Abstract
Purpose
Macular Integrity Assessment (MAIA) microperimetry is used widely in clinical trials and routine practice to assess paracentral scotoma. Current interpretation of MAIA is based on an assumed uniform 25 decibel (dB) cutoff for normal function irrespective of subject age and retinal location. We examined this convention by establishing an age- and loci-specific reference in healthy eyes and comparing this to the <25 dB cutoff.
Methods
Retrospective MAIA results from healthy eyes were analyzed for prevalence of loci with <25 dB. At each locus, a new reference cutoff was derived from quantile regression of sensitivity against age at the 2.5th percentile. Two clinical cases of serial MAIA testing were analyzed using the new approach and compared to the <25 dB cutoff.
Results
Fifty-four and 56 age-matched (range: 16–75 years) healthy eyes underwent small (37 loci) and large (68 loci) grid testing, respectively. Retinal sensitivity <25 dB was found in 5% of the small grid (1998 data points) and 10% of the large grid (3808 data points). These were found predominantly in older subjects and at the central point or in the perifoveal region. Quantile regression at each individual locus showed age-related decline with a median gradient of 0.6 dB/decade.
Conclusions
We caution against using <25 dB cutoff in MAIA interpretation and advocate an age- and loci-specific cutoff criterion.
Translational Relevance
Our study suggests that MAIA interpretation is influenced by the criterion used for defining abnormal pointwise measurement.
Keywords: macula, retinal sensitivity, MAIA, microperimetry, cone-rod dystrophy, hydroxychloroquine toxicity
Introduction
Microperimetry, which tracks eye movement in real time and presents the stimuli that compensate for eye movement, allows precise assessment of retinal sensitivities at specific retinal locations. The Macular Integrity Assessment (MAIA; CenterVue, Padova, Italy) is a commercially available microperimeter that has been shown to have a robust coefficient of repeatability,1 reliability, and intersession agreement.2 Hence, MAIA microperimetry has been used for investigating and monitoring paracentral scotoma in retinal clinical trials3–5 and in routine clinical care.6–8
Currently, clinical interpretation of MAIA results relies on the manufacturer's analysis printout. The printout shows the measured retinal sensitivity at each test loci (pointwise sensitivity [PS]) as well as the mean sensitivity (MS) across all test loci. The analysis printout also includes a “histogram of thresholds frequencies”, which shows the distribution of the subject's retinal sensitivities for the test superimposed on a Gaussian distribution of a “normal reference” derived from healthy eyes between 20 and 80 years of age as described in Section 18 of the manufacturer's operating manual. This curve indicates that a PS below 25 decibel (dB) is considered abnormal irrespective of subject or retinal location. The concept of a uniform cutoff in PS has not been supported by the literature. More specifically, it has been shown that, in MAIA, MS declines with age9,10 and PS declines with eccentricity11 in healthy eyes. Furthermore, age- and location-specific reference values in healthy eyes have long been used in conventional visual field examination in routine management of glaucoma and other optic neuropathies.12 Given the rapid increase in popularity of the MAIA device for analyzing macular diseases, we set out to explore the relevance of the uniform 25 dB cutoff in clinical interpretations.
This study investigated the prevalence of abnormal PS values, as defined by <25 dB, at various retinal locations in healthy eyes across a wide age range. We then determined age- and loci-specific cutoff thresholds at each locus using quantile regression in the same cohort of healthy eyes. Finally, two clinical cases illustrate the differences in MAIA PS interpretation between the use of the conventional <25 dB threshold versus the new age- and loci-specific cutoff determined using our cohort.
Methods
This retrospective study was approved by the human research ethics committee of The University of Western Australia (RA/4/20/5454) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each individual for their imaging and MAIA data to be used for research purpose.
Selection of Study Eyes
The MAIA microperimetry database was searched for eligible patients. Inclusion criteria were best-corrected visual acuity of 20/25 or better, normal retina and optic nerve head based on fundus examination, and normal retinal imaging investigations including optical coherence tomography (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany) and fundus autofluorescence (California; Optos plc, Dunfermline, UK) confirmed by the senior author (FKC). Exclusion criteria were history of eye surgery (e.g., cataract extraction, retinal laser), amblyopia, or history of medication use that may potentially affect photoreceptor function (i.e., hydroxychloroquine, antipsychotics, tamoxifen). Some of the individuals were healthy volunteers who were recruited into a previous prospective MAIA microperimetry study, and others were routine clinic patients with unilateral retinal disease. In patients with bilateral eligible eyes, MAIA data from one of two eyes were chosen at random for inclusion into the study. In patients with unilateral eye disease, the nondiseased eye was chosen if eligible. The first MAIA test performed by the patient was used for analysis.
