Abstract
Purpose
The aim of this study was to investigate the molecular basis of childhood glaucoma in Switzerland to recommend future targeted genetic analysis in the Swiss population.
Methods
Whole-exome sequencing and copy number variation (CNV) analysis was performed in a Swiss cohort of 18 patients from 14 unrelated families. Identified variants were validated by Sanger sequencing and multiplex ligation-dependent probe amplification. Breakpoints of structural variants were determined by a microarray. A minigene assay was conducted for functional analysis of a splice site variant.
Results
A diagnosis of primary congenital glaucoma was made in 14 patients, of which six (43%) harbored pathogenic variants in CYP1B1, one (7%) a frameshift variant in FOXC1, and seven (50%) remained without a genetic diagnosis. Three patients were diagnosed with glaucoma associated with nonacquired ocular anomalies, of which two patients with mild ocular features of Axenfeld-Rieger syndrome harbored a FOXC1 duplication plus an additional FOXC1 missense variant, and one patient with a Barkan membrane remained without genetic diagnosis. A diagnosis of juvenile open-angle glaucoma was made in one patient, and genetic analysis revealed a FOXC1 duplication.
Conclusions
Sequencing of CYP1B1 and FOXC1, as well as analysis of CNVs in FOXC1, should be performed before extended gene panel sequencing.
Translational Relevance
The identification of the molecular cause of childhood glaucoma is a prerequisite for genetic counseling and personalized care for patients and families.
Keywords: childhood glaucoma, primary congenital glaucoma, Axenfeld-Rieger syndrome, FOXC1, CYP1B1
Introduction
Childhood glaucoma is characterized by progressive and irreversible damage to retinal ganglion cells and may lead to blindness. Developmental abnormalities of the anterior segment of the eye can result in impaired outflow of aqueous humor through the trabecular meshwork into Schlemm's canal.1 Childhood glaucoma classification distinguishes primary congenital glaucoma (PCG) and juvenile open angle glaucoma (JOAG) from secondary types of glaucoma depending on the presence of acquired/ non-acquired ocular or systemic features.2 The primary form (PCG), characterized by isolated trabeculodysgenesis and an early disease onset, can present with tearing, photophobia, and blepharospasm. An enlarged globe (buphthalmos), increased cup-to-disc-ratio, corneal edema, and breaks in the Descemet's membrane (Haab's striae) are frequent clinical findings. In JOAG disease onset is between the ages of four and 40 years, presenting with a normal appearing anterior angle and without corneal or globe enlargement.2 Secondary forms of the disease include glaucoma associated with Peters’ anomaly, aniridia, ectopia lentis, and a number of syndromes including Axenfeld-Rieger syndrome (ARS).3 Variable presence of ocular features of ARS such as iris processes, posterior embryotoxon, corectopia, and iris hypoplasia, may cause the clinical distinction from PCG to be challenging.4
Type and incidence of childhood glaucoma varies according to population studied and degree of parental consanguinity (e.g. the incidence of PCG ranges from 1:1250 to 1:30,000).5–7 Ma et al.8 have recently presented an overview of genes associated with anterior segment disease and associated overlapping phenotypes. These include CYP1B1, FOXC1, PAX6, PITX2, FOXE3, PITX3, B3GLCT, COL4A1, PXDN, CPAMD8, LTBP2, all of which are associated with anterior segment anomalies which may lead to secondary glaucoma; of these, CYP1B1, FOXC1, and LTBP2 are specifically associated with PCG. The gene TEK is also associated with PCG. Souma et al.9 observed compromised aqueous humor outflow in Tek-null-mice, but there was markedly variable expressivity in human patients.9 A review of PCG-associated genes is provided in the Supplementary Material. The list for potential childhood glaucoma-associated genes can be extended for syndromic types of childhood glaucoma and the autosomal dominant JOAG gene MYOC.3,10 However, a genetic cause remains unknown in a large proportion of patients.1
In Switzerland, the genotype distribution of childhood glaucoma is unknown. Genetic analyses are not always paid for by health insurance providers, therefore in these circumstances the costs must be borne by the families. Medical treatment and screening strategies would be improved by knowledge of the underlying genetic causes of the disease. Thus our aim was to characterize a Swiss childhood glaucoma cohort by filtering whole-exome sequencing (WES) data with an extended gene list based on the results of a current literature search; correlate genotype with clinical parameters; and, on the basis of our findings, evaluate time- and cost-effective strategies for childhood glaucoma screening in Switzerland.
Materials and Methods
Patients
Patients with bilateral childhood glaucoma were recruited from the Departments of Ophthalmology at the University Hospital Zurich and University Hospital Basel, together with their families. Diagnosis of PCG, JOAG, or glaucoma associated with non-acquired ocular anomalies was made based on the Childhood Glaucoma Research Network Classification System Flowchart.2 Clinical examination results were obtained from patient records from the first and last visits: (1) presence of buphthalmos, (2) anterior segment morphology including presence of Haab's striae and/or iris abnormalities and horizontal corneal diameter, (3) optic disc cupping, (4) best-corrected distance or near visual acuity assessed with age-appropriate methods, (5) IOP measured using Tonopen, Goldmann, or Perkins tonometer according to the patient's age, either awake or under anesthesia, and (6) retinal nerve fiber layer (RNFL) thickness quantified using optical coherence tomography, when possible. The presence of a systemic disease or malformation and/or intellectual disability was noted. Number and type of surgeries were analyzed across the entire treatment period. Trabeculectomy and glaucoma drainage device implantation were categorized as fistulating surgeries. Treatment success was defined according to the Tube Versus Trabeculectomy Study.11 Demographic parameters and information about family history were recorded after direct questioning of the families. Affected family members were included in the study and their data were also extracted from their patient records. Blood samples were collected from all patients and their parents, as well as siblings (if available). Ethical approval was obtained (Cantonal Ethics Committee of Zurich, Ref-No. 2019-00108), and patients or guardians provided written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Exome Sequencing and Data Analysis
