Abstract
Purpose
The purpose of this study was to evaluate the safety and efficacy across time, of patients topically treated with Benozzi's method for presbyopia.
Methods
A nonrandomized case series retrospective study was developed, including patients with emmetropia with binocular uncorrected distance visual acuity (UDVA) of 25/20 or better, and with uncorrected near visual acuity (UNVA) at least Jaeger 2 or worse. The study was set in Buenos Aires, Argentina, from January 2011 to June 2018, with at least 1-year follow-up. Patients were treated with pilocarpine and diclofenac preservative-free eye drops (Benozzi Method; US 8.524.758 B2, EP1.938.839 B1), and the main outcome measured was binocular UNVA at different follow-up times. Other parameters, as the UDVA and presence of side effects, were evaluated.
Results
A total of 910 patients were included with a mean age at baseline of 48.67 ± 3.72 years old (range, 40–59 years). The baseline UNVA was 4.74 ± 1.53 and at 8 years of follow-up was decreased to 1.36 ± 0.48 (Jaeger scale). The mean binocular UDVA at baseline was 0.00 ± 0.01 logarithm of the minimum angle of resolution (logMAR) and after 8 years of follow-up was 0.03 ± 0.04 logMAR. All side effects reported (decrease of light perception, headaches, symptoms of ocular surface dryness, and dizziness) were spontaneously resolved in patients who continued with the treatment.
Conclusions
The efficacy of the pharmacological treatment of presbyopia to improve the UNVA without affecting the UDVA is shown. Side effects were well tolerated and resolved before 1 year of treatment.
Translational Relevance
This is a nonsurgical option for patients with emmetropic presbyopia who do not wish to wear glasses, which is a pharmacological treatment with eye drops.
Keywords: presbyopia, pharmacological treatment, spectacle independence, accommodation
Introduction
With aging, the performance of visual functions decreases.1,2 One of the first signs that mark “the pass of the time,” is presbyopia, when the progressive loss of accommodation starts to affect visual daily tasks.1–4 In a short time, people who still feel young, suffer the need to use spectacles for reading, and any other kind of visual activities in which near-sightedness is required. Many people take the “spectacle dependency” for granted and get used to it, but others do not and it, therefore, deteriorates their quality of life. For the latter, different surgical options are available, although not suitable for all cases.5–8
Corneal refractive surgery for presbyopia is growing, with the aid of new laser procedures and ablation profiles, but the limitation is the corneal structure itself, with many different possible complications, which sometimes causes irreversible effects.9–12 Yet, one “reversible” option is the implantation of phakic lens, which could be placed in the anterior or posterior chamber, in a relatively simple surgery.13,14 However, some complications have been described, principally with the anterior chamber lens, such as corneal endothelial cell loss, uveitis, glaucoma, or pupil deformation.15 In addition, complications could also occur with posterior chamber lens (glaucoma and cataract development).16,17 Another option for presbyopia management are pseudo-phakic procedures, with the evolution of a wide range of intraocular lens (IOLs) with multifocal effect.8,18 In young people with presbyopia and with a low refractive error, performing clear lens surgery is controversial.19,20 Even though it is possible to operate on healthy eyes with the intent to avoid spectacles for reading, it seems to be a risky medical option, as some major complications may occur.
The option of nonsurgical management of presbyopia, with eye drops was developed, studied, patented (Benozzi Method; US 8.524.758 B2- EP1.938.839 B1), and published by our group in 2012, proposing a treatment to improve accommodation in patients with emmetropic presbyopia, through parasympathetic stimulation combined with nonsteroidal anti-inflammatory drugs (NSAIDs).21 At a later time, more pharmacological options arose, due to the fact that there was still a large population with unsatisfied needs, for whom surgical options were not adequate.22–27 Because of that, the purpose of this work was to review the efficacy across time of patients topically treated with eye drops for presbyopia with the Benozzi's method.