Microperimetry Testing Protocol
All testing was performed with the MAIA microperimeter by trained ophthalmic assistants in a retinal clinic at a single institution. Routine MAIA testing was generally done prior to retinal examination and imaging. However, if testing was performed after eye examination or fundus imaging, the patient had to wait for 10 to 15 minutes in indoor room lighting and was given 2 to 3 minutes to adapt to the completely darkened perimetry room prior to MAIA testing.
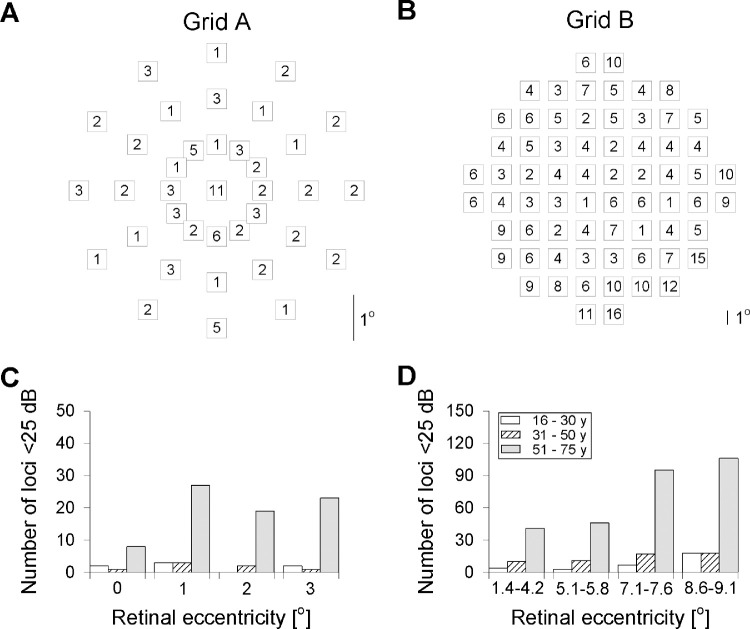
Results from two types of microperimetry grids were used to investigate the age- and loci-specific effect of cutoff thresholds. The smaller test, Grid A, predominantly samples the foveal (circular region of 2.5° radius) and the inner portion of the parafovea (rim between 2.5° and 4° radius). This grid consists of 37 test loci distributed in a radial pattern, sampling retinal locations at 0° and 1°, 2°, and 3° eccentricity from the fovea. The larger test, Grid B, on the other hand, consists of 68 test loci arranged in a square array centered on the fovea. Adjacent test loci are separated by 2° at 1°, 3°, 5°, 7°, and 9° from the vertical or horizontal meridian, similar to the 10-2 testing grid used in the Humphrey visual field. Every retinal location tested in Grids A and B was assigned a unique locus number (Supplementary Fig. S1).
Goldman III achromatic stimuli with stimulus duration of 200 ms were presented on a dim white background (1.27 cd/m2) one at a time. The dynamic range of the differential stimulus luminance is 0.08 to 317.04 cd/m2, corresponding to 36 to 0 dB of PS. Test strategy was 4-2 staircase for both grids. In some subjects, Grids A and B were performed at the same clinic visit, separated by a brief break of 1 to 2 minutes while the subject remained in the darkened room.
Statistical Analysis
Raw PS data were extracted for each study eye from the MAIA hardware with left eye data transformed to the right eye. The Shapiro-Wilk test was conducted to examine the distribution of PS at each test locus and revealed that, in most cases, the distribution was not normal. Hence, quantile regression was used as it is robust against nonparametric data.13,14 At each locus, quantile regression was used to derive the relationship between PS against age at the 2.5th and 97.5th percentiles. The area enclosed by the two lines was the 95% prediction interval.15 Therefore, given the age of a subject, the 2.5th percentile line was used to find the cutoff of normal PS (i.e., above the line) at a locus. All statistical tests were performed using R v.3.5.2.16 Quantile regressions were conducted with quantreg package v5.38 for R.17
Results
Subject Demographics
A total of 110 MAIA tests (21 with only Grid A, 23 with only Grid B, and 33 with both Grids A and B) from 77 healthy eyes of 77 subjects were selected for analysis. In Grid A (N = 54 eyes), the ratio of female to male was 25:29, with a median age of 55 year (range, 16 to 75). The ratio of female to male in Grid B (N = 56 eyes) was 19:37, and the median age was 54 years (range, 16 to 75). Among the 77 subjects, 26 had bilateral normal eye examination, 35 had unilateral retinal disease, and the remaining 16 were from healthy volunteers who had no fellow eye data (Supplementary Table S1). Refractive error information was available in 30 of 54 and 29 of 56 subjects in Grids A and B, respectively. In Grid A, the spherical error range was between −3.00 and +2.75 D, and the highest astigmatism was 4.00 D. In Grid B, spherical error range was between −4.50 and +2.75 D, and the highest astigmatism was +2.75 D.