DNA extraction was performed with the Chemagic DNA Blood Kit (Perkin Elmer, Waltham, MA, USA) and fragmented using the M220 Sonicator (Covaris, Woburn, MA, USA). Ligation of adapters was performed according to the IDT-Illumina TruSeq DNA Exome protocol (Illumina, San Diego, CA, USA). Exome target regions were captured according to the IDT-xGen hybridization capture of DNA libraries protocol (Integrated DNA Technologies, Coralville, IA, USA). Paired-end sequencing was performed on the NextSeq 550 (Illumina). Alignment of reads to the human genome (GRCh37) and variant calling was achieved by BaseSpace Onsite (Illumina). The sequencing coverage for the entire FOXC1 locus was 30 reads or higher (IDT-xGen exome research panel version 1). For annotation of variants AlamutBatch version 1.10 (Interactive Biosoftware, Rouen, France) was used. A gene list containing core childhood glaucoma genes, as well as additional candidate genes (selected through a literature search) was used for filtering of WES data in all patients (Supplementary Material Table S1). The search for disease-associated variants was restricted to listed genes and did not include analysis of WES data. Variants with heterozygous allele frequency (gnomAD heterozygous frequency all populations) <1% and homozygous allele frequency (gnomAD homozygous frequency all populations) <0.00001% were considered (https://gnomad.broadinstitute.org/). Synonymous and intronic variants were considered if within 20 nucleotides proximity to the intron-exon boundary. Missense variants were only considered if predicted pathogenic by at least two algorithms.12 Copy number variations (CNVs) of genes within the gene list were assessed using exome coverage depth data (Sequence Pilot version 5.0; JSI Medical Systems GmbH, Ettenheim, Germany). All identified variants were submitted to ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/). Haplotype analysis was performed according to previously published single-nucleotide polymorphisms in CYP1B1.13,14
Segregation Analysis
All substitutions or indels identified through WES were confirmed by Sanger sequencing, and available family members were sequenced for segregation analysis (Supplementary Figs. S1–S7). Potential de novo variants were confirmed by sequencing of parental DNA and excluding nonpaternity. Polymerase chain reaction (PCR) of CYP1B1 and FOXC1 was performed on Veriti 96 Well Thermal Cycler (Applied Biosystems, Waltham, MA, USA). The protocol used for CYP1B1 amplification has previously been published.15 PCR for FOXC1 was performed according to PCR Phusion High-Fidelity DNA Polymerase Protocol (Thermofisher Scientific, Waltham, MA, USA) using the GC-buffer and 2x S-Solution (Solis BioDyne, Tartu, Estonia). Big Dye Terminator Cycle Sequencing Kit version 1.1/3.1 (Thermofisher Scientific) was used for the Sanger reactions and a 3130xl Genetic Analyzer (Applied Biosystems) performed capillary sequencing. Sequences were visualized by Chromas version 2.6.6 (Technelysium, Brisbane, Australia). Primers are available on request. Multiplex ligation-dependent probe amplification (MLPA) was used to confirm FOXC1 CNVs using the SALSA MLPA P054-B2 FOXL2-TWIST1 probemix (MRC Holland, Amsterdam, The Netherlands) according to the manufacturer's instructions. MLPA amplicons were analyzed using a 3130xl Genetic Analyzer (Applied Biosystems) and Sequence Pilot version 5.0 (JSI medical systems).
Breakpoint Assessment
Breakpoints of CNVs were assessed using the Infinium CytoSNP-850K BeadChip version 1.2 (Illumina). Data were aligned to the human reference genome (GRCh38) by the BlueFuse Multi software version 4.5 (Illumina).
Minigene Assay for Intronic Variant in CYP1B1
A 4715-bp fragment including all of exon 2, intron 2, and exon 3 of CYP1B1 was amplified with TaKaRa LA Taq polymerase (Takara Bio, Kusatsu, Japan) according to the manufacturer's instructions, using 20 ng of genomic DNA. Primers were designed according to the In-Fusion HD Cloning kit (Takara Bio). Amplicons were inserted at the EcoRV restriction site of pcDNA 3.1(+) vector (Thermofisher), and subsequent transformation was performed according to the In-Fusion HD cloning kit. Sequences of reference- and variant-containing plasmid were verified by Sanger sequencing of exon 2, 3, and 200 base pairs down- and upstream of the exon-intron boundaries. HEK-293T cells (Thermofisher) were plated at 5 × 105cells/well in a six-well plate and transfected the following day by branched Polyethylenimine (PEI; Mw ∼25 000; Sigma-Aldrich, St. Louis, MO, USA). A total of 3 µg of plasmid DNA (2 µg of insert-containing plasmid vector and 1 µg empty vector) was transfected in a ratio PEI:DNA 3:1. Twenty hours after transfection, total RNA was extracted using NuceloSpin RNA plus Kit (Macherey-Nagel, Düren, Germany) and subsequently converted into cDNA using the SuperSript III Reverse Transcriptase (Thermofisher). PCR on cDNA was performed, and products were loaded on a 1% agarose gel. Real-time PCR was performed using SYBR 2x MasterMix (Life Technologies, Carlsbad, CA, USA). Triplicates of each reaction of 20 µL contained: 400 nmol/L primers and 100 ng cDNA. Quantification was performed on ABI Prims 7900HT Fast Real-time Sequence Detection System (Applied Biosystems) according to the manufacturer's instructions and analyzed using SDS2.2p1 software (Thermofisher). Data were normalized to RNA derived from empty control plasmid. For statistical analysis GraphPad PRISM version 6.07 (GraphPad Software, San Diego, CA, ISA) was used.
Results
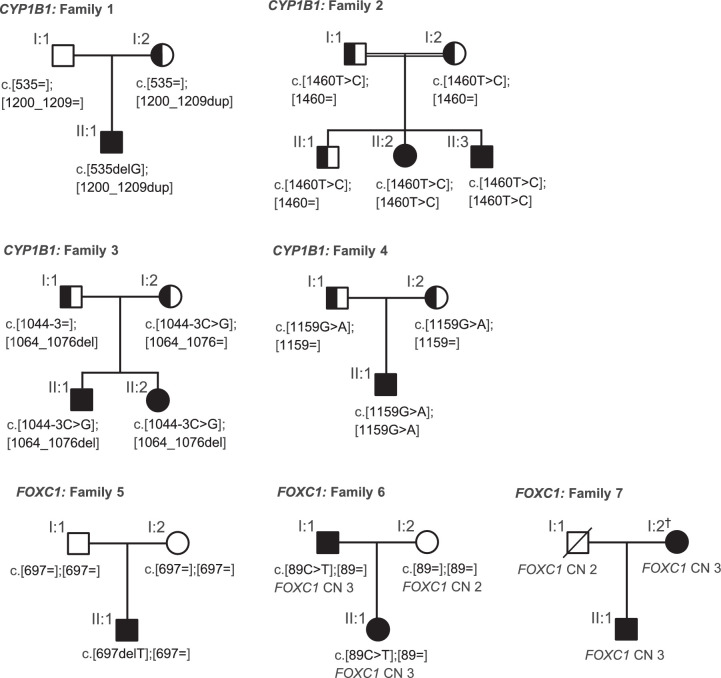
Our cohort included 18 patients from 14 unrelated families with bilateral childhood glaucoma (Table 2). A diagnosis of PCG was made in 14 patients. Three patients were diagnosed with glaucoma associated with nonacquired ocular anomalies; two related patients had ocular features of ARS, and one patient had a Barkan membrane. One patient was diagnosed with JOAG. Recessive variants in CYP1B1 and dominant variants in FOXC1 were identified in 50% of the families (Fig. 1). All of the listed variants (Table 1) segregated with the disease and were classified as disease-causing based on filtering criteria (see methods sections) and previous reports. In eight patients (seven families) no conclusively disease-causing variants were identified, nor did we identify any heterozygous variants in recessive childhood glaucoma genes.
