Abstract
Purpose
The purpose of this study was to investigate the relationship between the lamina cribrosa (LC) thickness (LCT) as assessed using enhanced depth-imaging (EDI) optical coherence tomography (OCT) and cognitive function in primary open-angle glaucoma (POAG).
Methods
The study consisted of 105 POAG eyes and 23 nonglaucomatous control eyes that completed neuropsychological tests. The optic nerve heads of the patients were imaged using EDI-OCT. B-scan images were constructed in three dimensions using maximum intensity projection (MIP), and the LCT was measured using the thin-slab MIP images. A comprehensive battery consisting of 15 neuropsychological tests was used to evaluate cognitive function.
Results
POAG eyes had smaller mean LCT as compared with control eyes (P < 0.001). Age and Mini Mental State Examination (MMSE) scores did not differ between the two groups. Linear regression analysis revealed that lower scores on the MMSE (P < 0.001), presence of glaucoma (P = 0.006), and a smaller global retinal nerve fiber layer thickness (P < 0.001) were independently associated with a smaller mean LCT. Davies’ test revealed a statistically significant breakpoint for the mean LCT (221.14 µm), below which a smaller MMSE score was significantly associated with a smaller mean LCT. In POAG eyes with a mean LCT smaller than the breakpoint (< 221.14 µm), not only the global cognition but also the visuospatial function and visual memory were worse than in those with a larger mean LCT (all P ≤ 0.003).
Conclusions
Impairment of cognitive function was observed in patients with POAG with a thinner LC. The role of LC imaging as a potential biomarker to monitor cognitive impairment needs further investigation.
Translational Relevance
LC thinning may reflect a shared mechanism of neurodegenerative diseases in the brain and optic nerve.
Keywords: lamina cribrosa thickness, cognitive impairment, primary open-angle glaucoma
Introduction
The possibility of a link between Alzheimer's disease (AD) and glaucoma has long been suggested. AD is a progressive neurodegenerative disorder of the brain white matter characterized by impairment of cognitive function secondary to neuronal cell loss.1,2 On the other hand, glaucoma is a progressive optic nerve degeneration accompanied by impaired visual function secondary to retinal ganglion cell (RGC) loss.3 It has been suggested that AD and glaucoma share several clinical characteristics; the incidence and severity of both conditions increase with age,4 and the prevalence of glaucoma is higher in patients with AD than those without.5 Loss of the superior and the inferior retinal nerve fiber layer (RNFL), which is one of the key features of glaucomatous optic nerve damage, has also been documented in patients with AD.3,6,7 Histological studies also have demonstrated a selective degeneration of the RGCs in the eyes of patients with AD, with characteristics similar to those in glaucomatous optic neuropathy.3,8–10 Given the similarity between AD and glaucoma, various overlapping pathogenic mechanisms have been suggested for the two diseases, including ischemia,11,12 neurotoxicity,13,14 and low cerebrospinal fluid (CSF) pressure.15,16
The lamina cribrosa (LC) is a connective tissue that lies within the deep optic nerve head (ONH) and structurally and nutritionally supports the RGC axons. Thinning of the LC has been implicated in the glaucomatous RGC damage.17,18 The RGC axons passing through a thinner LC are likely to have disturbed axoplasmic flow caused by an increased pressure gradient between the prelaminar (intraocular) and retrolaminar spaces. The disturbance of axoplasmic flow may in turn cause RGC apoptosis, finally resulting in an irreversible deterioration of visual function.
Our group recently found that a higher level of CSF tau, which is an early indicator of axonal degeneration and cognitive decline,19 was associated with a thinner LC in both healthy subjects and patients with AD. The findings indicated that an abnormal profile of CSF protein may be responsible not only for the neurodegeneration of the brain, but also for the thinning of the LC, which may be linked to increased susceptibility to glaucomatous optic nerve damage. This led to a new hypothesis for the common shared mechanism between AD and glaucoma: that both diseases are associated with the LC degeneration mediated by the abnormal profile of CSF protein. In addition, the LC thickness (LCT) could serve as a biomarker for neurodegeneration of the eye and brain simultaneously.
In the present study, we tested the hypothesis that degenerative changes in the optic nerve, including the LC, are associated with the neuronal loss in the brain that manifests as cognitive deficit. This was achieved by determining the relationship between cognitive function and the LCT in primary open-angle glaucoma (POAG).
Materials and Methods
This study was based on the Study for Elucidating Pathogenesis of Glaucoma with Aging by Assessment of Cognitive Function, which is a prospective study of patients with glaucoma performed at the Glaucoma Clinic of Seoul National University Bundang Hospital in collaboration with the Dementia Clinic of Seoul National University Bundang Hospital. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital. Informed written consent was obtained from all participants or their legally acceptable representatives in accordance with the Declaration of Helsinki.
Study Subjects
A database of patients with POAG included in the Study for Elucidating Pathogenesis of Glaucoma with Aging by Assessment of Cognitive Function between May 2012 and November 2015 was reviewed. The subjects were consecutively recruited to participate in the study. A database of control subjects without glaucoma was based on the separate study (Korean Longitudinal Study on Cognitive Aging and Dementia),20 which is a prospective study including patients with mild cognitive impairment.
POAG was defined as the presence of an open iridocorneal angle, glaucomatous optic nerve damage (i.e. the presence of focal neuroretinal rim thinning or notching, or an RNFL defect), and glaucomatous visual field defects without other ocular diseases or conditions that might cause abnormalities of the visual field. A glaucomatous visual field defect was defined as follows: (1) outside the normal limits on the glaucoma hemifield test; (2) three abnormal points, with a probability of being normal of P < 5%, and one abnormal point with a pattern deviation of P < 1%; or (3) a pattern SD of P < 5%. These visual field defects were confirmed on two consecutive reliable tests (fixation loss rate of ≤ 20% and false-positive and false-negative error rates of ≤ 25%).
The nonglaucomatous control subjects had an IOP of ≤ 21mm Hg with no history of increased IOP, an absence of a glaucomatous disc appearance, and a normal circumpapillary RNFL thickness profile on optical coherence tomography (OCT). Absence of a glaucomatous disc appearance was defined as an intact neuroretinal rim without disc hemorrhage, notches, or localized pallor.
The subjects underwent a complete ophthalmic examination, including visual acuity assessment, refraction, slit-lamp biomicroscopy, gonioscopy, Goldmann applanation tonometry, and dilated stereoscopic examination of the optic disc. They also underwent measurements of the central corneal thickness (Orbscan II; Bausch & Lomb Surgical, Rochester, NY) and axial length (IOL Master version 5; Carl Zeiss Meditec, Dublin, CA), stereo disc photography (EOS D60 digital camera; Canon, Utsunomiya-shi, Tochigi-ken, Japan), enhanced depth-imaging (EDI) volume scanning of the optic disc, and measurement of the circumpapillary RNFL thickness using spectral-domain OCT (Spectralis OCT; Heidelberg Engineering, Heidelberg, Germany), as well as standard automated perimetry (24-2 Swedish interactive threshold algorithm and Humphrey Field Analyzer II 750; Carl Zeiss Meditec).