Methods
Study Design
A nonrandomized case series retrospective study was developed, including all the patients with presbyopia treated with eye drops, from January 2011 to June 2018, with at least 1 year of follow-up. The study was performed following the tenets of Helsinki, in a private ophthalmology clinic (Centro de Investigación Avanzada de la Presbicia) in Buenos Aires city, Argentina. All the patients were previously informed about the characteristics of the pharmacological eye drop treatment and their potential side effects, as itching, burning, foreign body sensation, conjunctival redness, and/or headache. In addition, because the pupil size decreases during treatment, patients were advised about a potential light perception decrease. An informed consent was obtained from every patient before start the treatment. In that, patients give their consent to share the “treatment results” to the scientific community by congress and/or publication, always preserving their anonymity. The present study protocol was approved by the Ethical Committee of the Argentinian Presbyopia Society 0002/2019.
Exclusion and Inclusion Criteria, Parameters to Evaluate, and Statistics
A complete ophthalmic baseline assessment, including visual acuity, ocular surface, anterior segment, intraocular pressure (IOP), and ocular fundus (evaluated by binocular indirect ophthalmoscope), was performed to evaluate the following inclusion and exclusion criteria.
-
1.
Refractive errors were measured (auto-refracto-keratometer; Nidek ARK700K) and patients were excluded if one of the eyes has a cycloplegic spherical refraction greater than -0.50 D or 1.0 D and/or with cylinder refraction higher than 1.0 D.
-
2.
The uncorrected distance visual acuity (UDVA) was measured with Snellen charts and converted to the logarithm of the minimum angle of resolution (logMAR) and uncorrected near visual acuity (UNVA) was measured with reading chart at 45 cm distance (objectively measured), with Jaeger (J) standard notation (from J1 to J8). If binocular UDVA was lower than 25/20, those patients were also excluded.
-
3.
Only patients with UNVA of at least Jaeger 2 or worse reading performance were included, baseline age of 40 years or older, and less than 60 years old.
-
4.
Signs of ocular surface disease was evaluated by slit-lamp, staining, and grading it by Oxford scale, and cases were excluded if grade 3 or higher of baseline was detected.28
-
5.
Also done by slit-lamp, the lens was graded according to the Lens Opacities Classification System III to detect cataract development. In addition, patients were excluded if at the beginning of the study, NO3-NC3 or higher lens opacities were detected by slit-lamp, or also if any kind of corneal disease was detected (severe dry eye, corneal scares and/or haze, keratoconus, and previous corneal refractive surgery).29
-
6.
IOP was measured with Goldman tonometry and patients were excluded if IOP was higher than 21 mm Hg in one or both eyes.
-
7.
Patients with amblyopia, history of any kind of glaucoma, pseudophakia, macular disease, or any retinal disorder, were also excluded.
During the retrospective review, cases were separated according to the total years of follow-up. Considering that, 8 groups of patients were configured from 1 to 8 years of follow-up. The main outcome was binocular UNVA for each group, achieved at the end of their respective follow-up. Patients were followed during the first week, first month, and third month, and then every 6 months. Meanwhile, patients were continuously on the treatment. Every year, the same complete ophthalmic evaluation was performed to detect and rule-out side effects and/or if some baseline condition had changed (as cataracts, glaucoma, or retinal disease appears). In every visit, patients were asked about the occurrence of general and ocular side effects, as well as symptoms of any kind of discomfort, such as headaches, decrease of light perception (dimness) and/or ocular surface discomfort symptoms (dryness, itching, burning, foreign body sensation, and/or redness). If any of those side effects had appeared (or any other spontaneously expressed by patients), it was registered and followed up on to evaluate if it decreased or disappeared without specifically treating it or if the patient wanted to abandon the treatment. For patients with ocular surface discomfort, any kind of artificial tears and/or loteprednol etabonate 0.5% was allowed. These data were also analyzed.
Descriptive statistical results and graphics were analyzed and performed with XLMiner Analysis ToolPak software (Frontline Systems Inc.) and values were expressed as mean, SD and range.
Characteristics About the Treatment
The compounds contained in the eye drops were prepared in a private pharmaceutical laboratory, under sterile conditions, in 15 mL plastic drop-bottles, and stored in a specific refrigerated area until it was delivered to the patient. The use of eye drops was indicated twice daily, at the beginning of the day (at waking up), and 6 hours later. Patients were advised that they should not use spectacles during the treatment. Each patient received one bottle of pilocarpine and diclofenac preservative-free eye drops (Benozzi Method; US 8.524.758 B2- EP1.938.839 B1) a month. Once the bottle content was empty, the patient must return the bottle to the clinic and it was registered. Each bottle was numbered and identified with the patient data, as a traceability step. In addition, it was an indirect measurement of adherence to the treatment.