Frequency of Retinal Sensitivities below 25 dB in Healthy Eyes
A total of 1998 and 3808 measurements from Grids A and B, respectively, were analyzed. In Grid A, 91 of 1998 (5%) PS measurements fell below 25 dB (Fig. 1). Stratified by eccentricity and age, the central loci (0°) in those aged >50 years had the highest frequency of PS <25 dB (25%) while the loci with 2° eccentricity in those aged 16 to 30 years showed the least (0%) frequency of PS <25 dB (Table 1). In Grid B, 386 of 3808 (10%) PS measurements fell below 25 dB (Fig. 1). Stratified by eccentricity and age, peripheral loci (8.6°–9.1°) in those aged >50 years had the highest frequency of PS <25 dB (20%) while PS at 5.1° to 5.8° loci in those aged 16 to 30 were least likely to be <25 dB (Table 2). There was a significant increase in the proportion of loci with PS <25 dB with increasing retinal eccentricity (slope = 1.38, P < 0.05; Supplementary Fig. S2).
Figure 1.
Spatial distribution of the number of pointwise sensitivity (PS) measurements that fell below 25 dB in normal eyes is shown for the small (Grid A) and the large (Grid B) grids. The distribution of misclassified PS values is shown as histograms for each ring of retinal eccentricity and subdivided into three age groups (16 to 30, 31 to 50, and 50 to 75) for small (C) and large (D) grids.
Table 1.
Proportion of Loci below 25 dB (%) in Grid A Stratified by Subject Age and Retinal Eccentricity
| 0° | 1° | 2° | 3° | Total | |
|---|---|---|---|---|---|
| Age, y | 1 Locus | 12 Loci | 12 Loci | 12 Loci | 37 Loci |
| 16–30 (n = 13) |
2/13 (15.4) |
3/156 (1.9) |
0/156 (0) |
2/156 (1.3) |
7/481 (1.5) |
| 31–50 (n = 9) |
1/9 (11.1) |
3/108 (2.8) |
2/108 (1.9) |
1/108 (0.9) |
7/333 (2.1) |
| 51–75 (n = 32) |
8/32 (25.0) |
27/384 (7.0) |
19/384 (4.9) |
23/384 (6.0) |
77/1184 (6.5) |
| Total (N = 54) |
11/54 (20.4) |
33/648 (5.1) |
21/648 (3.2) |
26/648 (4.0) |
91/1998 (4.6) |
Table 2.
Proportion of Loci below 25 dB (%) in Grid B Stratified by Subject Age and Retinal Eccentricity
| 1.4°–4.2° | 5.1°–5.8° | 7.1°–7.6° | 8.6°–9.1° | Total | |
|---|---|---|---|---|---|
| Age, y | 16 loci | 16 loci | 20 loci | 16 loci | 68 loci |
| 16–30 (n = 7) |
4/112 (3.6) |
3/112 (2.7) |
7/140 (5.0) |
18/112 (16.1) | 32/476 (6.7) |
| 31–50 (n = 16) |
10/256 (3.9) |
11/256 (4.3) |
17/320 (5.3) |
18/256 (7.0) |
56/1088 (5.1) |
| 51–75 (n = 33) |
41/528 (7.8) |
46/528 (8.7) |
95/660 (14.4) |
106/528 (20.1) | 288/2244 (12.8) |
| Total (N = 56) |
55/896 (6.1) |
60/896 (6.7) |
119/1120 (10.6) | 142/896 (15.8) | 376/3808 (9.9) |
Loci- and Age-Specific Cutoff Defined by 2.5th Percentile
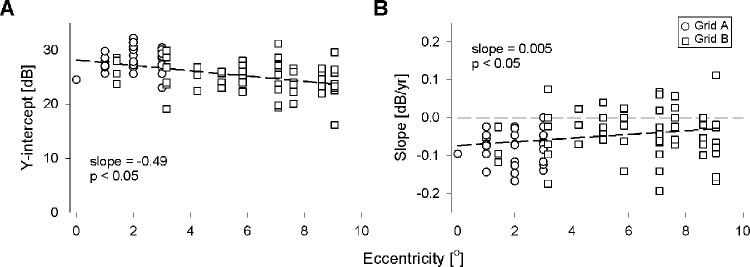
Figure 2 illustrates the derivation of y-intercept and slopes for the lower 2.5th percentile line at four test loci. At each locus, retinal sensitivities from all eyes were plotted against age, and quantile regression was performed at the 97.5th and 2.5th percentiles. Therefore, any data point that fell below the 2.5th percentile (i.e., lower dashed line) was defined as having abnormally low sensitivity for that age.
Figure 2.
Retinal sensitivity versus age in all healthy eyes are shown for locus number A2 at 1° (A) and locus number A26 at 3° of retinal eccentricity (B) in Grid A, as well as locus number B1 at 1.4° (C) and locus number B68 at 9.1° in Grid B. At each locus, regression of the 97.5th (upper dashed line) and 2.5th quantile (lower dashed line) is plotted. Inset shows location of the loci in test grids (filled square).