To be included in the present study, subjects were required to have a best-corrected visual acuity of at least 20/40, a spherical refraction of –8.0 to +4.0 diopters, and a cylinder correction of –3.0 to +3.0 diopters. Those with a history of ocular surgery other than cataract extraction and glaucoma surgery, a history of ocular trauma or uveitis, or a history of other intraocular diseases (e.g. diabetic retinopathy or retinal vessel occlusion) or neurological diseases (e.g. pituitary tumor) that could cause visual field defects were excluded. Subject with dementia diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) diagnostic criteria21 were excluded. Dementia diagnosis was performed independently of the results of cognitive function tests in the current study. Any subjects who had been diagnosed with other neurological or psychiatric disorders that may significantly affect cognitive function, those with a history of alcohol abuse, or other severe medical conditions that may affect cognition were also excluded. When both eyes of a subject were eligible for the study, one of the eyes was selected randomly.
Enhanced Depth-Imaging Spectral-Domain OCT of the Optic Nerve Head
The ONH was imaged using Spectralis OCT with the EDI technique. The details of the protocol for scanning the optic nerve using EDI OCT to evaluate the LC are described elsewhere.22,23 In brief, approximately 75 horizontal and vertical B-scan images covering the optic disc and separated by 30 to 34 µm (the scan-line distance was determined automatically by the instrument) were obtained for each eye. For each section, 42 OCT frames were averaged, which provided the best trade-off between image quality and patient cooperation.23
Measurement of Lamina Cribrosa Thickness
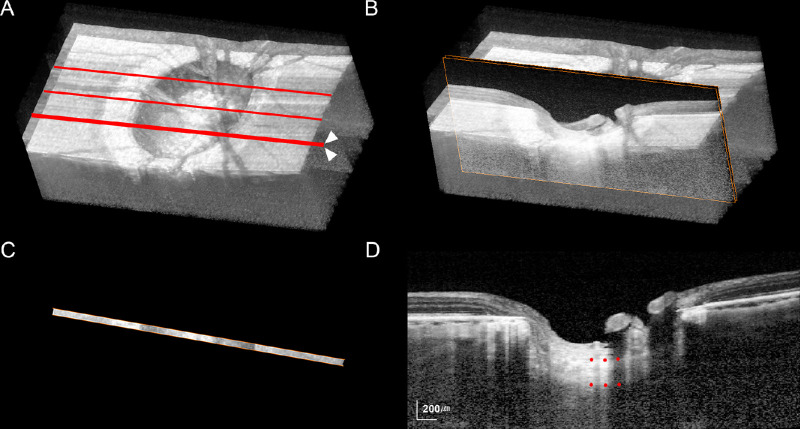
The LCT was measured in three planes in each eye (mid-horizontal, superior midperiphery, and inferior midperiphery) using thin-slab maximum intensity projection (MIP) images. Thin-slab MIP images were used because they allow a clearer detection of the posterior LC border.24 The technique of generating thin-slab MIP images is described in detail elsewhere.24 In brief, three-dimensional volumetric reconstruction of the ONH was performed from the B-scan images by MIP rendering using image-processing software (Amira 5.2.2; Visage Imaging, Berlin, Germany) (Fig. 1A). The thin-slab image was then obtained by selecting two planes (approximately 64 µm apart) inside the three-dimensional volumetric image data (Fig. 1B); only the data within these two planes were displayed in the thin-slab image (Fig. 1C). The LCT was measured as the distance between the anterior and posterior borders at the central three points (mutually separated by 100 µm) in each MIP thin-slab image along an axis perpendicular to the anterior LC surface at the measurement point (Fig. 1D). The measurements obtained from the three thin-slab images were used to calculate the mean LCT of the eye.
Figure 1.
Measurement of the lamina cribrosa (LC) thickness (LCT). (A, B) Maximum intensity projection (MIP) volumetric images constructed from B-scan images obtained using spectral-domain optical coherence tomography. (A) Red lines indicate the three locations where the thin volumetric sections were obtained. (B) Orange squares indicate the three B-scans that were selected to construct the thin-slab MIP image at the inferior midperiphery (arrowheads in A). (C, D) Thin-slab MIP images obtained from the three B-scans shown in B. The anterior and posterior borders of the LC were defined as the plane that best represented the margin of the hyper-reflective plate. The LCT was determined by measuring the distance between the anterior and posterior borders of the LC at the central three locations (red dots in D).
The LCT was measured using the manual caliper tool of the Amira 5.2.2 software by two observers (S.H.L. and E.J.L.) who were masked to the clinical information for the subject. All measurements were repeated three times each by the two observers, and the average of the six values was used for the main analysis.
Neuropsychological Assessments
Research neuropsychologists administered the Korean versions of the Consortium to Establish a Registry for Alzheimer's Disease Neuropsychological Assessment Battery (CERAD-K-N),25,26 the Benton Visual Retention Test (BVRT),27 and the Digit Span Test Forward (DST-F) and the Digit Span Test Backward (DST-B)28 to all subjects. The CERAD-K-N consists of nine neuropsychological tests, including the Categorical Fluency Test (CFT), the Modified Boston Naming Test (mBNT), the Mini Mental State Examination (MMSE), the Word List Memory Test (WLMT), the Constructional Praxis test (CPT), the Word List Recall Test (WLRT), the Word List Recognition Test (WLRcT), the Constructional Recall Test (CRT), and the Trail Making Test A and B (TMT-A and TMT-B). We evaluated depressive symptoms using the Korean version of the Geriatric Depression Scale (GDS).29 All neuropsychological assessments were performed within 1 week of obtaining the OCT scans.
Statistical Analysis
The interobserver and intraobserver agreements for the measurement of LCT were assessed by calculating the intraclass correlation coefficients (ICCs) and their confidence intervals (CIs). Linear regression analysis was used to investigate the factors influencing the LCT, first with a univariate model and then with a multivariate model that included variables from the univariate model with P < 0.10. Between-group comparisons were performed using Student's t-test for continuous variables and the χ2 test for categorical variables. General linear models (analysis of covariance and analysis of variance after adjustment for age, gender, and education) were applied to compare neuropsychological test scores between groups. Bonferroni's correction was applied to the raw data for the t test based on the number of comparisons within each analysis.
All of the statistical analyses were performed using the Statistical Package for the Social Sciences (version 22.0; SPSS, Chicago, IL). Except where stated otherwise, the data are presented as mean ± SD values, and a P value < 0.05 was accepted as significant.
Results
Baseline Characteristics
This study initially enrolled 109 subjects with POAG and 25 nonglaucomatous control eyes. Of these, 6 subjects (4 POAG and 2 control eyes) were excluded because of poor B-scan quality that did not allow delineation of the LC borders, yielding a final sample of 105 patients with POAG and 23 control subjects.