Table 2.
Clinical Details Of Patients With Childhood Glaucoma
| Findings at Diagnosis (OD/OS) | Surgeries | Findings at Last Visit (OD/OS) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient IDa | Diagnosis | Age (yrs) | IOP (mm Hg) | Anterior Segment (OU) | CD, horizontal (mm) | C/D-Ratio | OD | OS | Age (yrs) | Treatment successb | VA (Snellen decimal) | C/D-Ratio | Complications |
| 1 [II:1] | PCG | 0 | NA | NA | NA | NA | 1 × fist | 2 × fist | 5.8 | Failure/qualified 1 | LP/LP | NA/1.0 | Cat, RD/cat |
| 2 [II:2] | PCG | NA | NA | NA | NA | NA | 1 × trabeculotomy | 3 × fist, 2 × cyclo | 31.0 | Complete/complete | 0.03c/0.1c | NA/1.0 | None |
| 2 [II:3] | PCG | NA | NA | NA | NA | NA | NA | NA | 27.0 | Failure/failure | NLP/NLP | NA | Phthisis bulbi/Phthisis bulbi |
| 3 [II:1] | PCG | 0 | 38/34 | CE ++ | 12/12 | NA | 1 × fist, 1 × trabeculotomy, 4 × cyclo | 2 × fist, 1 × trabeculotomy, 3 × cyclo | 11.6 | Complete 4/qualified 3 | 0.2c/0.4c | 0.4/0.5 | Cat/cat |
| 3 [II:2] | PCG | 0 | 36/49 | CE ++ | 12/12 | NA | 1 × fist, 1 × trabeculotomy, 6 × cyclo | 1 × trabeculotomy, 4 × cyclo | 10.2 | Qualified 2/qualified 2 | HM/0.4c | 0.8/0.3 | Cat, RD/none |
| 4 [II:1] | PCG | 0 | 26 (EUA) | CE +++ | 12/12.5 | 0.2 | 2 × fist, 1 × trabeculotomy | 1 × fist, 1 × trabeculotomy | 3.9 | Qualified 1/qualified 4 | 0.3c/0.6 | 0.4/0.3 | None |
| 5 [II:1] | PCG, facial dysmorphism, ASD II | 0 | 21/22 | Buphthalmos, CE +++ | 12/11.5 | 0.2/0.2 | 1 × fist, 1 × trabeculotomy | 1 × trabeculotomy, 1 × cyclo | 0.7 | Qualified 4/qualified 4 | NA | 0.3/0.3 | None |
| 6 [I:1] | CG associated with ocular ARS features | 10 | NA | Iris hypoplasia, irido-corneal adhesions | NA | NA | 2 × trabeculotomy | 1 × trabeculotomy | 51.9 | Qualified 4/qualified 4 | 1.25/1.25 | 0.3/0.1 | None |
| 6 [II:1] | CG-suspect associated with ocular ARS features | 5.7 | 29/23 | Iris hypoplasia, cat (OD) | NA | 0.3 | 0 | 0 | 9.6 | Qualified 1/qualified 1 | 0.8c/1.25 | 0.3/0.3 | None |
| 7 [II:1] | JOAG, facial dysmorphism, developmental delay | 14.1 | 20/19 (EUA, UT) | Subepithelial haze + | 11.5/11 | 1/1 | 1 × fist, 1 × trabeculotomy, 1 × cyclo | 1 × fist, 1 × trabeculotomy, 1 × cyclo | 19.7 | Failure/qualified 4 | NLP/LP | 1.0/1.0 | Cat/cat |
| 8 [II:1] | PCG | 0.5 | 24/31 | Peripheral iris hypoplasia, CE +, Haab's striae +/- | 14/13 | 0.4/0.4 | 1 × trabeculotomy | 1 × trabeculotomy | 1.1 | Complete 4/complete 4 | 0.2 | 0.1/0.1 | None |
| 9 [II:1] | PCG | 0.2 | 46/40 | CE + | 12/12 | 0.7/0.6 | 1 × fist, 1 × trabeculotomy | 1 × trabeculotomy | 3.9 | Complete 2/complete 3 | 0.4/0.4 | 0.1/0.1 | None |
| 10 [II:1] | PCG | NA | NA | NA | NA | NA | 7 × cyclo | 7 × cyclo | 10.9 | Qualified 4/qualified 4 | NA | 0.2/NA | None |
| 11 [II:1] | PCG-late onset | 3.6 | 25/39 | CE +, Haab's striae | 14.5/15.3 | 0.4/1 | 1 × trabeculotomy | 1 × trabeculotomy | 7.1 | Complete 4/complete 4 | 1/0.02c | 0.3/0.9 | None |
| 11 [II:2] | PCG | 0.4 | 23/36 | CE +, Haab's striae | 12/13 | 0.4/0.6 | 1 × trabeculotomy | 1 × trabeculotomy | 3.1 | Complete 4/complete 4 | 0.5/0.6 | 0.1/0.1 | None |
| 12 [II:1] | PCG | 0.3 | 36/37 | CE ++, Haab's striae | 14/13.5 | 0.5/0.5 | 1 × fist, 1 × trabeculotomy | 1 × fist, 1 × trabeculotomy | 1.2 | Qualified 4/qualified 2 | 0.1/0.16 | 0.6/0.6 | None |
| 13 [II:1] | CG associated with Barkan membrane | 14.9 | 25/35 | Barkan membrane | NA | 0.6/1 | 0 | 0 | 24.0 | NA | 1/CF | 0.6/1.0 | None |
| 14 [II:1] | PCG | 0.3 | 20/18(EUA, UT) | Buphthalmos, CE +++ | 14.4/14 | 0.6/0.6 | 2 × fist, 1 × trabeculotomy, 9 × cyclo | 2 × fist, 1 × trabeculotomy, 9 × cyclo | 6.3 | Qualified 2/qualified 4 | NA | LP/0.1 | Cat/cat |
ARS, Axenfeld-Rieger syndrome; ASD, atrial septal defect; Cat, cataract; CD, corneal diameter; C/D, cup-to-disc; CF, counting fingers; CG, childhood glaucoma; CE, corneal edema; cyclo, cyclodestructive surgery; EUA, examination under anesthesia; fist, fistulating surgery; HM, hand movements; IOP, intraocular pressure; LP, light perception; NA, not available; NLP, no light perception; OD, oculus dexter; OS, oculus sinister; OU, oculus uterque; PCG, primary congenital glaucoma; RD, retinal detachment; UT, under topical treatment; VA, visual acuity; yrs, years
First numeral stands for family number. Roman numeral represents the generational affiliation of the index patient.