The slope and y-intercept were calculated for each locus; the median (range) y-intercept and median (range) slope for the lower 2.5th percentile in PS against age for all loci in Grid A were 27.22 (23.00 to 32.17) dB and −0.07 (−0.17 to +0.00) dB/year, respectively (Supplementary Table S2). The median (range) y-intercept and median (range) gradient for the lower 2.5th percentile in PS against age for all loci in Grid B were 24.97 (16.15 to 31.16) dB and −0.03 (−0.19 to +0.11) dB/year, respectively (Supplementary Table S3). The y-intercept showed a significant reduction with eccentricity for all loci tested (slope = −0.49, P < 0.05; Fig. 3). However, there was a less pronounced age-related decline in retinal sensitivity with increased test loci eccentricity (slope = 0.005, P < 0.05; Fig. 3). A graphical representation of the topographical variation in both parameters is also shown in Supplementary Figure S3.
Figure 3.
The y-intercept (A) and slope (B) for all loci plotted against retinal eccentricity. For each panel, the dashed line indicates linear regression, with the slope and the significance (P) of the linear model shown. Circles indicate loci from Grid A, and squares indicate loci from Grid B.
Comparing the <25 dB versus the Age- and Loci-Specific Cutoff in Two Clinical Cases
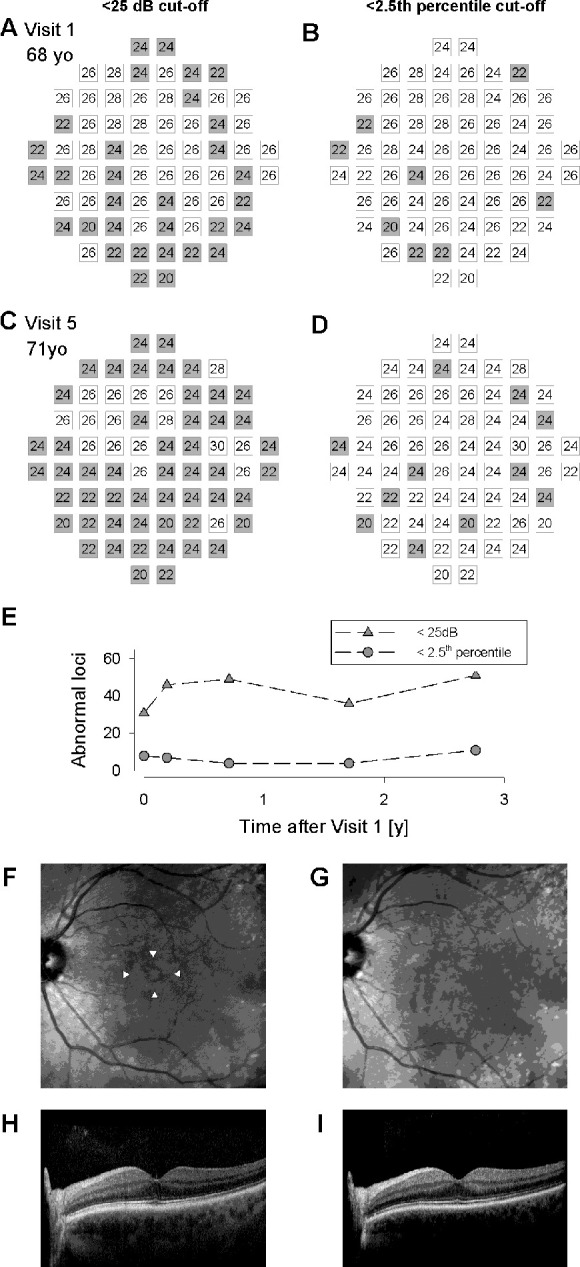
The first example is a 16-year-old girl with rod-cone dystrophy. The study (right) eye had a visual acuity of 6/9.5 at first examination. Five consecutive MAIA tests were performed at 6-monthly intervals using Grid A as part of a prospective natural history study. Only one test locus (1/37, 3%) at the superior portion of the test grid had abnormal PS at baseline by using the <25 dB cutoff criterion. In contrast, 13 test loci (35%) had abnormal PS based on the new age- and loci-specific criterion (Fig. 4). At the final visit, only 10 loci (37%) had abnormal PS using the <25 dB criterion compared to 22 loci (59%) using the new age- and loci-specific criterion. The use of our age- and loci-specific percentile criterion consistently revealed a greater number of abnormal loci across all five testing sessions. Infrared autofluorescence imaging in the same individual revealed a characteristic hyperautofluorescent ring centered on the fovea that had decreased in diameter from the first to the last visit (horizontal extent: 3.4 mm reduced to 3.2 mm). Horizontal optical coherence tomography (OCT) scan through the fovea also showed preservation of the ellipsoid band centrally but decreased horizontal extent between the first and last visits, indicating disease progression.