Results
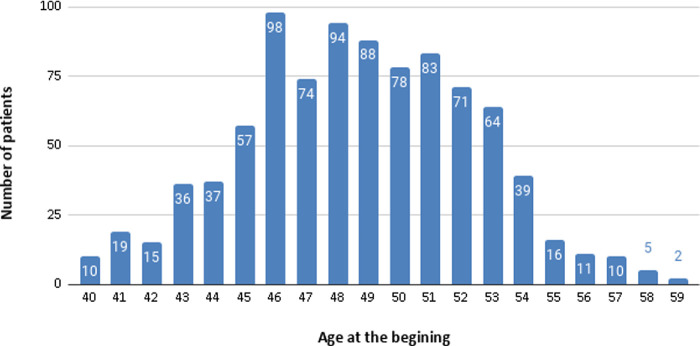
A total of 910 patients were found (427/483 women/men relation) with a mean age at baseline of 48.67 ± 3.72 years old (range, 40–59 years old). Of them, 26 (2.8%) abandoned the treatment for different reasons not related to the pharmacology treatment itself (passed away, cataract surgery, did not complete the appointment established, and changed their place of residence). Figure 1 shows the number of patients separated by their age when the treatment began.
Figure 1.
Patient groups according by their age when the treatment began.
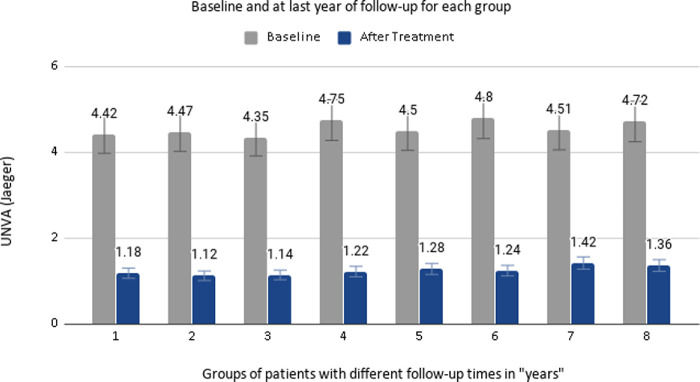
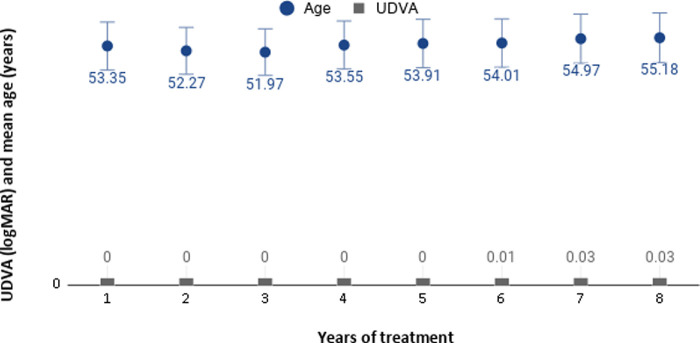
From the 910 included patients, their mean SE for the right eyes was 0.43 ± 0.39 D (range, -1.0 to 1.375) and 0.42 ± 0.41 (range, -0.75 to 1.5) for the left eyes. Table 1 shows the information for the eight different follow-up groups (from 1–8 years), including the age at baseline and at the end of the study, and the UNVA achieved with treatment, and the percentage of patients who had achieved UNVA between J1 and J2. Figure 2 shows the UNVA achieved for each follow-up group, before and after treatment. The mean binocular UDVA at baseline was 0.00 ± 0.01 logMAR (0.00–0.10) and after 8 years of follow-up was 0.03 ± 0.04 logMAR (0.00–0.10). Figure 3 shows the mean age from different groups of patients according their years of treatment and the mean UDVA achieved at each time point.
Table 1.