The clinical characteristics of the participants are summarized in Table 1. The POAG group had longer duration of education (P = 0.011), higher IOP (P < 0.001), and thinner global RNFL (P < 0.001) than the control group. The mean LCT and LCTs at all three locations were thinner in the POAG group as compared with the control group (P ≤ 0.004). In the POAG group, the mean LCT was 225.3 ± 27.4 µm (range, 162.3–302.7 µm). The LC was significantly thicker in the mid-horizontal plane (238.4 ± 27.6 µm) than in the superior midperipheral plane (218.1 ± 33.6 µm; P < 0.001) and the inferior midperipheral plane (219.6 ± 33.6 µm; P < 0.001). The intraobserver and interobserver ICCs for LCT measurements were 0.997 (95% CI, 0.987–0.999) and 0.963 (95% CI, 0.951–0.975), respectively.
Table 1.
Clinical Characteristics of Participants
| Variables | POAG Group (n = 105) | Control Group (n = 23) | P Value |
|---|---|---|---|
| Age, years | 72.2 ± 5.7 (60–93) | 74.9 ± 7.2 (68–92) | 0.097 |
| Gender, male/female | 48/57 | 7/16 | 0.133 |
| Education, years | 13.5 ± 3.4 (3–20) | 10.0 ± 6.0 (1–18) | 0.011 |
| Spherical equivalent, D | –0.29 ± 2.43 (–7.88 to 4.00) | n/a | n/a |
| Axial length, mm | 23.69 ± 0.89 (22.14–26.80) | 23.33 ± 1.41 (21.15–27.64) | 0.309 |
| Central corneal thickness, µm | 558.8 ± 30.3 (493–674) | n/a | n/a |
| IOP at exam, mm Hg | 15.2 ± 4.0 (9–37) | 10.8 ± 2.7 (8–17) | < 0.001 |
| VF MD, dB | –6.77 ± 6.41 (–27.97 to 1.42) | n/a | n/a |
| VF PSD, dB | 5.60 ± 4.02 (1.30–14.89) | n/a | n/a |
| Global RNFL thickness, µm | 75.7 ± 15.1 (34–111) | 93.5 ± 9.3 (80–113) | < 0.001 |
| Lamina cribrosa thickness, µm | |||
| Superior midperipheral | 218.1 ± 33.6 (129–314) | 241.2 ± 27.6 (185–312) | 0.003 |
| Mid-horizontal | 238.4 ± 27.6 (177–320) | 257.4 ± 28.5 (201–315) | 0.004 |
| Inferior midperipheral | 219.6 ± 33.6 (111–306) | 246.9 ± 33.7 (189–318) | 0.001 |
| Mean | 225.3 ± 27.4 (162.3–302.7) | 248.5 ± 26.8 (202.3–302.6) | < 0.001 |
POAG = primary open-angle glaucoma; D = diopter; IOP = intraocular pressure; VF = visual field; MD = mean deviation; dB = decibel; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer.
Data are mean ± SD (range) unless otherwise indicated.
The detailed results of neuropsychological tests are summarized in Table 2.
Table 2.
Summary of Cognitive Function Scores
| Cognitive Function Scores | |||
|---|---|---|---|
| Cognitive Function Tests | POAG Group (n = 105) | Control Group (n = 23) | P Value |
| Global cognition | |||
| MMSE | 26.8 ± 2.3 (19–30) | 26.4 ± 2.1 (24–30) | 0.410 |
| Attention/concentration | |||
| DST-F | 5.9 ± 1.5 (3–9) | n/a | n/a |
| DST-B | 3.7 ± 1.4 (0–7) | n/a | n/a |
| Language | |||
| CFT | 14.7 ± 4.2 (5–24) | 18.4 ± 4.1 (11–31) | 0.010 |
| mBNT | 11.7 ± 2.3 (6–15) | 11.0 ± 2.2 (6–13) | 0.216 |
| Visuospatial function | |||
| CPT | 10.2 ± 1.6 (4–11) | 9.4 ± 1.8 (7–11) | 0.049 |
| BVRT copy | 9.3 ± 1.2 (3–10) | n/a | n/a |
| Verbal memory | |||
| WLMT | 16.2 ± 4.0 (7–24) | 16.9 ± 3.9 (9–22) | 0.443 |
| WLRT | 5.2 ± 1.8 (1–9) | 5.8 ± 2.2 (3–10) | 0.155 |
| WLRcT | 8.2 ± 1.6 (4–10) | 9.1 ± 1.4 (6–10) | 0.033 |
| Visual memory | |||
| CRT | 6.3 ± 3.0 (0–11) | 7.5 ± 2.4 (3–11) | 0.087 |
| BVRT recall | 5.3 ± 2.1 (1–10) | n/a | n/a |
| Frontal function | |||
| TMT-A | 70.4 ± 53.2 (24–354) | 69.4 ± 59.2 (25–250) | 0.956 |
| TMT-B | 193.4 ± 104.0 (48–360) | 198.0 ± 93.9 (77–360) | 0.846 |
| Depression | |||
| GDS-KR | 10.4 ± 6.7 (1–27) | n/a | n/a |
POAG = primary open-angle glaucoma; MMSE = mini-mental state examination; DST-F = digit span test forward; DST-B = digit span test backward; CFT = categorical fluency test; mBNT = modified Boston naming test; CPT = constructional praxis test; BVRT = Benton visual retention test; WLMT = word list memory test; WLRT = word list recall test; WLRcT = word list recognition test; CRT = constructional praxis recall test; TMT = trail making test; GDS-KR = revised Korean version of geriatric depression scale.
Data are mean ± SD (range) unless otherwise indicated.
