Graphical abstract
Keywords: COVID-19, Traditional Chinese Medicine, Huoxiang Zhengqi dropping pills, Lianhua Qingwen granules, Prognosis
Abstract
Background
With the global epidemic of coronavirus disease (COVID-19), China has made progress in the prevention and control of the epidemic, and traditional Chinese medicine (TCM) has played a key role in dealing with the disease’s effects on the respiratory system. This randomized controlled clinical trial evaluated the clinical efficacy and prognosis of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in patients with COVID-19.
Methods
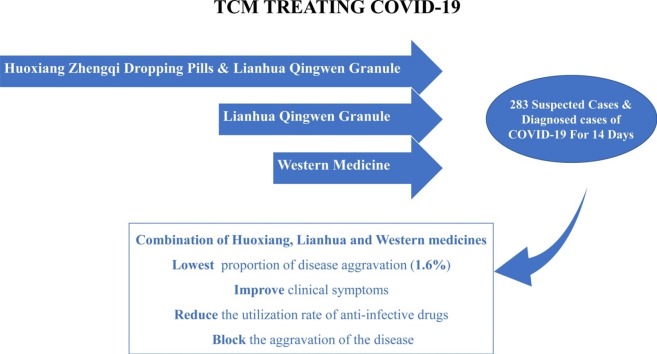
A total of 283 patients participated in this clinical trial, and participants were randomly assigned to receive either 1) Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules or 2) Linahua granules, both combined with western medicine, or 3) western medicine alone for 14 days. At the end of the trial, the improvement and resolution rates of clinical symptoms and the rate of patients who progressed to severe disease status were evaluated.
Results
After 14 days of treatment, there was no significant difference in the improvement rate of clinical symptoms among the three groups (P > 0.05). Huoxiang Zhengqi dropping pills combined with Lianhua Qingwen granules has advantages in the treatment of nausea, vomiting and limb soreness. During treatment, all participants were treated with western medicine, and there was a significant difference in the use of macrolides among the three groups (P < 0.05). Specifically, the utilization rate of antibiotics in the western medicine group was significantly greater than that of the other two groups. Among the 182 diagnosed patients who completed this clinical trial, 13 patients progressed to severe disease, including one case in the Huoxiang + Lianhua group (1.6 %), five cases in the Lianhua group (8.6 %), and seven cases in the western medicine group (11.1 %). There was no statistical differences in this rate among the three groups (P > 0.05). However, the proportion of patients who progressed to severe disease in the Huoxiang + Lianhua group was the lowest, suggesting that the combination of TCM with western medicine has a potential advantage in improving the prognosis of patients with COVID-19.
Conclusion
The use of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules combined with western medicine may have clinical advantages for COVID-19 patients in improving clinical symptoms, reducing utilization rate of anti-infective drugs, and improving patient prognosis, which could pave the way for the use of complementary medicine in treating this infection.
1. Introduction
Coronavirus disease (COVID-19) is an acute respiratory infectious disease caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [1]. As of April 4, 2020, it has spread to 206 countries, with a total of 976 249 diagnosed cases and 50 489 deaths and has been declared a global pandemic [2]. As a public health emergency of international concern [3], COVID-19 has had a serious socioeconomic impact worldwide [4]. The pathogen of COVID-19 is a novel coronavirus, which belongs to the β-coronavirus with severe acute respiratory syndrome-associated coronaviruses (Severe Acute Respiratory Syndrome-related coronavirus, SARS-CoV) [5]. The International Committee on Taxonomy of Viruses [6] announced the virus is classified as severe acute respiratory syndrome coronavirus 2 (Severe Acute Respiratory Syndrome Coronavirus-2, SARS-CoV-2). To date, the World Health Organization (WHO) has not recommended any specific vaccines or drugs for the prevention or treatment of COVID-19 [7]. Researchers are committed to developing new specialized treatments for COVID-19, but to date, they have little clinical evidence. At this stage, antiviral therapy is still the main treatment, which needs to be further optimized [8].