Uncorrected Near Visual Acuity (UNVA) for Each Group of Follow-Up Time, in Patients With Pharmacological Treatment for Presbyopia
| Age (years) | UNVA (Jaeger) | UNVA: J1 vs. J2, % | ||||||
|---|---|---|---|---|---|---|---|---|
| Years of Follow-up | Number of patients by Group | Baseline | Last Year Follow-up | Baseline | Last Year Follow-up | UNVA Improvement, % | J1 | J2 |
| 8 | 144 | 47.18 ± 3.66 | 55.18 ± 3.66 | 4.72 ± 1.52 | 1.36 ± 0.48 | 71.18 | 63.4 | 36.6 |
| (40 to 52) | (48 to 60) | (2 to 7) | (1 to 2) | |||||
| 7 | 175 | 47.96 ± 3.13 | 54.97 ± 3.13 | 4.51 ± 1.25 | 1.42 ± 0.49 | 68.51 | 57.2 | 42.8 |
| (41 to 54) | (48 to 61) | (2 to 7) | (1 to 2) | |||||
| 6 | 136 | 48.01 ± 4.47 | 54.01 ± 4.84 | 4.80 ± 1.42 | 1.24 ± 0.46 | 74.16 | 75.4 | 24.6 |
| (40 to 54) | (46 to 60) | (2 to 7) | (1 to 2) | |||||
| 5 | 128 | 48.91 ± 3.78 | 53.91 ± 3.78 | 4.50 ± 1.39 | 1.28 ± 0.43 | 71.55 | 75.2 | 24.8 |
| (40 to 55) | (45 to 60) | (2 to 7) | (1 to 2) | |||||
| 4 | 76 | 49.55 ± 3.73 | 53.55 ± 3.73 | 4.75 ± 1.44 | 1.22 ± 0.42 | 74.31 | 77.9 | 22.1 |
| (41 to 57) | (45 to 61) | (2 to 7) | (1 to 2) | |||||
| 3 | 77 | 48.97 ± 3.90 | 51.97 ± 3.90 | 4.35 ± 1.48 | 1.14 ± 0.35 | 73.79 | 84.6 | 15.4 |
| (42 to 57) | (45 to 60) | (2 to 7) | (1 to 2) | |||||
| 2 | 78 | 50.27 ± 3.91 | 52.27 ± 3.91 | 4.47 ± 1.35 | 1.12 ± 0.34 | 74.94 | 87.2 | 12.8 |
| (43 to 58) | (45 to 60) | (2 to 7) | (1 to 2) | |||||
| 1 | 96 | 48.31 ± 5.39 | 53.35 ± 5.75 | 4.42 ± 1.31 | 1.18 ± 0.39 | 73.30 | 94.8 | 5.2 |
| (43 to 59) | (44 to 60) | (2 to 7) | (1 to 2) | |||||
Figure 2.
Uncorrected distance visual acuity (UNVA) achieved for each follow up group, before and after treatment.
Figure 3.
The mean age from different groups of patients according their years of treatment and the mean uncorrected distance visual acuity (UDVA) achieved at each time point.
This table shows the number of participants for each group, age, and UNVA, at baseline and at the end of the study. It is also presented as the percentage of UNVA improvement after treatment, the difference between “before and after” treatment in Jaeger (J) scale, and the last column shows the percentage of the population achieving J1 or J2. Data were expressed as mean, SD, and range.
All the patients returned their treatment bottles, and none of them reported the necessity of use spectacles. The most frequent adverse effect was a decrease of light perception, which was subjectively reported by 241 patients (26%), followed by 119 patients reporting headaches (12.9%), 86 cases feeling ocular surface burning (9.3%), and 2 patients with dizziness. In their annual ophthalmic evaluation, no eyes were detected with IOP higher than 21 and none of the patients developed glaucoma, cataracts, and/or retinal disease. No ocular surface disease was detected in the annual control evaluated by the Oxford scale, however, the symptom of “burning” could be interpreted as some grade of dry eye disease. Other systemic side effects or symptoms were not detected and no patients abandoned the treatment due to side effects or discomfort. All the data are shown in Table 2.
Table 2.