Factors Associated With Lamina Cribrosa Thickness
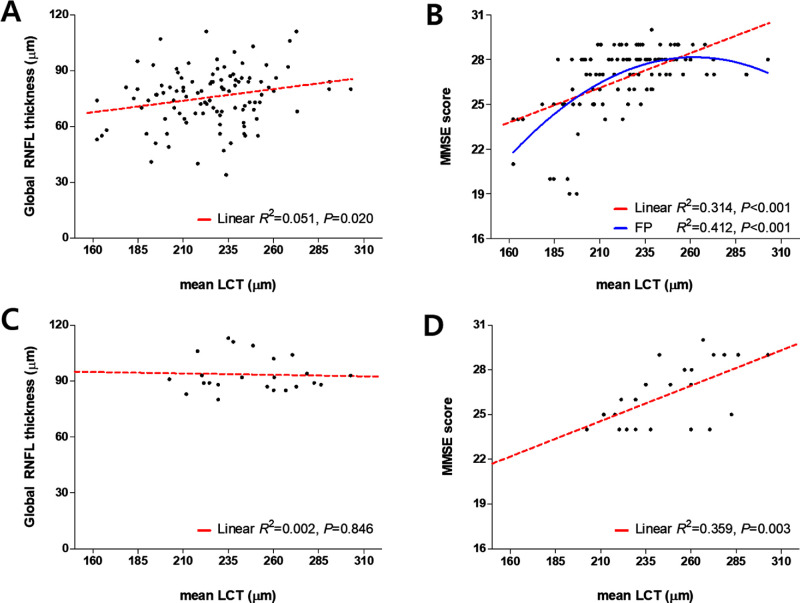
Factors associated with the mean LCT were assessed in the whole study subjects including both POAG (n = 105) and control (n = 23) groups (Table 3). In univariate analysis, a worse visual field mean deviation (MD; P = 0.044), a smaller global RNFL thickness (P = 0.001), presence of glaucoma (P < 0.001), lower scores on the MMSE (P < 0.001), DST-B (P < 0.001), CFT (P = 0.010), mBNT (P = 0.001), CPT (P = 0.014), BVRT copy (P < 0.001), WLMT (P = 0.001), WLRT (P < 0.001), CRT (P = 0.003), and BVRT recall (P < 0.001), and higher scores on the TMT-A (P < 0.001) and TMT-B (P < 0.001) were associated with a smaller measured LCT (Table 3). The multivariate analysis revealed that a smaller global RNFL thickness (P < 0.001), presence of glaucoma (P = 0.006), and a lower score on the MMSE (P < 0.001) were associated with a thinner LC (Table 3). Figure 2 shows the relationships between the LCT and the global RNFL thickness (Figs. 2A, 2C) and MMSE score (Figs. 2B, 2D) with the LCT set as an independent variable in the POAG (see Figs. 2A, 2B) and control (see Fig. 2C, 2D) groups. Despite of the difference in the LCT between groups, which was significantly smaller in the POAG group (Table 1), the trend of cognitive impairment being associated with smaller LCT was observed in both groups. Although the LCT in the POAG group was significantly related to the MMSE score when assessed using a linear model, the associations appeared to be nonlinear in the scattergram (see Fig. 2B), and so fractional polynomial analysis was performed using STATA (version 10.0; StataCorp, College Station, TX) to investigate whether the relationship was explained better by a nonlinear model.30 This analysis revealed that a fractional polynomial model was better than a linear model for explaining the relationship of the LCT with the MMSE score (R2 = 0.412 vs. 0.314 P = 0.001; see Fig. 2D).
Table 3.
Demographic Factors, Ocular Factors and Cognitive Function Test Scores Associated With the Lamina Cribrosa Thickness in POAG (n = 105) and Control (n = 23) Groups
| Univariate Analysis | Multivariate Analysisa | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Βeta | 95% CI | P Value | Βeta | 95% CI | P Value | VIF |
| Demographic characteristics | |||||||
| Age, for each year older | –0.454 | −1.284 to 0.377 | 0.282 | ||||
| Gender, male | 3.374 | −6.763 to 13.511 | 0.511 | ||||
| Ocular characteristics | |||||||
| Spherical equivalentb per 1 D increase | –1.615 | –3.797 to 0.566 | 0.145 | ||||
| Axial length, per 1 mm increase | 3.931 | −1.061 to 8.923 | 0.122 | ||||
| Central corneal thickness,b per 1 µm increase | 0.047 | –0.130 to 0.223 | 0.601 | ||||
| Baseline IOP, per 1 mm Hg increase | −0.796 | −2.032 to 0.441 | 0.205 | ||||
| MDb per 1 dB increase | 0.844 | 0.025 to 1.663 | 0.044 | 0.030 | −0.919 to 0.979 | 0.950 | 2.198 |
| PSD,b per 1 dB increase | –0.578 | –1.905 to 0.749 | 0.390 | ||||
| Global RNFL thickness, per 1 µm increase | 0.548 | 0.243 to 0.854 | 0.001 | 0.649 | 0.384 to 0.913 | < 0.001 | 1.299 |
| Presence of glaucoma | −23.190 | −35.628 to −10.752 | < 0.001 | −15.006 | −25.531 to −4.482 | 0.006 | 1.235 |
| Cognitive Function Tests, per 1 point increase | |||||||
| MMSE | 6.549 | 4.622 to 8.477 | < 0.001 | 7.801 | 6.128 to 9.473 | < 0.001 | 1.059 |
| DST-Fb | 0.151 | –3.482 to 3.784 | 0.935 | ||||
| DST-Bb | 7.400 | 3.713 to 11.087 | < 0.001 | −0.657 | −5.241 to 3.928 | 0.777 | 2.332 |
| CFT | 1.368 | 0.339 to 2.396 | 0.010 | −0.920 | −2.089 to 0.249 | 0.122 | 1.496 |
| mBNT | 3.599 | 1.468 to 5.731 | 0.001 | −0.701 | −3.096 to 1.695 | 0.563 | 1.891 |
| CPT | 3.735 | 0.771 to 6.699 | 0.014 | −1.836 | −5.028 to 1.356 | 0.256 | 1.647 |
| BVRT copyb | 9.018 | 4.840 to 13.196 | < 0.001 | 2.038 | −2.587 to 6.664 | 0.384 | 1.893 |
| WLMT | 2.056 | 0.834 to 3.277 | 0.001 | 0.416 | −1.262 to 2.094 | 0.624 | 2.752 |
| WLRT | 4.667 | 2.156 to 7.177 | < 0.001 | 0.264 | −3.600 to 4.128 | 0.892 | 2.972 |
| WLRcT | 2.476 | −0.764 to 5.716 | 0.133 | ||||
| CRT | 2.534 | 0.869 to 4.199 | 0.003 | −0.305 | −2.056 to 1.445 | 0.730 | 1.696 |
| BVRT recallb | 5.098 | 2.806 to 7.390 | < 0.001 | 1.551 | −1.226 to 4.327 | 0.270 | 2.245 |
| TMT-A | −0.177 | −0.265 to −0.089 | < 0.001 | −0.023 | −0.137 to 0.091 | 0.688 | 2.286 |
| TMT-B | −0.090 | −0.137 to −0.043 | < 0.001 | −0.024 | −0.069 to 0.021 | 0.295 | 1.384 |
POAG = primary open-angle glaucoma; CI = confidence interval; VIF = variance inflation factor; D = diopter; IOP = intraocular pressure; MD = mean deviation; dB = decibel, PSD = pattern standard deviation; RNFL = retinal nerve fiber layer, MMSE = mini-mental state examination; DST-F = digit span test forward; DST-B = digit span test backward; CFT = categorical fluency test; mBNT = modified Boston naming test; CPT = constructional praxis test; BVRT = Benton visual retention test; WLMT = word list memory test; WLRT = word list recall test; WLRcT = word list recognition test; CRT = constructional praxis recall test; TMT = trail making test.
Variables with P < 0.1 in the univariate analysis were included in the multivariate model.