Figure 4.

Distribution of relative scotoma (gray squares) as defined by the conventional <25 dB cutoff (A, C) or the age- and loci-specific 2.5th percentile cutoff (B, D). The change in the number of loci classified as a relative scotoma over 2 years using either of the two cutoff definitions (E). Filled triangles indicate the use of the conventional <25 dB cutoff while filled circles indicate the use of the new 2.5th percentile cutoff. Infrared autofluorescence images in the same subject at first (F) and last (G) visit with Grid A result overlaid to show that all test loci were within the hyperautofluorescent ring even at the last visit. The horizontal extent of the hyperautofluorescent ring from the first visit is shown underneath the ring and is replicated in G to show the reduction in the horizontal extent of the ring. Horizontal OCT scans at first (H) and last (I) visit.
The second example is a 68-year-old female patient who was undergoing regular screening for hydroxychloroquine toxicity. The patient had already been receiving the medication for 19 years (cumulative dose of 2000 g) when she was first assessed using the MAIA. At the initial visit, visual acuity was 6/7.5 in the study (left) eye with normal pupillary response and normal Ishihara test result. Infrared reflectance imaging showed a faint hyporeflective ring at the parafovea indicating early toxicity despite preservation of the ellipsoid band (Fig. 5). Hydroxychloroquine was ceased and the patient was monitored over the next 3 years. MAIA performed at the first visit showed diffused retinal function abnormality across the entire macula using the conventional <25 dB cutoff (31/68; 46% of loci abnormal). By using the same <25 dB criterion for the most recent MAIA test, one may conclude that there was continued progression of drug toxicity, as suggested by the increasing number of loci with abnormal PS (31/68 increased to 51/68, with 20 additional loci). However, using the age- and loci-specific cutoff, the minor PS defect at the first visit (8/68, 12% loci abnormal) remained relatively stable at the last visit (8/68 increased to 11/68, with only three additional loci). At the latest visit, visual acuity was 6/6 with no changes detected in OCT, and the parafoveal hyperreflective ring in the en face reflectance image had disappeared. Multifocal electroretinography (ERG) performed at the first and last visits showed generally reduced amplitude density in both eyes but no noticeable difference between the two visits (Supplementary Fig. S4).
Figure 5.
Distribution of relative scotoma (gray squares) as defined by the conventional <25 dB cutoff (A, C) or the age- and loci-specific 2.5th percentile cutoff (B, D). The change in the number of loci classified as a relative scotoma over 3 years using either of the two cutoff definitions (E). Filled triangles indicate the use of the conventional <25 dB cutoff while filled circles indicate the use of the new 2.5th percentile cutoff. Infrared reflectance images in the same subject at first (F) and last (G) visit showing disappearance of the hyporeflective ring (marked by white arrowheads). Horizontal OCT scans at first (H) and last (I) visit showed continuous ellipsoid band in the macula at both visits.
Discussion
Approximately 5% (Grid A) to 10% (Grid B) of the MAIA measurements in healthy eyes can be classified as abnormal using the conventional uniform <25 dB cutoff threshold. Increased frequency of misclassification was observed in older subjects, at the foveal center and with an increase in retinal eccentricity. Quantile regression of PS against age at each test locus showed a range of y-intercepts and slopes of the 2.5th percentile lines at a given retinal eccentricity. We illustrated, with two clinical cases, the potential disparity in clinical interpretation of MAIA results when different cutoff definitions are used.