Side Effects of Pharmacological Treatment of Presbyopia
| Amount of Cases | Occurred | Resolved | |
|---|---|---|---|
| Headaches | 119 | Day 1 | Day 7 |
| Dimness | 241 | Day 1 | Month 12 |
| Ocular surface symptoms | 86 | Day 1 | Month 6 |
| Other: dizziness | 2 | Day 1 | Day 3 |
This table shows the presented side effects and the time they were resolved.
Discussion
The present study, reviewing the result of presbyopia management with eye drops with 8 years of follow-up, shows excellent results without severe adverse effects. This pharmacological treatment, self-administered twice a day, seems to be sufficient to give spectacle independence for near visual tasks, in people with emmetropia with presbyopia and is an efficient treatment for patients in their 40s until their 60s.
Surgical options, as corneal refractive procedures, phakic, and pseudophakic IOLs, are improving and increasing patient's ability to avoid spectacles. However, limitations exist to recommend some of those procedures for patients with presbyopia, with low refractive error without cataracts. So, there is a wide active population over the world, aged from the 40s to the 60s, with an unsatisfied need, due to the decrease of their physiological accommodation due to aging, because spectacle dependency after 40 could, in a different manner, affect their quality of life.30,31 For this group of people, presbyopia management with nonsurgical options utilizing pharmacological eye drops is growing.
The first publication proposing a new potential pharmacological treatment for presbyopia management was from our group, in 2012.21 Previous experimental animal studies have shown how the effects of the parasympathetic stimulation with pilocarpine generates the ciliary muscle spasmodic contraction and enhancement of lens thickness, which increases the focal depth.32,33 However, near vision improvement decreased distance vision because the lens cannot change its thickness or position.34,35 However, by combining NSAIDs with parasympathetic agonists, the intensity of the contraction of the pupil and the ciliary muscle was decreased, allowing the lens to change shape and position increasing vision performance at all distances.36 This can explain why our patients reached good near and distance vision. This effect is achieved by the change of the shape and position of the lens, which consequently provokes accommodative capacity related to the parasympathetic activity. However, more studies will be necessary to show the mechanism of action, as anterior chamber measurements with ultrasound biomicroscopy, performed before, during, and after treatment. In that study, our group showed preliminary results in 100 subjects, which were followed during 5 years, treated with parasympathetic stimulation combined with NSAIDs using drops composed of pilocarpine and diclofenac. Treatment was indicated 3 times daily, and 20 patients presented ocular burning and discomfort right after drop instillation, but only one of them abandoned treatment due to that cause. The age of the patients was between 45 and 50 years old during the treatment, and all of them had good near and distance vision, without systemic diseases or complications.
While our group continued to study different pharmacological concentrations, formulations, and instillation indications, and moving forward with regulatory issues and patent activities, other studies were published. An interesting review about pharmacological treatments of presbyopia was published by Renna et al. in 2017.37 They only found four papers: our work from 2012, the work of Crawford et al. (2014), Abdelkader et al. in 2015, and one work from their group (Renna et al; 2016).22,23,25 With similar pharmacological principles, the difference remains principally in the drug formulations, instillations protocols, and side effects. However, to our knowledge, until the present, no treatment was approved by any regulatory agency in any country. In addition, the present study is the first presenting results with a large population (910 patients) with the longest follow-up, showing remarkable results with pharmacological eye drops for presbyopia management without affecting distance vision.
The last published work regarding pharmacological treatment for presbyopia, as of the time this report was sent to be reviewed, was a prospective consecutive interventional study, performed by Vargas et al., evaluating optical quality and pupil diameter in 117 patients with presbyopia, aged between 41 and 65 years old.27 They were treated with a novel therapy with drops composed by pilocarpine 0.247% phenylephrine 0.78%, polyethylene glycol 0.09%, nepafenac 0,023%, pheniramine 0.034%, and naphazoline 0.003%. The study has a very short follow-up time (2 hours), where the authors found that treatment had a promising effect, improving near vision in 92.3% of patients. Interestingly, they found that the pupil diameter outcomes changed significantly according to the age group, where pupil diameter, was able to change under different light stimulus maintaining a dynamic pupil (dynamic pseudo-accommodation). This phenomenon was not specifically evaluated by our group and it will be interesting to measure with our pharmacological composition. Nevertheless, it is necessary to remark that it is indeed the ciliary muscle contraction that allows us to improve near vision and not the stenopeic effect secondary to the pupil contraction.
