications. Am J Ophthalmol. 1988; 105: 361–365. [DOI] [PubMed] [Google Scholar]
- 12. Mah-Sadorra JH, Najjar DM, Rapuano CJ, et al.. Serratia corneal ulcers: a retrospective clinical study. Cornea. 2005; 24: 793–800. [DOI] [PubMed] [Google Scholar]
- 13. Templeton WC III, Eiferman RA, Snyder JW, et al.. Serratia keratitis transmitted by contaminated eyedroppers. Am J Ophthalmol. 1982; 93: 723–726. [DOI] [PubMed] [Google Scholar]
- 14. Penland RL, Wilhelmus KR. Stenotrophomonas maltophilia ocular infections. Arch Ophthalmol. 1996; 114: 433–436. [DOI] [PubMed] [Google Scholar]
- 15. Hovding G, Sjursen H.. Bacterial contamination of drops and dropper tips of in-use multidose eye drop bottles. Acta Ophthalmol (Copenh). 1982; 60: 213–222. [DOI] [PubMed] [Google Scholar]
- 16. Raynaud C, Laveran H, Rigal D, et al.. Bacterial contamination of eyedrops in clinical use. J Fr Ophtalmol. 1997; 20: 17–24. [PubMed] [Google Scholar]
- 17. Tasli H, Cosar G.. Microbial contamination of eye drops. Cent Eur J Public Health. 2001; 9: 162–164. [PubMed] [Google Scholar]
- 18. Wessels IF, Bekendam P, Calvin WS, et al.. Open drops in ophthalmology offices: expiration and contamination. Ophthalmic Surg Lasers. 1999; 30: 540–546. [PubMed] [Google Scholar]
- 19. Brudieu E, Duc DL, Masella JJ, et al.. Bacterial contamination of multi-dose ocular solutions. A prospective study at the Grenoble Teaching Hospital. Pathol Biol (Paris). 1999; 47: 1065–1070. [PubMed] [Google Scholar]
- 20. Clark P, Ong P, Stanley CB. Contamination of diagnostic ophthalmic solutions in primary eye care settings. Mil Med. 1997; 162: 501–506. [PubMed] [Google Scholar]
- 21. Centers for Disease Control and Prevention. CDC twenty four seven. Saving lives, protecting people. Methicillin-resistant Staphylococcus aureus (MRSA). Available at: https://www.cdc.gov/mrsa/community/environment/index.html. Accessed: May 16, 2019.
- 22. Special Pathogens Laboratory—Pseudomonas aeruginosa. Available at: https://www.specialpathogenslab.com/perch/resources/pseudomonas-fact-sheet.pdf. Accessed: May 16, 2019.
- 23. Pond K & World Health Organization. Water, Sanitation and Health Team. Water recreation and disease: plausibility of associated infections: acute effects, sequelae and mortality. IWA Publishing; 2005;191–229. ISBN: 1843390663. https://apps.who.int/iris/handle/10665/43338 [Google Scholar]
- 24. Environmental Health & Safety—The University of Iowa - Adenovirus and Adenoviral Vectors. Available at: https://ehs.research.uiowa.edu/adenovirus-and-adenoviral-vectors. Accessed: May 16, 2019.
- 25. Rahman MQ, Tejwani D, Wilson JA, Butcher I, Ramaesh K. Microbial contamination of preservative free eye drops in multiple application containers. Br J Ophthalmol. 2006; 90: 139–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsegaw A, Tsegaw A, Abula T, Assefa Y. Bacterial contamination of multi-dose eye drops at Ophthalmology Department, University of Gondar, Northwest Ethiopia. Middle East Afr J Ophthalmol. 2017; 24: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Costa AX, Gama RM, Kitadai SPS, Andrade EP, Ferro GBR, Gomes JAP. Drop volume of artificial tear solutions: pharmacoeconomic study. Rev Bras Oftalmol. 2015; 74: 339–44. [Google Scholar]
- 28. Gaynes BI, Singa RM, Cao Y. Dosage variability of topical ocular hypotensive products: a densitometric assessment. J Glaucoma. 2009; 18: 149–152. [DOI] [PubMed] [Google Scholar]
- 29. Gaynes BI, Singa RM, Schaab G, Sorokin Y. Impact of administration angle on the cost of artificial tear solutions: does bottle positioning minimize wastage? J Ocul Pharmacol Ther. 2007; 23: 196–201. [DOI] [PubMed] [Google Scholar]
- 30. Roizenblatt R, Freitas D, Belfort Jr R, Hofling-Lima AL, Prata Jr JA. Impacto econômico no tratamento do glaucoma: volume de gotas de colírios antiglaucomatosos brasileiros e norte-americanos. Arq Bras Oftalmol. 2001; 64: 143–6. [Google Scholar]
- 31. U.S. Pharmacopeial Convention. USP chapter pharmaceutical compounding—sterile preparations. In: USP 37–NF 32. Rockville, MD: U.S. Pharmacopeial Convention; 2014. [Google Scholar]
- 32. Somner JEA, Cavanagh DJ, Wong KKY, Whitelaw M, Thomson T, Mansfield D. Disposable drops: the risks, financial and environmental costs of reusing them. Eye. 2010; 24: 361–363. [DOI] [PubMed] [Google Scholar]
- 33. Spencer WH. Pseudomonas aeruginosa infections of the eye. Calif Med. 1953; 79: 438–443. [PMC free article] [PubMed] [Google Scholar]
- 34. Teweldemedhin M, Gebreyesus H, Atsbaha AH, Asgedom SW, Saravanan M. Bacterial profile of ocular infections: a systematic review. BMC Ophthalmol. 2017; 17: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Birkhoff M, Marx D. New devices for dispensing ophthalmic treatments may be the key to managing the life-cycle of established products with low investments in filling technology. IPI. 2010; 2: 44–46. [Google Scholar]
- 36. Perry HD, Donnenfeld ED.. Issues in the use of preservative-free topicals. Manag Care. 2003; 12: 39–41. [PubMed] [Google Scholar]
- 37. Awwad S, Mohamed Ahmed AHA, Sharma G, et al.. Principles of pharmacology in the eye. Br J Pharmacol. 2017; 174: 4205–4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