Despite the widespread use of MAIA microperimetry as a clinical tool and endpoint measure, there is still no standardized method of data analysis. The most commonly used method is to examine the mean sensitivity value across the entire grid,7,18–20 with some groups reporting overall median sensitivity instead due to the lack of normal distribution.21,22 Changes in PS have also been analyzed,23 but this suffers from large test-retest variability even in healthy eyes.1–3,24,25 Since neither of these approaches take into account the expected normal values, meaningful analysis and interpretation require comparing microperimetry data between a diseased and a healthy control group.1,8,26–28 However, such an approach is not feasible when evaluating individual patients to determine eligibility for a clinical trial or disease progression on an individual basis. The alternative to using MS or PS is to examine deviation from the expected lower bound of normal range. For example, retinal sensitivity values of <25 dB in one or more loci were used as a selection criterion in a randomized clinical trial of novel laser treatment in age-related macular degeneration.29 In late disease stage, the number of scotoma within the grid was used to assess disease severity.30 We investigated the appropriateness of using a lower bound of 25 dB across all ages and all loci. In our data set, we found that up to 5% to 10% of the PS measurements in healthy individuals could be interpreted as a relative scotoma if a fixed <25 dB cutoff was used, a much higher proportion than the 2.5% as expected from a threshold that is 2 standard deviations below the mean. The percentage of misclassification was particularly high in older individuals and at greater retinal eccentricity. There was also a disproportionally large number of data points in the central locus of Grid A that were <25 dB, which could be attributed to the internal fixation point of the MAIA device interfering with sensitivity measurement.31
To our knowledge, only two studies have reported on a reference database for MAIA microperimetry. One study recruited 237 healthy eyes aged 10 to 70 years (mean = 31) using a different grid pattern from the two that were used in our study and reported MS values across the entire grid instead of PS.9 The other study collected MAIA sensitivity in 237 test loci from 60 subjects aged 19 to 50 years (median = 26) and modeled normative values using spatial extrapolation.32 In comparison to the previous study, our lower 2.5th percentile cutoff was less than the lower 5th percentile for the same retinal locus in a 50-year-old eye. One reason behind this discrepancy may be the difference in the median age of the cohort. Since our cohort was older (median age for Grids A and B was both 54 years) and we took into account age-related decline using quantile regression, it is not surprising that our study estimated lower retinal sensitivities than the previous work. Other studies have also reported MAIA values from healthy eyes as control groups in their studies.4,8,19,25,33 The mean MS reported ranges from 24.9 to 29.5 dB. It is important to note that direct comparing of MS values reported by different groups to our data is not appropriate as the test grids differ. However, three studies used the same test grid across different age groups. 4,8,19 One study reported a mean (SD) MS of 29.2 (1.5) dB in 32 normal eyes with a mean (SD) age of 24.1 (3.1) years. Two other investigations conducted using the same test grid reported mean (SD) MS of 28.7 (1.7) and 27.1 (3.6) dB in participants aged 65.4 (6.6) and 71.7 (7.4) years, respectively, which supports our assumption of age-related decline in MAIA sensitivity. From these observations, we advocate for analysis of MAIA data that consider the age- and loci-specific reference range. Our data suggest that the effect of aging on retinal sensitivity decline may differ across retinal eccentricity. Therefore, our findings have particular importance for studies that select older patients or define trial endpoints based on the number of loci below this <25 dB threshold in a test grid that contains a central foveal stimulus or a large testing grid that samples the perifoveal regions.
In our case of rod-cone dystrophy, overlay of MAIA loci onto retinal imaging showed that only the region within the hyperautofluorescent ring was stimulated by the test grid. Although this region was characterized by intact ellipsoid band, recent data showed reduced cone cell density in this zone using adaptive optics scanning laser ophthalmoscopy.34 Although we did not examine the cone density in our study, the finding of widespread relative scotoma by using an age- and loci- specific cutoff is consistent with previous studies showing structural changes. In the case of the patient with a large cumulative dose of hydroxychloroquine, the use of an age- and loci-specific cutoff leads to the conclusion that there was no progression of suspected toxicity. This is consistent with multimodal imaging analysis and multifocal ERG in this patient and is in keeping with previous reports of stable functional deficit in those with minimal toxicity at the time of drug cessation.35,36 Note that representative cases are shown in the study to demonstrate the difference in clinical interpretation using the age- and location-specific reference than the 25 dB cutoff. A prospective cohort study is necessary to determine the appropriate reference range for determining the ground truth microperimetry classification. This will enable a comprehensive, cohort-based investigation into the sensitivity and specificity of our proposed threshold in normal and diseased cohorts.
Although our results illustrate the importance of establishing an age- and loci-specific reference range and using these cutoff thresholds for individualized disease progression assessment, this study was based on MAIA data collected from a mixed cohort of patients, including some with fellow eye retinal pathology. Therefore, several limitations need be considered. First, the test-retest variability of the data set was not addressed, and this is a key factor pertinent to psychophysical tests such as microperimetry. The MAIA data used for the analysis were based on the subjects’ first and, often, only test result. Further investigation is needed to ascertain whether using data from the first or subsequent visits will affect the establishment of a reference MAIA database given some evidence showing that learning effect can occur between the first and second testing sessions.1,37 Second, as the lowest age in our cohort was only 16 years, our results should not be extrapolated to interpret MAIA results in children. It has been reported that microperimetry sensitivity values in children tend to be lower19; hence, further study is required to establish reference database for young subjects. Similarly, the oldest subject in this study was only 75 years. The data presented here do not cover the group of patients (i.e., >75 years old) who are more likely to develop age-related macular degeneration. Therefore, an older group of control subjects needs to be included in future studies of normative range to generate data that can be used for examining the effect of drusen on retinal function in a geriatric population. Third, the cohort examined was relatively small, with few individuals in each decade, and testing differed between subjects (i.e., some subjects contributed to both grids while some contributed to one grid only). Finally, we assumed a linear decline in PS, but our data suggested that the decline may be more rapid from the age of 50 years. This again requires the inclusion of healthy >75-year-old subjects for validation. Despite these limitations, this is the first report of age- and loci-specific reference PS values across 18° of central retina. To create a ground truth reference database, future study design has to prospectively recruit healthy subjects with no systemic disease and no retinal or optic nerve abnormality based on high-resolution imaging such as OCT angiography and adaptive optics imaging of ganglion and photoreceptor cells. In addition, ocular biometry should also be measured to control for its effect on retinal sensitivity. Retinal function such as multifocal electroretinography should also be measured to ascertain whether the age-related decline in retinal sensitivity is confounded by aging of the brain. To reduce the learning effect, prospective studies should also include a practice session.