These variables were not available for control group.
Values with statistical significance are shown in boldface.
Figure 2.
Scattergrams showing the relationships of the LCT with the ocular factor and cognitive function test. (A, C) Global retinal nerve fiber layer (RNFL) thickness, and the scores on (B, D) the Mini Mental State Examination (MMSE) in the primary open-angle glaucoma (POAG) A, B and control C, D groups. Red dashed and blue solid lines were obtained using linear regression and fractional polynomial (FP) models, respectively. The best-fitting FP model for the relationship between the MMSE score and mean LCT was described by y = 9.021 + 0.000847 x2 – 0.000002163 x3.
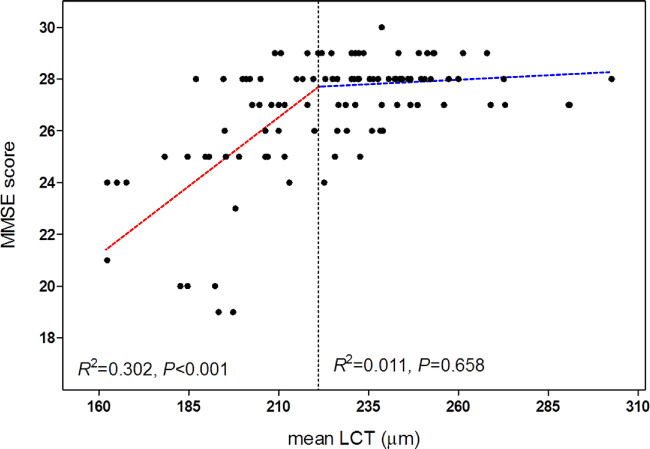
Davies’ test was then used to investigate whether there was a statistically significant breakpoint for the LCT that would better explain its relationship with the MMSE score in the POAG eyes.31 This test was implemented using the R statistical language (version 3.3.1; available from http://www.R-project.org, accessed October 20, 2016). This analysis identified 221.14 µm as a significant breakpoint (R2 = 0.420; P < 0.001; Fig. 3). The slope for eyes with an LCT larger than this breakpoint was not significant (R2 = 0.011; P = 0.658), whereas in eyes with an LCT less than this breakpoint, the LCT was significantly smaller for lower MMSE scores (R2 = 0.302; P < 0.001; see Fig. 3).
Figure 3.
Scattergram showing the relationship between the lamina cribrosa thickness (LCT) and the Mini Mental State Examination (MMSE) score in the POAG group. The dashed black line indicates the breakpoint where the slope becomes statistically significant. Note that the line becomes approximately horizontal at the point where LCT exceeds 221.14 µm.
Effects of Lamina Cribrosa Thickness on Neuropsychological Test Results
The POAG subjects were divided into two groups based on the LCT breakpoint: eyes with a thinner LC (LCT < 221.14 µm) and those with a thicker LC (LCT ≥ 221.14 µm). Although the clinical characteristics were largely comparable between these two LCT groups (Table 4), significant intergroup differences were found in some of the cognitive function scores, specifically in those associated with visual function. Global cognition, as represented by the MMSE score (P < 0.001) as well as visuospatial function (BVRT copy score, P = 0.001) and visual memory (BVRT recall score, P = 0.003) were significantly worse in eyes with a thinner LC than in those with a thicker LC (Table 5).
Table 4.
Comparison of Clinical Characteristics Between the POAG Groups Classified Based on the Breakpoint for the Lamina Cribrosa Thickness
| POAG Eyes with a Thinner LC | POAG Eyes with a Thicker LC | ||
|---|---|---|---|
| Variables | (LCT < 221.14 µm, n = 43) | (LCT ≥ 221.14 µm, n = 62) | P Value |
| Age, yearsa | 72.7 ± 5.9 | 71.8 ± 5.5 | 0.474 |
| Education, yearsa | 13.1 ± 3.9 | 13.9 ± 3.2 | 0.253 |
| Female, n (%)b | 26 (60.5) | 31 (50.0) | 0.290 |
| Spherical equivalent (D)a | 0.3 ± 2.2 | –0.6 ± 3.5 | 0.137 |
| Axial length, mma | 23.6 ± 1.1 | 24.0 ± 1.1 | 0.172 |
| Central corneal thickness, µma | 558.1 ± 29.1 | 570.0 ± 35.0 | 0.137 |
| IOP at exam, mm Hga | 14.8 ± 4.7 | 15.5 ± 3.4 | 0.388 |
| VF MD, dBa | –8.1 ± 6.2 | –5.8 ± 6.5 | 0.072 |
| VF PSD, dBa | 6.0 ± 3.9 | 5.3 ± 4.1 | 0.372 |
| Global RNFL thickness, µma | 72.7 ± 14.6 | 77.8 ± 15.1 | 0.088 |
POAG = primary open-angle glaucoma; LC = lamina cribrosa; LCT = lamina cribrosa thickness; D = diopters; IOP = intraocular pressure; VF = visual field; MD = mean deviation; dB = decibel; PSD = pattern standard deviation; RNFL = retinal nerve fiber layer.
Data are mean ± SD (range) unless otherwise indicated.
Comparisons were performed by Student t test.
This comparison was performed by Pearson's χ2 test.
Table 5.