Complete success: no medication and at least light perception, IOP >5/<22 mm Hg (1), IOP 6–18 mm Hg (2), IOP 6–16 mm Hg (3), IOP 6–14 mm Hg (4). Qualified success: medication and at least light perception, IOP >5/<22 mm Hg (1), IOP 6–18 mm Hg (2), IOP 6–16 mm Hg (3), IOP 6–14 mm Hg (4). Failure: IOP ≤5 mm Hg/>21 mm Hg or loss of light perception.
Abnormal value for respective age.
Figure 1.
Pedigrees and segregation of CYP1B1 and FOXC1 pathogenic variants. All affected family members, as well as all unaffected family members who were available for genetic testing, are shown (with the exception of family 6). The complete pedigrees are shown in Supplementary Figures S9–S12. Sequence variations of CYP1B1 (families 1–4) are numbered to transcript NM_000104.3 and for FOXC1 (families 5–7) to transcript NM_001453.2. CN, copy number. †Manifests neither glaucoma nor features of Axenfeld-Rieger syndrome.
Table 1.
Disease-Causing Variants Identified in Patients and Demographic Details
| Patient ID* | Sex | Origin† | Diagnosis | Gene | Sequence Variation | Region/Size | Predicted Protein Change | Allele Frequency‡ | PP§ | Haplotype | First Report |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 [II:1] | m | Portugal | PCG | CYP1B1 | c.[535delG];[1200_1209dup] | Exons 2; 3 | p.[(Ala179Argfs*18)];[(Thr404Serfs*30)] | 0.00004;0.0002 | — | CCGGTA | Belmouden et al.15; Stoilov et al.18 |
| 2 [II:2], 2 [II:3] | f, m | Somalia | PCG | CYP1B1 | c.[1460T>C];[1460T>C] | Exon 3 | p.[(Leu487Pro)];[(Leu487Pro)] | 0 | 5/5 | CCGGTA | Firasat et al.14 |
| 3 [II:1], 3 [II:2] | m, f | Switzerland | PCG | CYP1B1 | c.[1044-3C>G];[1064_1076del] | Intron 2; Exon 3 | p.[(?)];[(Arg355Hisfs*69)] | 0; 0.0002 | — | CCGCCG; CCGGTA | This study; Stoilov et al.20 |
| 4 [II:1] | m | Switzerland | PCG | CYP1B1 | c.[1159G>A];[1159G>A] | Exon 3 | p.[(Glu387Lys)];[(Glu387Lys)] | 0.0003 | 4/5 | CCGGTA | Stoilov et al.18 |
| 5 [II:1] | m | Italy | PCG, facial dysmorphism, ASD II | FOXC1 | c.[697delT];[697=] | Exon 1 | p.[(Cys233Alafs*82)];[Cys233=] | 0 | — | — | This study |
| 6 [I:1], 6 [II:1] | m, f | Switzerland/Germany | CG associated with ocular ARS features | FOXC1 | c.[89C>T];[89=] | Exon 1 | p.[(Ala30Val)];[Ala30=] | 0 | 4/5 | This study | |
| FOXC1, FOXQ1, FOXF2, GMDS partial | g.[(1307929_1318643)_(1837594_1856280)dup];[(1307929_1318643)_(1837594_1856280)=] | 519-548 kb | — | — | — | — | Chanda et al.16 | ||||
| 7 [I:2], 7 [II:1] | f, m | Switzerland | No glaucoma, JOAG + facial dysmorphism + developmental delay | FOXC1, FOXQ1, FOXF2, GMDS partial | g.[(1307929_1347995)_(1713458)dup];[(1307929_1347995)_(1713458)=] | 366-406 kb | — | — | — | — | This study |
| 8 [II:1] | m | Macedonia | PCG | — | — | — | — | — | — | — | — |
| 9 [II:1] | m | Switzerland/Greece | PCG | — | — | — | — | — | — | — | — |
| 10 [II:1] | m | Senegal | PCG | — | — | — | — | — | — | — | — |
| 11 [II:1], 11 [II:2] | m, f | Switzerland | PCG-late onset, PCG | — | — | — | — | — | — | — | — |
| 12 [II:1] | m | Serbia | PCG | — | — | — | — | — | — | — | — |
| 13 [II:1] | f | Switzerland | CG associated with Barkan membrane | — | — | — | — | — | — | — | — |
| 14 [II:1] | m | Cameroon/ Switzerland | PCG | — | — | — | — | — | — | — | — |
ARS, Axenfeld-Rieger syndrome; ASD, atrial septal defect; CG, childhood glaucoma; f, female; JOAG, juvenile open angle glaucoma; m, male; PCG, primary congenital glaucoma; PP, pathogenicity prediction.
First numeral stands for family number. Roman numeral represents the generational affiliation of the index patient.
Geographic origin of ancestors (three generations).
gnomAD heterozygous frequency all populations.
Number of algorithms with pathogenic prediction of variant/number of algorithms with available prediction; considered were align Grantham variation and Grantham deviation (AGVGD), sorting intolerant from tolerant prediction (SIFT), multivariate analysis of protein polymorphism (MAPP), MutationTaster, Polyphen2.
SNPs rs2617266, rs10012, rs1056827, rs1056836, rs1056837 and rs1800440 were used for haplotype construction.13,14
Sequence variations of CYP1B1 are numbered to transcript NM_000104.3 and for FOXC1 to transcript NM_001453.2. The reference sequence accession number of duplicated regions in family 6 and 7 is NC_000006.12.