Measures to deal with the epidemic locally in China have obtained phased achievements, and traditional Chinese medicine (TCM) has been incorporated into COVID-19 treatment guidelines [9], playing an indispensable role in the management of the disease. At the beginning of the epidemic in China, a clinical screening study on the effective prescriptions for the prevention and treatment of COVID-19 by TCM was rapidly launched. By February 17, 2020, a total of 60 107 diagnosed cases were treated with TCM, accounting for 85.20 % of all cases. The proportion of cases who received TCM that were cured, discharged, and had symptom improvement accounted for 87 %, demonstrating an optimum clinical efficacy of TCM in the treatment of COVID-19 [10]. As such, Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules have been recommended for the treatment of COVID-19 in China. They are highly recognized for their remarkable efficacy in fighting SARS [11] and influenza [12], having significant effects on improving symptoms such as fever, cough, sputum production, and dsypnea [13,14]. Therefore, this clinical trial evaluated the effectiveness of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in the treatment of clinical symptoms and prognosis of patients with COVID-19, in order to provide new clinical evidence for the treatment of COVID-19.
2. Materials and methods
A randomized, controlled, non-blinded design was used in this study. All study participants came from the isolation treatment site in Wuhan, Hubei Province. The study was approved by the Ethics Committee of Hubei Hospital for Traditional Chinese Medicine (HBZY2020-C01-01). Written informed consent was obtained from each of the study participants. This study was registered with the China Clinical Trial Registration Center (registration number ChiCTR2000029601).
2.1. Subjects
2.1.1. Diagnostic criteria
The diagnostic criteria of COVID-19 for suspected and diagnosed cases were based on Diagnosis and Treatment Guideline for COVID-19 (Trial 7th Edition) released by the National Health Commission of the People’s Republic of China.
2.1.1.1. Suspected cases
Patients suspected of COVID-19 were identified using comprehensive analysis of the following: (1) Epidemiological history such as (a) Travel history or residence in Wuhan city and surrounding areas, or other communities where COVID-19 cases had been reported within 14 days before onset of illness; (b) Contact history with diagnosed COVID-19 patient(s) (i.e. showing positive nucleic acid test) within 14 days before onset of illness; (c) Contact history with any patient(s) who had fever or respiratory symptoms who are from Wuhan and the surrounding areas, or from communities with reported cases within 14 days before onset of COVID-19. (2) Clinical manifestations include (a) fever and/or respiratory symptoms; (b) imaging characteristics of pneumonia: multiple small patchy shadows and interstitial changes were present in the early stage, especially in the extrapulmonary bands, which then develops bilateral multiple ground-glass infiltrates ; in severe cases, lung consolidation may occur, and pleural effusion is rare; (c) the total white blood cell count is normal or decreased in early onset, the lymphocyte count is normal or decreased.
If the patient reported any one of those in the epidemiological history and meets any two of the clinical manifestations, or if there is no definite epidemiological history, but they meet all three of the clinical manifestations, the patient labeled as a suspected case of COVID-19.
2.1.1.2. Diagnosed cases
Patients were defined as a diagnosed case of COVID-19 if they met the criteria for asuspected case, and one of the following etiological criteria: (a) real-time fluorescent reverse transcription polymerase chain reaction (RT-PCR) of respiratory or blood samples for detection of novel coronavirus nucleic acids; (b) Respiratory or blood specimens are genetically sequenced and highly homologous to known new coronaviruses; (c) serum novel coronavirus-specific Immunoglobulin M (IgM) and Immunoglobulin G (IgG) antibodies are positive; (d) serum novel coronavirus-specific IgG antibodies change from negative to positive or its value in the recovery period is > 4 times than its value in the acute period.
Improvement of symptoms refers to the relief of clinical symptoms; while the progression of the disease refers to the diagnosed patients clinically worsening.
2.1.2. Inclusion and exclusion criteria
Patients who met the following criteria were included in this study: (1) suspected and diagnosed cases of COVID-19 meeting the diagnostic criteria mentioned previously; (2) 18–85 years old, regardless of sex; (3) provided informed consent.
Patients who met any one of the following conditions were excluded from the study: (1) clear evidence of bacterial infection; (2) severe primary diseases, such as heart, kidney, lung, endocrine, blood, metabolism, or gastrointestinal tract diseases, which may affect the patient's participation in the trial or affect the outcome of the study; (3) family history of mental illness or previous mental illness; (4) allergies or multiple drug allergies; (5) pregnant or lactating women.