Comparison of the Cognitive Function Scores Between the POAG Groups Classified Based on the Breakpoint for the Lamina Cribrosa Thickness
| Model 1a | Model 2b | |||||
|---|---|---|---|---|---|---|
| POAG Eyes with a | POAG Eyes with a | |||||
| Thinner LC (LCT | Thicker LC (LCT | |||||
| < 221.14 µm, n = 43) | ≥ 221.14 µm, n = 62) | F | P Value | F | P Value | |
| Global cognition | ||||||
| MMSE | 25.5 ± 2.8 | 27.7 ± 1.1 | 29.397 | < 0.001 | 27.884 | < 0.001 |
| Attention/concentration | ||||||
| DST-F | 5.7 ± 1.7 | 5.9 ± 1.6 | 0.268 | 0.606 | 0.259 | 0.612 |
| DST-B | 2.5 ± 1.5 | 3.9 ± 1.0 | 2.711 | 0.103 | 2.614 | 0.109 |
| Language | ||||||
| CFT | 14.5 ± 3.8 | 14.8 ± 4.4 | 0.480 | 0.490 | 0.463 | 0.498 |
| mBNT | 10.9 ± 2.5 | 12.2 ± 2.0 | 3.800 | 0.054 | 3.662 | 0.058 |
| Visuospatial function | ||||||
| CPT | 9.7 ± 2.2 | 10.6 ± 0.9 | 5.018 | 0.027 | 4.832 | 0.030 |
| BVRT copy | 8.8 ± 1.6 | 9.7 ± 0.6 | 11.735 | 0.001 | 11.252 | 0.001 |
| Verbal memory | ||||||
| WLMT | 15.5 ± 4.0 | 16.7 ± 3.9 | 0.255 | 0.615 | 0.246 | 0.621 |
| WLRT | 4.9 ± 1.8 | 5.4 ± 1.8 | 0.268 | 0.606 | 0.259 | 0.612 |
| WLRcT | 8.1 ± 1.5 | 8.3 ± 1.6 | 0.000 | 0.982 | 0.000 | 0.983 |
| Visual memory | ||||||
| CRT | 6.1 ± 3.4 | 6.5 ± 2.7 | 0.011 | 0.917 | 0.010 | 0.919 |
| BVRT recall | 4.4 ± 2.4 | 5.9 ± 1.7 | 9.358 | 0.003 | 8.986 | 0.003 |
| Frontal function | ||||||
| TMT-A | 85.9 ± 66.6 | 59.7 ± 38.5 | 2.573 | 0.112 | 2.481 | 0.118 |
| TMT-B | 232.1 ± 109.4 | 166.6 ± 91.7 | 5.577 | 0.020 | 5.368 | 0.022 |
| Depression | ||||||
| GDS-KR | 10.2 ± 6.4 | 10.6 ± 6.9 | 0.004 | 0.951 | 0.004 | 0.951 |
POAG = primary open-angle glaucoma; LC = lamina cribrosa; LCT = lamina cribrosa thickness; MMSE = mini-mental state examination; DST-F = digit span test forward; DST-B = digit span test backward; CFT = categorical fluency test; mBNT = modified Boston naming test; CPT = constructional praxis test; BVRT = Benton visual retention test; WLMT = word list memory test; WLRT = word list recall test; WLRcT = word list recognition test; CRT = constructional praxis recall test; TMT = trail making test; GDS-KR = revised Korean version of geriatric depression scale.
Values are expressed as mean ± SD. Statistically significant values are bold.
Bonferroni correction was applied to raw data for measurements in the 15 cognitive function tests. Values that were significant after Bonferroni correction (P < 0.0033; 0.05/15) are shown in bold.
Comparison was performed using analysis of variance (ANCOVA) with the covariates of age, gender, and education.
Comparison was performed using analysis of variance (ANOVA) after adjustment for age, gender, and education.
Representative Cases
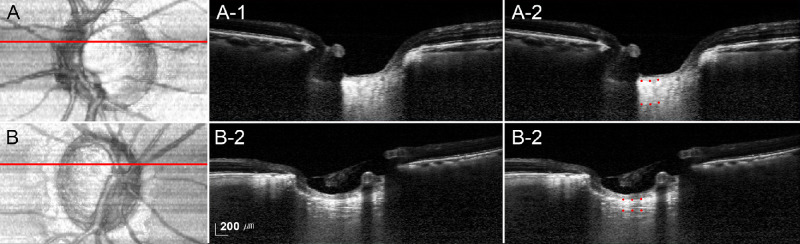
Figure 4 shows representative cases with POAG presenting the relationship between MMSE scores and the LCT. The LC was predominantly thicker in eyes with higher MMSE scores (mean LCT = 261.33 µm, MMSE score = 29; Fig. 4A) than in eyes with lower MMSE scores (mean LCT = 162.33 µm, MMSE score = 21; Fig. 4B).
Figure 4.
Representative eyes with a thicker lamina cribrosa (LC, A, A-1, A-2) and with a thinner LC (B, B-1, B-2). (A, B) Enface views of the maximum intensity projection (MIP) volumetric images. Red lines indicate the location where the superior thin-slab MIP images were obtained. A-1, B-1 Thin-slab MIP images obtained at the superior midperiphery of the optic nerve head (red lines). A-2, B-2 Same images as in A-1 and B-1 with labels. Red dots indicate the anterior and posterior borders of the LC. The Mini Mental State Examination (MMSE) scores were 29 A and 21 B, respectively; note that the LC is notably thicker (mean LCT = 261.33 µm) in the former patient (mean LCT = 162.33 µm).
Discussion
A possible link between the pathomechanisms of AD and glaucoma has long been postulated.8,10 This study found that thinning of the LC was significantly associated with worse cognitive function (as indicated by a lower MMSE score), which was independent of the severity of glaucoma. This finding suggests that cognitive impairment and glaucomatous damage share a common mechanism of LC thinning. The finding provides additional insight into the shared etiology between AD and glaucoma, by demonstrating that structural changes of the LC within the ONH could be associated with neurodegeneration in both the eyes and the brain. To the best of our knowledge, this is the first study to document the relationship between LCT and cognitive function in patients with POAG.
The hypothesis tested in the current study was based on our previous findings that an abnormal profile of CSF protein was associated with a thinner LC in both healthy subjects and patients with AD.19 In addition, cognitive function was found to be positively correlated with the LCT, which might indicate that thinning of the LC may be connected to cognitive impairment. The present study found that the LCT was indeed smaller in patients with POAG with lower MMSE scores. With the POAG group having a thinner LC than control group at all locations, the LCT became smaller with lower MMSE in both groups. It can be speculated that pathological changes in the brain neural tissue accompany degeneration of the LC tissue, or that accumulation of abnormal proteins in the brain and CSF affects the instability of the LC, which finally leads to LC thinning. ONH axons passing through a thinner LC may be subjected to higher stresses induced by a higher translaminar pressure gradient, resulting in an increased susceptibility to glaucomatous axonal damage.
The role of astrocytes could provide an explanation for a shared etiology between cognitive dysfunction and glaucoma. Astrocytes are present in both the ONH and brain, and they help to maintain the extracellular environment as well as providing adjacent neurons with neurotrophic support.32,33 Astrocytes in the eye are important for maintaining the structural elements of the ONH, surrounding the cribrosal beams, and producing collagens and elastins that comprise the core of the laminar beams.34–36 In addition, the close relationship between the astrocytes and the retinal vasculature highlights the potential role of astrocytes in regulating blood flow in the ONH and retina, as they do in the central nervous system.11 Therefore, degradation of astrocytes may lead not only to thinning of the LC but also impaired regulation of blood flow.
On the other hand, astrocytes are able to organize communication pathways and neuronal plasticity in the brain via the removal and degradation of β-amyloid peptides, which are key proteins in the pathogenesis of AD.37,38 Thus, astrocyte damage could result in the dysregulation of amyloid beta protein in the extracellular space, which might result in mechanical stress on the adjacent axons that causes diffuse injury leading to neuronal death.39 Together, these findings indicate that astrocyte dysfunction may be a clue to understand the mechanism of glaucoma and cognitive dysfunction shared by LC thinning.
It has been suggested that a smaller RNFL thickness is correlated with cognitive impairment.7,40 Although we also found a significant linear correlation between the RNFL thickness and the MMSE score in the present study (R2 = 0.052, P = 0.019; data not presented), the correlation was weaker than that between the LCT and the MMSE score (R2 = 0.314, P < 0.001). In patients where RNFL thinning is already present (e.g. glaucoma, retinal diseases, and optic nerve diseases), a smaller RNFL thickness may not be an ideal parameter for predicting cognitive impairment. The stronger association found between the LCT and cognitive function (compared to RNFL thickness) in the present study may indicate that the LCT is a better indicator of cognitive function, specifically in glaucomatous eyes.