Primary Congenital Glaucoma
Fourteen patients were diagnosed with PCG, of which six (43%) harbored pathogenic variants in CYP1B1, one (7%) a frameshift variant in FOXC1, and seven (50%) remained without a genetic diagnosis. All patients had typical features of PCG and received trabeculotomy or fistulating surgeries with variable clinical outcomes (Table 2). A novel CYP1B1 splice site variant and previously reported pathogenic variants, either in homozygosity or compound heterozygosity, were identified in families 1 to 4 (Table 1). Consanguinity was reported in only one (family 2) of the two families with homozygous CYP1B1 variants. The poor visual outcome of affected patients in family 2 may have been caused or exacerbated by the limited access to medical care in their country of residence during childhood. Review of the patient record in patient 5 [II:1] harboring the FOXC1 frameshift variant (NM_001453.2:c.697delT;p.(Cys233Alafs*82)) revealed dysmorphic facial features (midfacial hypoplasia) and a congenital heart defect (atrial septal defect type 2), features also associated with ARS. A de novo origin or mosaicism in the parents was found for the CYP1B1 frameshift variant (NM_000104.3:c.535delG; p.(Ala179Argfs*18)) in patient 1 [II:1] and the FOXC1 frameshift variant in patient 5 [II:1] (NM_001453.2:c.697delT;p.(Cys233Alafs*82)) (Supplementary Figs. S1 and S5).
Glaucoma Associated With Nonacquired Ocular Anomalies
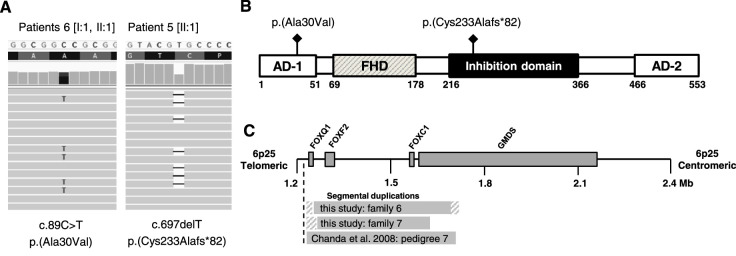
Two related patients (family 6) diagnosed with glaucoma associated with ARS harbored FOXC1 variations, and one patient (patient 13 [II:1]) with glaucoma associated with a Barkan membrane remained without a genetic diagnosis. Family 6 consisted of an affected father (patient 6 [I:1]) and a daughter (patient 6 [II:1]) with mild iris hypoplasia, as well as iris-strands bridging the irido-corneal angle (observed only in the father). Glaucoma was diagnosed at the age of 10 years in the father. Because the daughter had normal RNFL thickness and normal perimetry, she was classified as a glaucoma-suspect (Table 2). Genetic analysis revealed duplications of FOXC1. According to microarray data (Infinium cyto-850K BeadChip), the size of the duplicated region was between 519 and 548 kb, including the duplication of FOXQ1, FOXF2, and parts of GMDS, which are flanking genes. The region was likely to be identical to a previously reported duplication (Fig. 3C).16 An additional missense variant (NM_001453.2:c.89C>T; p.(Ala30Val)) co-segregated with the duplicated allele, a finding that has not been described previously for FOXC1 variants. According to coverage data analysis, the variant was present only on one FOXC1 allele (36% of reads contained the variant). Together with the genetic results, a diagnosis of glaucoma associated with ocular ARS features was made. No systemic features of ARS were identified in family 6.
Figure 3.
FOXC1 variants associated with childhood glaucoma. (A) Missense and de novo frameshift variants identified by exome sequencing. Thirty-six percent of reads contain the missense variant c.89C>T. (B) Location of identified variants within FOXC1. Scheme adapted from Medina-Trillo et al.31 (C) Extent of segmental duplications shown in a schematic representation of partial chromosome 6p25. Shaded areas indicate breakpoint regions. Scheme adapted from Chanda et al.16
JOAG
Patient 7 [II:1] was diagnosed with JOAG at age 14 years. Genetic analysis revealed a FOXC1 duplication. Diagnosis was likely to have been made some time after disease onset because of severe global developmental delay. Marked optic atrophy was observed, but with the exception of subepithelial corneal haze, the anterior segment was normal. Despite several surgeries, treatment had only limited success, and the patient was legally blind (Table 2). Furthermore, patient 7 [II:1] had dysmorphic facial features (prominent forehead, as well as a protruding lower lip), features that may be associated with ARS. A novel FOXC1 duplication which ranged between 366 to 406 kb in size and included the duplication of neighboring genes FOXQ1, FOXF2, and parts of GMDS was identified by genetic analysis (Fig. 3C). His mother, in whom the duplication was also detected, showed no evidence of ocular abnormality (including RNFL thickness and perimetry) apart from subepithelial corneal haze comparable to that observed in her son.
Minigene Assay for Novel CYP1B1 Variant
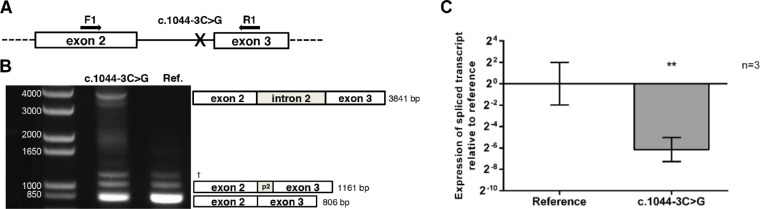
A novel CYP1B1 splice site variant (NM_000104.3:c.1044-3C>G) identified in family 3 was functionally tested in a cellular system. Real-time PCR from minigene assays revealed a 98% reduction of correctly spliced CYP1B1 for the c.1044-3C>G containing transcript compared to the reference (ΔΔ-CT = 6.15 ± 1.13 SD, P = 0.0095) (Fig. 2C). Intron retention was exclusively shown for the c.1044-3C>G containing transcript and was verified by Sanger sequencing (Fig. 2B, Supplementary Fig. S8). Interestingly, the Human Splicing Finder score predicted a 12.7% reduced acceptor function compared to the reference sequence.
Figure 2.