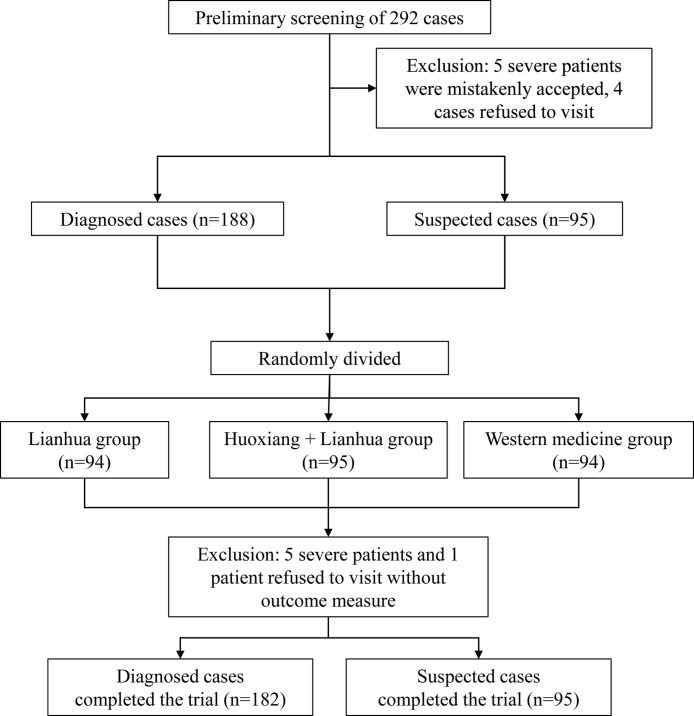
In this study, a total of 292 patients were recruited from the isolation treatment site in Wuhan, Hubei Province from February 5–10, 2020. Aside from the criteria above, patients who met the following criteria were also excluded in the final data analysis: (1) those who did not meet the diagnosis and inclusion criteria; (2) those who were lost to follow up without any available data during observation; and (3) those who could not adhere to the prescribed treatment and did not complete observation period for any reason. Among the recruited patients, 5patients with severe illness were mistakenly initially accepted into the study and 4 patients were excluded because of refusal to cooperate. A total of 283 subjects who met the inclusion criteria entered this clinical trial. All patients were randomly divided into three treatment groups: Huoxiang + Lianhua group, Lianhua group, and western medicine group at a ratio of 1:1:1 (Fig. 1 ).
Fig. 1.
Flowchart of screening, randomization and treatment.
2.2. Study medication
Huo Xiang Zhengqi dropping pills (Chinese medicine Z20000048, Tianjin Tasly Pharmaceutical Group Co., Ltd, specification: 2.6 g in each bag) are film-coated drop pills, which are yellowish-brown to brown in color once their coating is removed, fragrant, pungent, and slightly sweet and bitter to taste. Each pill is composed of Pogostemon cablin (Blanco) Benth, Atractylodes lancea (Thunb.) DC., Magnolia officinalis Cortex, Angelicae dahurica Radix, Poria cocos (Schw.) Wolf, Areca catechu L., Pinellia ternate (Thunb.) Breit., Glycyrrhizae Radix et Rhizoma, Perilla frutescens, and Citrus reticulata. The drug quality standards complied with the provisions of part I of the 2015 edition of the Chinese Pharmacopoeia.
Lianhua Qingwen granules (Chinese medicine Z20100040, Beijing Yiling Pharmaceutical Co., Ltd., specification: 6 g per bag) is made up of brown granules, with a mild fragrance and bitter taste. It is composed of Forsythia suspensa (Thunb.) Vahl, Ephedra sinica Stapf, Lonicera japonica Thunb., Isatis indigotica Fortune, Mentha haplocalyx Briq., Dryopteris crassirhizoma Nakai, Rhodiola rosea L., Gypsum Fibrosum, Pogostemon cablin (Blanco) Benth., Rheum palmatum L., Houttuynia cordata Thunb., and Glycyrrhiza uralensis Fisch. Armeniaca sibirica (L.) Lam.
The drug quality standards for both medicines were complied with the provisions of Part I of the 2015 Edition of the Chinese Pharmacopoeia.
2.3. Intervention and efficacy evaluation
Random numbers were generated by SAS statistical analysis software (version 9.2), and patients were grouped using a block random method. Data analysis was completed independently by professional statisticians. All study participants received the western medicine treatment plan recommended by the novel coronavirus's pneumonia diagnosis and treatment program (Seventh Edition) jointly issued by the State Health Commission and the State Administration of Traditional Chinese Medicine. The specific recommendations were as follows: 1) Antiviral treatment with oral oseltamivir (75 mg per tablet) one tablet, once a day; Arbidol (100 mg per tablet) taken orally, two tablets three times a day; Ribavirin (100 mg per tablet) taken orally, one and a half tablets three times a day; 2) Antimicrobial therapy: strengthen bacteriological monitoring and use antibiotics when there is evidence of a secondary bacterial infection using oral penicillins, cephalosporins, ofloxacin, and macrolide etc.