Vargas et al. does not assess subjective symptoms secondary to the small pupil size diameter. In our experience, some patients suffer from that, referring to it as a decrease of light perception (dimness), which was worse at night. In our study, it was reported in 26% of cases.27 This symptom was reported at the beginning of treatment and was resolved and not reported after month 12 of follow-up. Patients perceiving that discomfort continued the treatment because they appreciate spectacle independence. Pupil size was not objectively measured, but a pupil size decrease was expected, due to the pharmacological effect of pilocarpine. In addition, all the patients were advised that their pupil size would be decreased while under treatment.
There are still more aspects to resolve (i.e. headaches were described by different groups and this was the second most frequent side-effect found in the present series). It was described as tolerable and patients expressed that it spontaneously resolved 15 to 20 minutes after drops were instilled, secondary to the parasympathetic stimulation. Moreover, headaches were not reported after day 7 of follow-up. Pharmacological interactions with other ophthalmological chronic topical treatments, such as glaucoma, or ocular surface disease (dry eye), must be specifically studied.
Ocular surface disease in this study was not relevant, and burning was presented in 9.3% of patients, which was resolved with the appropriate treatment of the ocular surface disease. Nevertheless, patients tolerated the treatment well and none dropped from the study. Moreover, our group developed and published, in 2018, results from a prospective study, evaluating the ocular surface with the Schirmer test, tear film break up time test, staining evaluation, and cytology impression, suggesting that the proposed pharmacological treatment for presbyopia showed no changes in tear production and even could produce some ocular surface staining amelioration, after 1 year of follow-up.38
Baseline assessment of intraocular pressure was part of the annual routine ophthalmic evaluation. In the presented series, none of the large number of patients developed IOP higher than 21 mm Hg. Scientific evidence presented in this work and the known pharmacological activity from the principal compound used in the eye drops (pilocarpine and diclofenac), should not increase the IOP, it even could decrease it.39 However, it would be interestingly to perform a case control study to evaluate if this treatment in patients with different kinds of glaucoma could have a “neutral or beneficial” interaction related to the aqueous humor drainage.
Finally, new image technologies will improve our knowledge about the accommodation changes, presented across time, during the proposed pharmacological treatment of presbyopia. In addition, it would be of interest to measure the visual field and quality of vision (as aberrometry, contrast sensitivity, and glare evaluations) to determine if this treatment was able to modify it or not. However, all of the patients with the proposed pharmacological treatment for presbyopia have achieved and maintained for the full 8 years an UNVA between Jaeger 1 and 2. The Jaeger values improved between 68.5% and 74.9% for different groups. A slight change occurred between 6 and 8 years of follow-up in the mean UDVA (from 0.01 to 0.03, respectively), with a range at 8 eight years from 0 to 0.10 logMAR (equivalent to 20/20 - 20/25 in Snellen scale). This change could be associated with the normal aging process and not secondary to the effect of the treatment. Moreover, patients still have enough distance vision to avoid spectacles not only for distance, but also for near vision.
Conclusion
Until the present, this work represents the first scientific evidence that presbyopia could be efficiently and safely managed without spectacles or surgeries. It was obtained with a specific pharmacological treatment, instilling drops twice a day, in a group of patients with emmetropia with 8 years of follow-up without relevant side effects. All the patients achieved remarkable improvements of UNVA and UDVA was not clinically affected.
New lines of studies, including potential focus on the emmetropic population, are ongoing by our group in a prospective manner, with a multicentric study being proposed soon, to verify our positive results.