CYP1B1 variant c.1044-3C>G alters splicing in a minigene assay. (A) Scheme of minigene construction and position of forward (F1) and reverse (R1) primer used for endpoint PCR. (B) Qualitative analysis (endpoint PCR): PCR plateau phase shows intron retention in transcript containing the c.1044-3C>G variant compared to the reference. Additional bands represent alternative spliced transcript (1161 bp) and correctly spliced transcript (806 bp). p2, partial intron 2. †Nonsequenceable DNA-fragment. (C) Quantitative analysis (linear phase PCR): Real-time PCR data representing fold difference of spliced transcript relative to reference. n, biological replicates; error bars represent ± SD; ** represents significance level according to Student's t-test, P ≤ 0.01.
Discussion
In our Swiss cohort of 18 patients from 14 unrelated families diagnosed with childhood glaucoma, WES including CNV analysis revealed pathogenic variants in CYP1B1 in four (29%) of the families; FOXC1 pathogenic variants or CNVs were detected in three (21%) of the families. In seven families (50%), no conclusively or potentially disease-causing sequence variant was identified.
The ethnic heterogeneity of our cohort was reflected by identification of pathogenic variants in CYP1B1 previously found in Portuguese and Moroccan (p.(Ala179Argfs*18)), Portuguese and Spanish (p.(Glu387Lys)), Spanish and Turkish (p.(Thr404Serfs*30)), Turkish (p.(Arg355Hisfs*69)), and Pakistani (p.(Leu487Pro)) patients.14,15,17–20 Interestingly, the novel intronic variant c.1044-3C>G was the only pathogenic variant found on the CCGCCG haplotype, which, compared with the more common ancestral CCGGTA haplotype, is less frequently associated with pathogenic variants in CYP1B1 (Table 1).13,21 The intronic variant c.1044-3C>G was the first CYP1B1 splice site variant functionally analyzed in a cellular system. Reduction of the correctly spliced transcript by 98% compared to the reference, as observed in our minigene assays, is most likely to occur because of nonsense-mediated mRNA decay induced by stop codons in the retained intron, although this hypothesis remains untested. Because the majority of CYP1B1 pathogenic variants reported to date result in loss of function due to alterations in protein stability, abundance, or enzymatic activity, we assume that the reduced amount of correctly spliced transcript in the presence of the c.1044-3C>G variant is disease-causing.22,23
Clinical manifestations were consistent with features of PCG in all patients with pathogenic CYP1B1 variants. Several studies have attempted to compare the clinical phenotype and genotype in PCG patients with and without pathogenic variants in CYP1B1; however, results to date are inconclusive.17,24,25 More promising is the approach taken by Hollander et al.26 and García-Antón et al.,22 namely assessing the histologic angle tissue and enzyme activity. Their results suggest that CYP1B1 null alleles lead to an underdeveloped post-trabecular outflow pathway, making these patients more suitable candidates for fistulating rather than nonfistulating surgery. In keeping with this, patient 1 [II:1] (harboring two null alleles) had undergone fistulating surgeries on both eyes; however, without success in the right eye and with only qualified success in the left eye. The small sample size and variable follow-up period make a meaningful genotype-phenotype correlation between the patients with and without pathogenic CYP1B1 variants within our cohort challenging. However, concordant with findings in Saudi Arabian families, more frequent postoperative complications and lower treatment success rates were apparent in the patients with, compared to those without, pathogenic CYP1B1 variants (Table 2).24
Patients carrying FOXC1 variants showed considerable inter- and intrafamilial variability of clinical manifestations. These ranged from subepithelial haze in patients 7 [I:2, II:1], JOAG in patient 7 [II:1], iris hypoplasia and glaucoma in patients 6 [I:1, II:1], and PCG in patient 5 [II:1]. FOXC1 is a member of forkhead box family transcription factors involved in the formation of the anterior segment.27 The whole range of gene alterations has been described as causes of autosomal dominant anterior segment defects, often classified as part of the ARS-spectrum.27–30 A dosage-dependent mechanism for phenotype variability has been described previously.31,32 According to this model, variants displaying 50% to 60% or 130% to 150% of transcriptional activity result in goniodysgenesis associated with glaucoma, whereas activity levels beyond these thresholds lead to more severe anterior segment anomalies and nonocular tissue involvement (resulting in systemic features such as sensorineural hearing loss, congenital heart defects, dysmorphic features, intellectual disability, and dental and umbilical anomalies). FOXC1 duplications, presumed to have 150% of transcriptional activity, are concordant with this proposal, as carriers have previously been shown to develop glaucoma.28 Our findings are also largely consistent with this model, as FOXC1 duplication carriers (patients 6 [I:1, II:1] and 7 [I:2, II:1]) had less severe ocular phenotypes compared with patient 5 [II:1] carrying the de novo frameshift variant p.(Cys233Alafs*82), who presented with congenital onset glaucoma, congenital heart defect, and midfacial hypoplasia. The frameshift variant p.(Cys233Alafs*82) results in a protein lacking part of the inhibition domain (Fig. 3B). Such variants may reveal increased (over 150%) transcriptional activation despite reduced stability of the protein, according to Medina-Trillo et al.31 Our findings in patient 5 [II:1] confirm the association of frameshift variants located in the inhibition domain with a congenital glaucoma phenotype and additional extraocular findings.33,34 FOXC1 duplications were previously associated with iris hypoplasia and glaucoma, yet patient 7 [II:1] had a macroscopically normal iris stroma. In addition, patient 7 [II:1] showed global developmental delay and a prominent forehead, as well as a protruding lower lip, features associated with ARS but not previously described for FOXC1 duplications.28 Genetic alterations other than FOXC1 duplication cannot be excluded as a cause for the developmental delay. The absence of glaucoma and typical ocular ARS-features in the 54-year-old mother of patient 7 [II:1] is unexpected because high penetrance of ocular findings has been postulated for ARS in general, as well as for FOXC1 duplications.28,29,35 In