We found a statistically significant breakpoint for LCT, below which there was a significant association between the LCT and the MMSE score. A subgroup analysis performed based on the breakpoint for LCT revealed that POAG eyes with a thinner LC had worse visuospatial function (BVRT copy score), and visual memory (BVRT recall score), as well as worse global cognitive function (MMSE score). The BVRT is a widely used test that assesses visual memory, visual perception, and visuoconstructional abilities. In the recall condition (i.e. BVRT recall), subjects are shown 10 figures for 10 seconds each and then asked to draw the figures from memory. In the copying condition (i.e. BVRT copy), subjects are shown figures and asked to copy them while they are still being presented. The presence of adequate copying ability with impaired recall ability suggests a short-term visual memory deficit, whereas impaired copying suggests deficits in visual perception and constructional skills.41,42 The findings of the present study indicate that visual perception is specifically impaired in patients with POAG with a thinner LC. The visual field MD tended to be worse in the subgroup with a thinner LC, which could explain the impaired visual function in this group. However, it should be noted that not only the cognitive domains associated with visuospatial function but also those associated with visual memory were impaired in subjects with a thinner LC. Recognition and associative memory for visuospatial stimuli is mainly processed in the medial temporal lobe.43,44 Given that this lobe is the region affected during the earliest stages of AD,45 impaired visuospatial function and visual memory in patients with a thinner LC may again indicate shared pathophysiological processing between AD and the thinning of the LC. Given that the visual field was only marginally worse in eyes with a thinner LC, it appears that impaired cognitive functioning in several specific domains might be due to a mechanism shared with the LC thinning, rather than being caused by long-standing visual deterioration.
When interpreting these results, it should be remembered that the RNFL thickness differed only marginally between the two LCT groups. RNFL thinning is a well-established finding in patients with impaired cognitive function, and is considered a retrograde degeneration secondary to neuronal loss in the brain.46,47 Given that the LC is a connective tissue that is unlikely to be directly affected by retrograde axonal degeneration, the decrease in LCT with cognitive impairment may indicate an independent mechanism of LC thinning associated with pathological changes in the brain.
On the other hand, it is noteworthy that there were fewer data points below the breakpoint for LCT than above it. This suggests that the LCT does not influence the impairment of cognitive function in a larger proportion of patients with POAG whose LC is thicker than the breakpoint. However, our data do not guarantee that eyes with an LCT smaller than the breakpoint are not at risk of developing cognitive impairment.
It has been suggested that morphologic change of the ONH associated with myopia may affect LCT.17,48 Although axial length was not a significant parameter influencing the LCT in our study, the result showed a potential association of longer axial length with larger LCT (see Table 3). The relationship between myopia and LCT is controversial between studies.17,48 Further study, including larger number of the myopic population, could provide a more definite answer for this issue.
Measurement of LCT could be challenging, particularly in subjects having thick neuroretinal rim with abundant retinal vessels, because of poor visibility of the posterior LC border. We adapted both EDI and MIP rendering to improve the visibility of posterior LC, and successfully measured the LCT with excellent interobserver reproducibility in both the POAG and healthy groups.
The present study was limited by the relatively small number of control subjects. The study was initially designed to include patients with glaucoma, thus, control subjects were recruited later from another study. This resulted in the small sample size of the control group and the difference in some items in the cognitive function tests as well. On the other hand, the scope of the present study was to link the LC tissue and cognition, and so other optic nerve head structures (i.e. prelaminar tissue) were not considered. Future studies including various optic nerve head parameters may provide further insight into more detailed relationship between optic nerve head morphology and cognitive function. Finally, the relatively fair cognitive function of our subjects might have hampered the investigation of a direct relationship between AD and glaucoma. Despite this limitation, we consider that the findings of our study are meaningful in suggesting LCT as an indicator for cognitive impairment from an earlier stage.
In conclusion, this study has demonstrated a significant correlation between impaired cognitive function and thinner LC in patients with POAG. Cognitive impairment was independently associated with a thinner LC in addition to the smaller RNFL thickness, which suggests a common mechanism underlying cognitive dysfunction and glaucoma is shared by the thinning of the LC. The LCT could be a potential marker not only for diagnosing glaucoma but also for monitoring neurodegenerative diseases, such as AD. Both AD and glaucoma are neurodegenerative diseases of the brain and the eyes, and further study is required to determine whether these two diseases share a common pathogenic mechanism.
Acknowledgments
Disclosure: S.H. Lee, None; J.W. Han, None; E.J Lee, None; T.-W. Kim, None; H. Kim, None; K.W. Kim, None
References
- 1. Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology. 1995; 45: 883–888. [DOI] [PubMed] [Google Scholar]
- 2. Bozzali M, Falini A, Franceschi M, et al.. White matter damage in Alzheimer's disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002; 72: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004; 363: 1711–1720. [DOI] [PubMed] [Google Scholar]
- 4. Kim CS, Seong GJ, Lee NH, Song KC. Prevalence of primary open-angle glaucoma in central South Korea the Namil study. Ophthalmology. 2011; 118: 1024–1030. [DOI] [PubMed] [Google Scholar]
- 5. Tamura H, Kawakami H, Kanamoto T, et al.. High frequency of open-angle glaucoma in Japanese patients with Alzheimer's disease. J Neurol Sci. 2006; 246: 79–83. [DOI] [PubMed] [Google Scholar]
- 6. Kromer R, Serbecic N, Hausner L, Froelich L, Aboul-Enein F, Beutelspacher SC. Detection of retinal nerve fiber layer defects in Alzheimer's disease using SD-OCT. Front Psychiatry. 2014; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu D, Zhang L, Li Z, et al.. Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer's disease. BMC Neurol. 2015; 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer's disease. N Engl J Med. 1986; 315: 485–487. [DOI] [PubMed] [Google Scholar]