In addition to western medicine, participants in Huoxiang + Lianhua group were treated with Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules. Participants took one bag of Huoxiang Zhengqi dropping pills twice a day and one bag of Lianhua Qingwen granules three times a day. Subjects in Lianhua only group were treated with Lianhua Qingwen granules and western medicine, taking one bag of Lianhua Qingwen three times a day. Finally, participants in the western medicine group were only treated with western medicine and were not treated with TCM.
All subjects underwent symptom assessment and body temperature and vital signs monitoring (blood pressure, heart and respiration rates). Medication compliance was recorded, as well as adverse events, before and after 14 days of treatment. The main outcome measures were clinical symptom improvement and disappearance rates after 14 days of treatment, and the secondary outcome measure was the proportion of patients who progressed to severe status despite the same duration of treatment.
2.4. Safety monitoring
According to a clinical trial making use of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in the early treatment of influenza and other diseases, it was observed that their use was safe in these clinical populations. Therefore, safety-wise, the overall risk level of this study was low. As previously mentioned, all medications and adverse events were recorded in this study, taking note of their severity, occurrence, and remission time.
2.5. Statistical analysis
FAS sets were used for statistical analysis. Data entry was carried out using SAS software (version 9.2), which was done independently by four staff members, twice. Analysis of variance was used for the comparison of measurement data between groups, and chi-square test was used for the comparison of grade data between groups. When P < 0.05, the results were statistically significant.
3. Results
3.1. Subject characteristics
In this study, 292 patients participated in the preliminary screening, of which 5 patients with severe illness were mistakenly accepted and 4 patients were excluded because of refusal to visit. A total of 283 subjects who met the inclusion criteria were enrolled in this clinical trial, including 188 diagnosed COVID-19 patients and 95 patients suspected to have COVID-19. All patients were randomly divided into three groups with a ratio of 1:1:1: Huoxiang + Lianhua group (n = 95), Lianhua group (n = 94), and western medicine group (n = 94). At the end of the trial, a further 5 patients were with severe disease were excluded and 1 patient refused to complete their data collection visit. A total of 182 diagnosed patients completed this study.
General data of diagnosed and suspected patients are presented in Table 1 , and the data of diagnosed subjects are presented in Table 2 . Participants’ past medical history were recorded, including hypertension, diabetes, hyperlipidemia, coronary heart disease, chronic obstructive pulmonary disease (COPD), and bronchial asthma. Primary initial symptoms included fever, fatigue, cough, and diarrhea. There was no significant difference in the baseline data of sex, age, height, weight, past medical history, initial symptoms, disease diagnosis, and medication between the patients diagnosed with and suspected to have COVID-19 among the three treatment groups (P > 0.05).
Table 1.
Comparisons of characteristics in diagnosed and suspected patients.
| Term | LHQW (N = 94) | LHQW + HXZQ (N = 95) | WM (N = 94) | P-value |
|---|---|---|---|---|
| Male (%) | 58 (61.7) | 47 (49.5) | 50 (53.2) | 0.224 |
| Age ( ± s) | 54.58 ± 13.76 | 54.31 ± 11.63 | 54.06 ± 13.90 | 0.964 |
| Height [cm, ( ± s)] | 168.61 ± 7.05 | 167.73 ± 7.24 | 167.12 ± 7.56 | 0.372 |
| Weight [kg, ( ± s)] | 66.48 ± 8.70 | 64.65 ± 9.12 | 64.90 ± 11.39 | 0.382 |
| Smoking (%) | 13 (13.8) | 13 (13.7) | 9 (9.6) | 0.602 |
| Past medical history (%) | 38 (40.4) | 38 (40.0) | 35 (37.2) | 0.888 |
| Bronchial asthma | 1 (1.1) | 2 (2.1) | 0 (0.0) | 0.775 |
| Chronic obstructive pulmonary disease | 1 (1.1) | 2 (2.1) | 1 (1.1) | 1.000 |
| Coronary artery disease | 4 (4.3) | 2 (2.1) | 3 (3.2) | 0.649 |
| High blood pressure | 21 (22.3) | 17 (17.9) | 16 (17.0) | 0.609 |
| Diabetes | 5 (5.3) | 9 (9.5) | 7 (7.4) | 0.552 |
| Hyperlipidemia | 6 (6.4) | 7 (7.4) | 5 (5.3) | 0.847 |
| Others | 13 (13.8) | 14 (14.7) | 14 (14.9) | 0.975 |
| Initial symptoms (%) | 86 (91.5) | 91 (95.8) | 80 (85.1) | 0.038 |
| Fever | 69 (73.4) | 73 (76.8) | 65 (69.1) | 0.490 |
| Cough | 36 (38.3) | 38 (40.0) | 36 (38.3) | 0.969 |
| Diarrhea | 4 (4.3) | 2 (2.1) | 4 (4.3) | 0.670 |
| Lack of strength | 35 (37.2) | 27 (28.4) | 31 (33.0) | 0.435 |
| Chest tightness and shortness of breath | 5 (5.3) | 13 (13.7) | 6 (6.4) | 0.082 |
| Disease diagnosis (%) | 0.748 | |||
| Diagnosed case | 58 (61.7) | 61 (64.2) | 63 (67.0) | – |
| Suspected case | 36 (38.3) | 34 (35.8) | 31 (33.0) | – |
Table 2.