mice, penetrance of clinical abnormalities was shown to depend on the genetic background, which may explain (among other factors) variable disease severity and penetrance in humans.36 Furthermore, the pathogenicity of duplicated FOXQ1, FOXF2, and parts of GMDS remains elusive, and their role in embryonic development should be elucidated by further investigation.37 It remains unknown whether the mother harbors additional genetic variations that somehow act as protecting factors. Furthermore, although there is a strong indication for FOXC1 duplications being pathogenic, the possibility of a linked locus harboring the actual disease-causing alteration cannot be excluded. The two families in our cohort carrying FOXC1 duplications add to cases of FOXC1-associated Axenfeld-Rieger spectrum with development of glaucoma at a young age, whereas patient 5 [II:1] with the frameshift variant may be classified as a “FOXC1-associated primary congenital glaucoma or as “ARS with PCG”.4
The prevalence of CYP1B1 pathogenic variants in our cohort was comparable to other European populations.17,19,38 Previous studies investigated the prevalence of FOXC1 pathogenic variants among childhood glaucoma patients.34,39,40 Siggs et al.34 recently analyzed a large Italian-Australian cohort of PCG patients without CYP1B1 variants, and found 6.1% of disease-causing FOXC1 variants among these patients. In our study, the respective percentage is higher (14%; one of seven PCG families without a CYP1B1 variant). In general, it needs to be acknowledged that exome sequencing cannot reliably identify all forms of genetic variation (e.g. deep intronic variants, structural variants including copy number variants in noncoding regions), and that this may explain a proportion of unsolved cases in studies like ours using WES as a diagnostic approach.41
Our findings highlight the need for routine implementation of genetic testing in affected patients in Switzerland. Although the relatively small cohort size is a limiting factor of the study, our results suggest that Sanger sequencing of the entire CYP1B1 and FOXC1 genes complemented by MLPA of FOXC1 should be performed as a first genetic diagnostic workup in patients presenting with childhood glaucoma in Switzerland, before the use of extended gene panels. In cases of dominant pedigrees only FOXC1 may be tested, whereas in sporadic cases both CYP1B1 and FOXC1 should be tested given the occurrence of de novo FOXC1 variants. However, if disease onset is after the age of four years, and additional acquired or nonacquired ocular anomalies are lacking, testing may include the JOAG gene MYOC, despite the fact that no pathogenic variants in this gene were recorded in our cohort. Further, if detailed phenotype assessment reveals distinct nonacquired ocular anomalies such as ectopia lentis, aniridia, cataract, or Peters’ anomaly, screening should be extended to additional disease-associated genes. Thus clinical diagnosis should guide genetic testing. However, it is important to emphasize that in patients carrying FOXC1 pathogenic variants a specific review of systemic features of ARS should be made. Finally, sharing of clinical and genetic data on international childhood glaucoma registries like the Childhood Glaucoma Research Network may be recommended to further accelerate research in the field of childhood glaucoma.2
Supplementary Material
Acknowledgments
The authors thank Corina Röscheisen, Rike Michels, and Sven Hirsch-Hoffmann for contributing clinical data. The study was coordinated by Alice Hakim-Brunner. Alessandro Maspoli, Fatma Kivrak-Pfiffner, and Urs Graf were responsible for patient sample administration and DNA-extraction. Additional genetic analyses were performed by Cordula Haas. James V.M. Hanson is acknowledged for editorial comments.
Supported by a grant from the Iten-Kohaut-Foundation to the University Hospital Zurich Foundation and a donation from the EMDO-Stiftung.
Disclosure: E. Lang, None; S. Koller, None; L. Bähr, None; M. Töteberg-Harms, None; D. Atac, None; F. Roulez, None; A. Bahr, None; K. Steindl, None; S. Feil, None; W. Berger, None; C. Gerth-Kahlert, None
References
- 1. Lewis CJ, Hedberg-Buenz A, DeLuca AP, Stone EM, Alward WLM, Fingert JH. Primary congenital and developmental glaucomas. Hum Mol Genet. 2017; 26: R28–R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thau A, Lloyd M, Freedman S, Beck A, Grajewski A, Levin A V. New classification system for pediatric glaucoma: implications for clinical care and a research registry. Curr Opin Ophthalmol. 2018; 29: 385–394. [DOI] [PubMed] [Google Scholar]
- 3. Gupta V, Jamieson R, Schimmenti L, Grigg J, Mackey DA. 3. Genetics. In: Weinreb RN, Grajewski A, Papadopoulos M, Grigg J, Freedman S, eds. Childhood Glaucoma. 9th ed. Amsterdam, The Netherlands: Kugler Publications; 2013: 45–52. [Google Scholar]
- 4. Traboulsi EI. Overlapping phenotypes in congenital ocular malformations and the importance of molecular testing. JAMA Ophthalmol. 2019; 137: 355–357. 10.1001/jamaophthalmol.2018.5638. [DOI] [PubMed] [Google Scholar]
- 5. Sharafieh R, Child AH, Sarfarazi M. Molecular genetics of primary congenital glaucoma. In: Traboulsi EI, ed. Genetic Diseases of the Eye. 2nd ed. New York: Oxford University Press; 2012: 295–310. [Google Scholar]
- 6. Papadopoulos M, Cable N, Rahi J, et al.. The British Infantile and Childhood Glaucoma (BIG) Eye Study. Invest Ophthalmol Vis Sci. 2007; 48: 4100–4106. [DOI] [PubMed] [Google Scholar]
- 7. Aponte EP, Diehl N, Mohney BG. Incidence and clinical characteristics of childhood glaucoma: a population-based study. Arch Ophthalmol. 2010; 128: 478–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ma AS, Grigg JR, Jamieson R V. Phenotype–genotype correlations and emerging pathways in ocular anterior segment dysgenesis. Hum Genet. 2018; 138(8–9): 899–915. [DOI] [PubMed] [Google Scholar]
- 9. Souma T, Tompson SW, Thomson BR, et al.. Angiopoietin receptor TEK mutations underlie primary congenital glaucoma with variable expressivity. J Clin Invest. 2016; 126: 2575–2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kubota R, Noda S, Wang Y, et al.. A novel myosin-like protein (myocilin) expressed in the connecting cilium of the photoreceptor: molecular cloning, tissue expression, and chromosomal mapping. Genomics. 1997; 41: 360–369. [DOI] [PubMed] [Google Scholar]
- 11. Gedde SJ, Feuer WJ, Shi W, et al.. Treatment outcomes in the primary tube versus trabeculectomy study after 1 year of follow-up. Ophthalmology. 2018; 125: 650–663. [DOI] [PubMed] [Google Scholar]