Acknowledgments
Disclosure: G. Benozzi, None; C. Perez, None; J. Leiro, None; S. Facal, None; B. Orman, None
References
- 1. Petrash JM. Aging and age-related diseases of the ocular lens and vitreous body. Invest Ophthalmol Vis Sci. 2013; 54: ORSF54–ORSF59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charman WN. Virtual Issue Editorial: Presbyopia - grappling with an age-old problem. Ophthalmic Physiol Opt. 2017; 37: 655–660. [DOI] [PubMed] [Google Scholar]
- 3. Bourne RRA, Flaxman SR, Braithwaite T, et al.. Vision Loss Expert Group. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: a systematic review and meta-analysis. Lancet Glob Health. 2017; 5: e888–e897. [DOI] [PubMed] [Google Scholar]
- 4. Chan VF, MacKenzie GE, Kassalow J, Gudwin E, Congdon N. Impact of presbyopia and its correction in low- and middle-income countries. Asia Pac J Ophthalmol (Phila). 2018; 7: 370–374. [DOI] [PubMed] [Google Scholar]
- 5. Wolffsohn JS, Davies LN. Presbyopia: effectiveness of correction strategies. Prog Retin Eye Res. 2019; 68: 124–143. [DOI] [PubMed] [Google Scholar]
- 6. Greenwood M, Bafna S, Thompson V. Surgical correction of presbyopia: lenticular, corneal, and scleral approaches. Int Ophthalmol Clin. 2016; 56: 149–, 66. [DOI] [PubMed] [Google Scholar]
- 7. Stojanovic NR, Feingold V, Pallikaris IG. Combined cataract and refractive corneal inlay implantation surgery: comparison of three techniques. J Refract Surg. 2016; 32: 318–, 25. [DOI] [PubMed] [Google Scholar]
- 8. Alio JL, Plaza-Puche AB, Férnandez-Buenaga R, Pikkel J, Maldonado M. Multifocal intraocular lenses: an overview. Surv Ophthalmol. 2017; 62: 611–634. [DOI] [PubMed] [Google Scholar]
- 9. Pallikaris IG, Panagopoulou SI.. PresbyLASIK approach for the correction of presbyopia. Curr Opin Ophthalmol. 2015; 26: 265–272 [DOI] [PubMed] [Google Scholar]
- 10. Stival LR, Figueiredo MN, Santhiago MR. Presbyopic excimer laser ablation: a review. J Refract Surg. 2018; 34: 698–710. [DOI] [PubMed] [Google Scholar]
- 11. Shah DN, Melki S.. Complications of femtosecond-assisted laser in-situ keratomileusis flaps. Semin Ophthalmol. 2014; 29: 363–, 75. [DOI] [PubMed] [Google Scholar]
- 12. Wang J, Ren Y, Liang K, Jiang Z, Tao L, Ambrósio R Jr. Post-LASIK ectasia: twenty years of a conundrum. Semin Ophthalmol. 2019; 34: 66–68. [DOI] [PubMed] [Google Scholar]
- 13. Kamiya K, Takahashi M, Takahashi N, Shoji N, Shimizu K. Monovision by implantation of posterior chamber phakic intraocular lens with a central hole (hole ICL) for early presbyopia. Sci Rep. 2017; 7: 11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bianchi GR. Initial results from a new model of posterior chamber implantable phakic contact lens: IPCL V2.0. Med Hypothesis Discov Innov Ophthalmol. 2019; 8: 57–63. [PMC free article] [PubMed] [Google Scholar]
- 15. Menezo JL, Peris-Martinez C, Cisneros-Lanuza AL, Martinez-Costa R. Rate of cataract formation in 343 highly myopic eyes after implantation of three types of phakic intraocular lenses. J Refract Surg. 2004; 20: 317–324 [DOI] [PubMed] [Google Scholar]
- 16. Fernandes P, Gonzalez-Meijome JM, Madrid-Costa D, Ferrer-Blasco T, Jorge J, Montes-Mico R. Implantable collamer posterior chamber intraocular lenses: a review of potential complications. J Refract Surg. 2011; 27: 765–776. [DOI] [PubMed] [Google Scholar]
- 17. Mastropasqua L, Toto L, Nubile M, Falconio G, Ciancaglini M. Long-term complications of bilateral posterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2004; 30: 901–904. [DOI] [PubMed] [Google Scholar]
- 18. Greenstein S, Pineda R 2nd. The quest for spectacle independence: a comparison of multifocal intraocular lens implants and pseudophakic monovision for patients with presbyopia. Semin Ophthalmol. 2017; 32: 111–115. [DOI] [PubMed] [Google Scholar]