In conclusion, our data suggest the limitation of the current approach in analyzing PS based on a fixed <25 dB cutoff. From a cohort of healthy eyes, we estimated the age- and loci-specific lower 2.5th percentile cutoffs for PS at 105 positions in the macula. We showed, using two clinical examples, the efficacy of using an age- and loci-specific criterion defined using our healthy cohorts instead of a uniform 25 dB cutoff in interpreting MAIA results.
Supplementary Material
Acknowledgments
FKC receives funding from the National Health and Medical Research Council (Centre of Research Excellence Grant 116360, Fellowships 1054712, 1142962). DAM is supported by a National Health and Medical Research Council practitioner fellowship (1154518).
Disclosure: J. Charng, None; P.G. Sanfilippo, None; M.S. Attia, None; M. Dolliver, None; S. Arunachalam, None; A.L. Chew, None; E.N. Wong, None; D.A. Mackey, None; F.K. Chen, None
References
- 1. Wong EN, Morgan WH, Chen FK. Intersession test-retest variability of 10-2 MAIA microperimetry in fixation-threatening glaucoma. Clin Ophthalmol. 2017; 11: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molina-Martin A, Pinero DP, Perez-Cambrodi RJ. Reliability and intersession agreement of microperimetric and fixation measurements obtained with a new microperimeter in normal eyes. Curr Eye Res. 2016; 41: 400–409. [DOI] [PubMed] [Google Scholar]
- 3. Chew EY, Clemons TE, Jaffe GJ, et al.. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology. 2019; 126: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cocce KJ, Stinnett SS, Luhmann UFO, et al.. Visual function metrics in early and intermediate dry age-related macular degeneration for use as clinical trial endpoints. Am J Ophthalmol. 2018; 189: 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lam BL, Davis JL, Gregori NZ, et al.. Choroideremia gene therapy phase 2 clinical trial: 24-month results. Am J Ophthalmol. 2019; 197: 65–73. [DOI] [PubMed] [Google Scholar]
- 6. Battu R, Khanna A, Hegde B, Berendschot TT, Grover S, Schouten JS. Correlation of structure and function of the macula in patients with retinitis pigmentosa. Eye (Lond). 2015; 29: 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chew AL, Sampson DM, Chelva E, Khan JC, Chen FK. Perifoveal interdigitation zone loss in hydroxychloroquine toxicity leads to subclinical bull's eye lesion appearance on near-infrared reflectance imaging. Doc Ophthalmol. 2018; 136: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roh M, Lains I, Shin HJ, et al.. Microperimetry in age-related macular degeneration: association with macular morphology assessed by optical coherence tomography. Br J Ophthalmol. 2019; 103: 1769–1776. [DOI] [PubMed] [Google Scholar]
- 9. Molina-Martin A, Pinero DP, Perez-Cambrodi RJ. Normal values for microperimetry with the MAIA microperimeter: sensitivity and fixation analysis in healthy adults and children. Eur J Ophthalmol. 2017; 27: 607–613. [DOI] [PubMed] [Google Scholar]
- 10. Spry PG, Johnson CA.. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001; 78: 436–441. [DOI] [PubMed] [Google Scholar]
- 11. Acton JH, Bartlett NS, Greenstein VC. Comparing the Nidek MP-1 and Humphrey field analyzer in normal subjects. Optometry Vision Sci. 2011; 88: 1288–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987; 105: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 13. Cade BS, Noon BR.. A gentle introduction to quantile regression for ecologists. Front Ecol Environ. 2003; 1: 412–420. [Google Scholar]
- 14. Koenker R. Quantile Regression. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- 15. Koenker R, Machado JAF.. Goodness of fit and related inference processes for quantile regression. J Am Stat Assoc. 1999; 94: 1296–1310. [Google Scholar]
- 16. Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. [Google Scholar]
- 17. Koenker R. quantreg: quantile regression. Available at: http://wwwr-projectorg, Accessed 2019.08.08.