- 12. Gerth-Kahlert C, Koller S, Hanson JVM, et al.. Genotype-phenotype analysis of a novel recessive and a recurrent dominant snrnp200 variant causing retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2019; 60: 2822–2835. [DOI] [PubMed] [Google Scholar]
- 13. Stoilov IR, Costa VP, Vasconcellos JPC, et al.. Molecular genetics of primary congenital glaucoma in Brazil. Invest Ophthalmol Vis Sci. 2002; 43: 1820–1827. [PubMed] [Google Scholar]
- 14. Firasat S, Riazuddin SA, Khan SN, Riazuddin S. Novel CYP1B1 mutations in consanguineous Pakistani families with primary congenital glaucoma. Mol Vis. 2008; 14(October 2008): 2002–2009. [PMC free article] [PubMed] [Google Scholar]
- 15. Belmouden A, Melki R, Hamdani M, et al.. A novel frameshift founder mutation in the cytochrome P450 1B1 (CYP1B1) gene is associated with primary congenital glaucoma in Morocco. Clin Genet. 2002; 62: 334–339. [DOI] [PubMed] [Google Scholar]
- 16. Chanda B, Asai-Coakwell M, Ye M, et al.. A novel mechanistic spectrum underlies glaucoma-associated chromosome 6p25 copy number variation. Hum Mol Genet. 2008; 17: 3446–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cardoso MS, Anjos R, Vieira L, Ferreira C, Xavier A, Brito C. CYP1B1 gene analysis and phenotypic correlation in portuguese children with primary congenital glaucoma. Eur J Ophthalmol. 2015; 25: 474–477. [DOI] [PubMed] [Google Scholar]
- 18. Stoilov I, Akarsu AN, Alozie I, et al.. Sequence analysis and homology modeling suggest that primary congenital glaucoma on 2p21 results from mutations disrupting either the hinge region or the conserved core structures of cytochrome P4501B1. Am J Hum Genet. 1998; 62: 573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. López-Garrido MP, Medina-Trillo C, Morales-Fernandez L, et al.. Null CYP1B1 genotypes in primary congenital and nondominant juvenile glaucoma. Ophthalmology. 2013; 120: 716–723. [DOI] [PubMed] [Google Scholar]
- 20. Stoilov I, Akarsu AN, Sarfarazi M. Identification of three different truncating mutations in cytochrome P4501B1 (CYP1B1) as the principal cause of primary congenital glaucoma (Buphthalmos) in families linked to the GLC3A locus on chromosome 2p21. Hum Mol Genet. 1997; 6: 641–647. [DOI] [PubMed] [Google Scholar]
- 21. Chitsazian F, Tusi BK, Elahi E, et al.. CYP1B1 mutation profile of Iranian primary congenital glaucoma patients and associated haplotypes. J Mol Diagnostics. 2007; 9: 382–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. García-Antón MT, Salazar JJ, De Hoz R, et al.. Goniodysgenesis variability and activity of CYP1B1 genotypes in primary congenital glaucoma. PLoS One. 2017; 12(4): e0176386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chavarria-Soley G, Sticht H, Aklillu E, et al.. Mutations in CYP1B1 cause primary congenital glaucoma by reduction of either activity or abundance of the enzyme. Hum Mutat. 2008; 29: 1147–1153. [DOI] [PubMed] [Google Scholar]
- 24. Abu-Amero KK, Osman EA, Mousa A, et al.. Screening of CYP1B1 and LTBP2 genes in Saudi families with primary congenital glaucoma: genotype-phenotype correlation. Mol Vis. 2011; 17(November): 2911–2919. [PMC free article] [PubMed] [Google Scholar]
- 25. De Melo MB, Mandal AK, Tavares IM, et al.. Genotype-phenotype correlations in CYP1B1-associated primary congenital glaucoma patients representing two large cohorts from India and Brazil. PLoS One. 2015; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hollander DA, Sarfarazi M, Stoilov I, Wood IS, Fredrick DR, Alvarado JA. Genotype and phenotype correlations in congenital glaucoma: CYP1B1 mutations, goniodysgenesis, and clinical characteristics. Am J Ophthalmol. 2006; 142: 993–1004. [DOI] [PubMed] [Google Scholar]
- 27. Nishimura DY, Swiderski RE, Alward WLM, et al.. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat Genet. 1998; 19: 140–147. [DOI] [PubMed] [Google Scholar]
- 28. Lehmann OJ, Ebenezer ND, Jordan T, et al.. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am J Hum Genet. 2000; 67: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Seifi M, Walter MA. Axenfeld-Rieger syndrome. Clin Genet. 2018; 93: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 30. Genetics M. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000; 130: 107–115. [DOI] [PubMed] [Google Scholar]
- 31. Medina-Trillo C, Sánchez-Sánchez F, Aroca-Aguilar JD, et al.. Hypo- and hypermorphic FOXC1 mutations in dominant glaucoma: transactivation and phenotypic variability. PLoS One. 2015; 10: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Walter MA. PITs and FOXes in ocular genetics: the Cogan lecture. Invest Ophthalmol Vis Sci. 2003; 44: 1402–1405. [DOI] [PubMed] [Google Scholar]
- 33. Souzeau E, Siggs OM, Zhou T, et al.. Glaucoma spectrum and age-related prevalence of individuals with FOXC1 and PITX2 variants. Eur J Hum Genet. 2017; 25: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Siggs OM, Souzeau E, Pasutto F, et al.. Prevalence of FOXC1 variants in individuals with a suspected diagnosis of primary congenital glaucoma. JAMA Ophthalmol. 2019; 137: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alward WLM. Axenfeld-Rieger syndrome in the age of molecular genetics. Am J Ophthalmol. 2000; 130: 107–115. [DOI] [PubMed] [Google Scholar]
- 36. Smith RS, Zabaleta A, Kume T, et al.. Haploinsufficiency of the transcription factors FOXC1 and FOXC2 results in aberrant ocular development. Hum Mol Genet. 2000; 9: 1021–1032. [DOI] [PubMed] [Google Scholar]
- 37. de Vos IJHM, Stegmann APA, Webers CAB, Stumpel CTRM. The 6p25 deletion syndrome: An update on a rare neurocristopathy. Ophthalmic Genet. 2017; 38: 101–107, 10.3109/13816810.2016.1164191. [DOI] [PubMed] [Google Scholar]
- 38. Chouiter L, Nadifi S.. Analysis of CYP1B1 Gene Mutations in Patients with Primary Congenital Glaucoma. J Pediatr Genet. 2017; 06: 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chakrabarti S, Kaur K, Rao KN, et al.. The transcription factor gene FOXC1 exhibits a limited role in primary congenital glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 75–83. [DOI] [PubMed] [Google Scholar]
- 40. Tanwar M, Kumar M, Dada T, Sihota R, Dada R. MYOC and FOXC1 gene analysis in primary congenital glaucoma. Mol Vis. 2010; 16: 1996–2006. [PMC free article] [PubMed] [Google Scholar]
- 41. Tan R, Wang Y, Kleinstein SE, et al.. An evaluation of copy number variation detection tools from whole-exome sequencing data. Hum Mutat. 2014; 35: 899–907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.