- 19. Alió JL, Grzybowski A, Romaniuk D. Refractive lens exchange in modern practice: when and when not to do it? Eye Vis (Lond). 2014; 1: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Westin O, Koskela T, Behndig A. Epidemiology and outcomes in refractive lens exchange surgery. Acta Ophthalmol. 2015; 93: 41–45. [DOI] [PubMed] [Google Scholar]
- 21. Benozzi J, Benozzi G, Orman B. Presbyopia: a new potential pharmacological treatment. Med Hypothesis Discov Innov Ophthalmol. 2012; 1: 3–5. [PMC free article] [PubMed] [Google Scholar]
- 22. Crawford KS, Garner WH, Burns W. Dioptin™: a novel pharmaceutical formulation for restoration of accommodation in presbyopes. Invest Ophthalmol Vis Sci. 2014; 55: 3765.24939034 [Google Scholar]
- 23. Abdelkader A. Improved presbyopic vision with miotics. Eye Contact Lens. 2015; 41: 323–327. [DOI] [PubMed] [Google Scholar]
- 24. Garner WH, Garner MH. Protein disulfide levels and lens elasticity modulation: applications for presbyopia. Invest Ophthalmol Vis Sci. 2016; 57: 2851–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Renna A, Vejarano LF, De la Cruz E, Alió JL. Pharmacological treatment of presbyopia by novel binocularly instilled eye drops: a pilot study. Ophthalmol Ther. 2016; 5: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsuneyoshi Y, Higuchi A, Negishi K, Tsubota K. Suppression of presbyopia progression with pirenoxine eye drops: experiments on rats and non-blinded, randomized clinical trial of efficacy. Sci Rep. 2017; 7: 6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vargas V, Vejarano F, Alió JL. Near vision improvement with the use of a new topical compound for presbyopia correction: a prospective, consecutive interventional non-comparative clinical study. Ophthalmol Ther. 2019; 8: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003; 22: 640–650. [DOI] [PubMed] [Google Scholar]
- 29. Chylack LT Jr, Wolfe JK, Singer DM, et al.. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. Arch Ophthalmol. 1993; 111: 831–836. [DOI] [PubMed] [Google Scholar]
- 30. Fricke TR, Tahhan N, Resnikoff S, et al.. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018; 125: 1492–1499. [DOI] [PubMed] [Google Scholar]
- 31. Sheeladevi S, Seelam B, Nukella PB, Borah RR, Ali R, Keay L. Prevalence of refractive errors, uncorrected refractive error, and presbyopia in adults in India: a systematic review. Indian J Ophthalmol. 2019; 67: 583–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. Am J Physiol. 1998; 275: R1885–R1897. [DOI] [PubMed] [Google Scholar]
- 33. Wendt M, Glasser A.. Topical and intravenous pilocarpine stimulated accommodation in anesthetized rhesus monkeys. Exp Eye Res. 2010; 90: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Croft MA, Kaufman PL, Erickson-Lamy K, Polansky JR. Accommodation and ciliary muscle muscarinic receptors after echothiophate. Invest Ophthalmol Vis Sci. 1991; 32: 3288–3297. [PubMed] [Google Scholar]
- 35. Ostrin LA, Glasser A.. Effects of pharmacologically manipulated amplitude and starting point on Edinger-Westphal-stimulated accommodative dynamics in rhesus monkeys. Invest Ophthalmol Vis Sci. 2007; 48: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003; 217: 89–98. [DOI] [PubMed] [Google Scholar]
- 37. Renna A, Alio JL, Vejarano LF. Pharmacological treatments of presbyopia: a review of modern perspectives. Eye Vis (Lond). 2017; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Facal S, Leiro J, Gualtieri A, et al.. Ocular surface evaluation in patients treated with pharmacological treatment for presbyopia. Int J Ophthalmic Pathol. 2018; 7: 2. [Google Scholar]
- 39. Barany EH. The mode of action of pilocarpine on outflow resistance in the eye of a primate (Cercopithecus ethiops). Invest Ophthalmol. 1962; 1: 712–727 [PubMed] [Google Scholar]