- 9. Blanks JC, Hinton DR, Sadun AA, Miller CA. Retinal ganglion cell degeneration in Alzheimer's disease. Brain Res. 1989; 501: 364–372. [DOI] [PubMed] [Google Scholar]
- 10. Sadun AA, Bassi CJ. Optic nerve damage in Alzheimer's disease. Ophthalmology. 1990; 97: 9–17. [DOI] [PubMed] [Google Scholar]
- 11. Mackenzie PJ, Cioffi GA. Vascular anatomy of the optic nerve head. Can J Ophthalmol. 2008; 43: 308–312. [DOI] [PubMed] [Google Scholar]
- 12. Kalaria RN, Ballard C. Overlap between pathology of Alzheimer disease and vascular dementia. Alzheimer Dis Assoc Disord. 1999; 13(Suppl 3): S115–S123. [DOI] [PubMed] [Google Scholar]
- 13. Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H. Vitreous fluid levels of beta-amyloid ((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol. 2005; 49: 106–108. [DOI] [PubMed] [Google Scholar]
- 14. Gupta N, Fong J, Ang LC, Yucel YH. Retinal tau pathology in human glaucomas. Can J Ophthalmol. 2008; 43: 53–60. [DOI] [PubMed] [Google Scholar]
- 15. Berdahl JP, Allingham RR, Johnson DH. Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology. 2008; 115: 763–768. [DOI] [PubMed] [Google Scholar]
- 16. Silverberg G, Mayo M, Saul T, Fellmann J, McGuire D. Elevated cerebrospinal fluid pressure in patients with Alzheimer's disease. Cerebrospinal Fluid Res. 2006; 3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci. 2004; 45: 2660–2665. [DOI] [PubMed] [Google Scholar]
- 18. Quigley HA, Hohman RM, Addicks EM, Massof RW, Green WR. Morphologic changes in the lamina cribrosa correlated with neural loss in open-angle glaucoma. Am J Ophthalmol. 1983; 95: 673–691. [DOI] [PubMed] [Google Scholar]
- 19. Lee EJ, Kim TW, Lee DS, et al.. Increased CSF tau level is correlated with decreased lamina cribrosa thickness. Alzheimers Res Ther. 2016; 8: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kim TH, Park JH, Lee JJ, et al.. Overview of the Korean longitudinal study on cognitive aging and dementia. Alzheimers Dement. 2013; 9: P626–P627. [Google Scholar]
- 21. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 22. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008; 146: 496–500. [DOI] [PubMed] [Google Scholar]
- 23. Lee EJ, Kim TW, Weinreb RN, Park KH, Kim SH, Kim DM. Visualization of the lamina cribrosa using enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2011; 152: 87–95.e81. [DOI] [PubMed] [Google Scholar]
- 24. Lee EJ, Kim TW, Weinreb RN. Improved reproducibility in measuring the laminar thickness on enhanced depth imaging SD-OCT images using maximum intensity projection. Invest Ophthalmol Vis Sci. 2012; 53: 7576–7582. [DOI] [PubMed] [Google Scholar]
- 25. Lee DY, Lee KU, Lee JH, et al.. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J Int Neuropsychol Soc. 2004; 10: 72–81. [DOI] [PubMed] [Google Scholar]
- 26. Lee JH, Lee KU, Lee DY, et al.. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J Gerontol B Psychol Sci Soc Sci. 2002; 57: P47–P53. [DOI] [PubMed] [Google Scholar]
- 27. Sivan AB. Benton visual retention test. San Antonio, TX: Psychological Corporation; 1992. [Google Scholar]
- 28. Wechsler D. WMS-R: Wechsler memory scale-revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 29. Bae JN, Cho MJ. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J Psychosom Res. 2004; 57: 297–305. [DOI] [PubMed] [Google Scholar]
- 30. Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology–with an emphasis on fractional polynomials. Methods Inf Med. 2005; 44: 561–571. [PubMed] [Google Scholar]
- 31. Davies RB. Hypothesis testing when a nuisance parameter is present only under the alternative. Biometrika. 1987; 74: 33–43. [Google Scholar]
- 32. Ransom BR, Fern R. Does astrocytic glycogen benefit axon function and survival in CNS white matter during glucose deprivation? Glia. 1997; 21: 134–141. [PubMed] [Google Scholar]
- 33. Goss JR, O'Malley ME, Zou L, Styren SD, Kochanek PM, DeKosky ST. Astrocytes are the major source of nerve growth factor upregulation following traumatic brain injury in the rat. Exp Neurol. 1998; 149: 301–309. [DOI] [PubMed] [Google Scholar]
- 34. Hernandez MR. Extracellular matrix macromolecules of the lamina cribrosa: a pressure-sensitive connective tissue. J Glaucoma. 1993; 2: 50–57. [PubMed] [Google Scholar]
- 35. Trivino A, Ramirez JM, Salazar JJ, Ramirez AI, Garcia-Sanchez J. Immunohistochemical study of human optic nerve head astroglia. Vision Res. 1996; 36: 2015–2028. [DOI] [PubMed] [Google Scholar]
- 36. Ye H, Hernandez MR. Heterogeneity of astrocytes in human optic nerve head. J Comp Neurol. 1995; 362: 441–452. [DOI] [PubMed] [Google Scholar]
- 37. Bard F, Cannon C, Barbour R, et al.. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000; 6: 916–919. [DOI] [PubMed] [Google Scholar]
- 38. Wyss-Coray T, Loike JD, Brionne TC, et al.. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003; 9: 453–457. [DOI] [PubMed] [Google Scholar]
- 39. Vickers JC, Dickson TC, Adlard PA, Saunders HL, King CE, McCormack G. The cause of neuronal degeneration in Alzheimer's disease. Prog Neurobiol. 2000; 60: 139–165. [DOI] [PubMed] [Google Scholar]
- 40. Iseri PK, Altinas O, Tokay T, Yuksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol. 2006; 26: 18–24. [DOI] [PubMed] [Google Scholar]
- 41. Moses JA., Jr Factor structure of Benton's tests of visual retention, visual construction, and visual form discrimination. Arch Clin Neuropsychol. 1986; 1: 147–156. [PubMed] [Google Scholar]
- 42. Hinton VJ, Dobkin CS, Halperin JM, et al.. Mode of inheritance influences behavioral expression and molecular control of cognitive deficits in female carriers of the fragile X syndrome. Am J Med Genet. 1992; 43: 87–95. [DOI] [PubMed] [Google Scholar]
- 43. Barr WB. Examining the right temporal lobe's role in nonverbal memory. Brain Cogn. 1997; 35: 26–41. [DOI] [PubMed] [Google Scholar]
- 44. Milner B. Psychological aspects of focal epilepsy and its neurosurgical management. Adv Neurol. 1975; 8: 299–321. [PubMed] [Google Scholar]
- 45. Didic M, Barbeau EJ, Felician O, et al.. Which memory system is impaired first in Alzheimer's disease? J Alzheimers Dis. 2011; 27: 11–22. [DOI] [PubMed] [Google Scholar]
- 46. Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett. 2007; 420: 97–99. [DOI] [PubMed] [Google Scholar]
- 47. Ascaso FJ, Cruz N, Modrego PJ, et al.. Retinal alterations in mild cognitive impairment and Alzheimer's disease: an optical coherence tomography study. J Neurol. 2014; 261: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 48. Lee EJ, Kim TW, Weinreb RN, Suh MH, Kim H. Lamina cribrosa thickness is not correlated with central corneal thickness or axial length in healthy eyes: central corneal thickness, axial length, and lamina cribrosa thickness. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 847–854. [DOI] [PubMed] [Google Scholar]