- 18. Hagag AM, Mitsios A, Gill JS, et al.. Characterisation of microvascular abnormalities using OCT angiography in patients with biallelic variants in USH2A and MYO7A. Br J Ophthalmol. 2020; 104: 480–486. [DOI] [PubMed] [Google Scholar]
- 19. Jones PR, Yasoubi N, Nardini M, Rubin GS. Feasibility of Macular Integrity Assessment (MAIA) microperimetry in children: sensitivity, reliability, and fixation stability in healthy observers. Invest Ophthalmol Vis Sci. 2016; 57: 6349–6359. [DOI] [PubMed] [Google Scholar]
- 20. Welker SG, Pfau M, Heinemann M, Schmitz-Valckenberg S, Holz FG, Finger RP. Retest reliability of mesopic and dark-adapted microperimetry in patients with intermediate age-related macular degeneration and age-matched controls. Invest Ophthalmol Vis Sci. 2018; 59: AMD152–AMD159. [DOI] [PubMed] [Google Scholar]
- 21. Denniss J, Baggaley HC, Brown GM, Rubin GS, Astle AT. Properties of visual field defects around the monocular preferred retinal locus in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 2652–2658. [DOI] [PubMed] [Google Scholar]
- 22. Steinberg JS, Sassmannshausen M, Pfau M, et al.. Evaluation of two systems for fundus-controlled scotopic and mesopic perimetry in eye with age-related macular degeneration. Transl Vis Sci Technol. 2017; 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Han RC, Jolly JK, Xue K, MacLaren RE. Effects of pupil dilation on MAIA microperimetry. Clin Exp Ophthalmol. 2017; 45: 489–495. [DOI] [PubMed] [Google Scholar]
- 24. Dimopoulos IS, Tseng C, MacDonald IM. Microperimetry as an outcome measure in choroideremia trials: reproducibility and beyond. Invest Ophthalmol Vis Sci. 2016; 57: 4151–4161. [DOI] [PubMed] [Google Scholar]
- 25. Pfau M, Lindner M, Fleckenstein M, et al.. Test-retest reliability of scotopic and mesopic fundus-controlled perimetry using a modified MAIA (Macular Integrity Assessment) in normal eyes. Ophthalmologica. 2017; 237: 42–54. [DOI] [PubMed] [Google Scholar]
- 26. Parodi MB, Triolo G, Morales M, et al.. Mp1 and Maia fundus perimetry in healthy subjects and patients affected by retinal dystrophies. Retina. 2015; 35: 1662–1669. [DOI] [PubMed] [Google Scholar]
- 27. Sato S, Hirooka K, Baba T, Tenkumo K, Nitta E, Shiraga F. Correlation between the ganglion cell-inner plexiform layer thickness measured with cirrus HD-OCT and macular visual field sensitivity measured with microperimetry. Invest Ophthalmol Vis Sci. 2013; 54: 3046–3051. [DOI] [PubMed] [Google Scholar]
- 28. Vujosevic S, Smolek MK, Lebow KA, Notaroberto N, Pallikaris A, Casciano M. Detection of macular function changes in early (AREDS 2) and intermediate (AREDS 3) age-related macular degeneration. Ophthalmologica. 2011; 225: 155–160. [DOI] [PubMed] [Google Scholar]
- 29. Lek JJ, Brassington KH, Luu CD, et al.. Subthreshold nanosecond laser intervention in intermediate age-related macular degeneration: study design and baseline characteristics of the laser in early stages of age-related macular degeneration study (report number 1). Ophthalmol Retina. 2017; 1: 227–239. [DOI] [PubMed] [Google Scholar]
- 30. Vujosevic S, Pucci P, Casciano M, et al.. Long-term longitudinal modifications in mesopic microperimetry in early and intermediate age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2017; 255: 301–309. [DOI] [PubMed] [Google Scholar]
- 31. Denniss J, Astle AT.. Central perimetric sensitivity estimates are directly influenced by the fixation target. Ophthalmic Physiol Opt. 2016; 36: 453–458. [DOI] [PubMed] [Google Scholar]
- 32. Denniss J, Astle AT.. Spatial interpolation enables normative data comparison in gaze-contingent microperimetry. Invest Ophthalmol Vis Sci. 2016; 57: 5449–5456. [DOI] [PubMed] [Google Scholar]
- 33. Cassels NK, Wild JM, Margrain TH, et al.. Microperimetry in age-related macular degeneration: an evidence-base for pattern deviation probability analysis in microperimetry. Transl Vis Sci Technol. 2019; 8: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakatake S, Murakami Y, Funatsu J, et al.. Early detection of cone photoreceptor cell loss in retinitis pigmentosa using adaptive optics scanning laser ophthalmoscopy. Graefes Arch Clin Exp Ophthalmol. 2019; 257: 1169–1181. [DOI] [PubMed] [Google Scholar]
- 35. Marmor MF, Hu J.. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014; 132: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 36. Mititelu M, Wong BJ, Brenner M, Bryar PJ, Jampol LM, Fawzi AA. Progression of hydroxychloroquine toxic effects after drug therapy cessation: new evidence from multimodal imaging. JAMA Ophthalmol. 2013; 131: 1187–1197. [DOI] [PubMed] [Google Scholar]
- 37. Wu Z, Ayton LN, Guymer RH, Luu CD. Intrasession test-retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013; 54: 7378–7385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.