Comparisons of characteristics in diagnosed patients.
| Term | LHQW (N = 58) | LHQW + HXZQ (N = 61) | WM (N = 63) | P-value |
|---|---|---|---|---|
| Male (% | 35 (60.3) | 33 (54.1) | 35 (55.6) | 0.773 |
| Age ( ± s) | 52.86 ± 13.95 | 56.07 ± 12.10 | 53.90 ± 13.92 | 0.411 |
| Height [cm, ( ± s)] | 168.64 ± 6.57 | 167.79 ± 7.42 | 167.10 ± 7.85 | 0.512 |
| Weight [kg, ( ± s)] | 66.37 ± 9.62 | 64.88 ± 8.94 | 66.46 ± 12.34 | 0.643 |
| Smoking (%) | 10 (17.2) | 9 (14.8) | 8 (12.7) | 0.781 |
| Past medical history (%) | 24 (41.4) | 22 (36.1) | 25 (39.7) | 0.831 |
| Bronchial asthma | 1 (1.7) | 2 (3.3) | 0 (0.0) | 0.423 |
| Chronic obstructive pulmonary disease | 1 (1.7) | 1 (1.6) | 1 (1.6) | 1.000 |
| Coronary artery disease | 2 (3.4) | 1 (1.6) | 3 (4.8) | 0.694 |
| High blood pressure | 12 (20.7) | 10 (16.4) | 11 (17.5) | 0.819 |
| Diabetes | 1 (1.7) | 3 (4.9) | 6 (9.5) | 0.164 |
| Hyperlipidemia | 3 (5.2) | 4 (6.6) | 4 (6.3) | 1.000 |
| Others | 9 (15.5) | 8 (13.1) | 11 (17.5) | 0.798 |
| Initial symptoms (%) | 51 (87.9) | 58 (95.1) | 53 (84.1) | 0.142 |
| Fever | 43 (74.1) | 46 (75.4) | 43 (68.3) | 0.635 |
| Cough | 19 (32.8) | 24 (39.3) | 25 (39.7) | 0.680 |
| Diarrhoea | 1 (1.7) | 1 (1.6) | 3 (4.8) | 0.622 |
| Lack of strength | 23 (39.7) | 18 (29.5) | 19 (30.2) | 0.421 |
| Chest tightness and shortness of breath | 2 (3.4) | 5 (8.2) | 5 (7.9) | 0.564 |
| Disease diagnosis (%) | ||||
| Diagnosed case | 58 (100.0) | 61 (100.0) | 63 (100.0 %) | – |
| Suspected case | – | – | – |
3.2. Symptom improvements
Improvement in clinical symptoms of patients diagnosed with and suspected to have COVID-19 is presented in Table 3 . There was no significant difference in the improvement rate of clinical symptoms among the three groups before and after treatment (P > 0.05). All treatment schemes were found to improve patients' fever and diarrhea. Treatment utilizing Huoxiang Zhengqi dropping pills combined with Lianhua Qingwen granules had an advantage in the treatment of nausea, vomiting, and limb soreness.
Table 3.
Improvement of clinical symptoms in diagnosed and suspected patients.
| Term | LHQW (N = 94) |
LHQW + HXZQ (N = 95) |
WM (N = 94) |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Day7 Improvement rate | Day14 Improvement rate |
Number | Day7 Improvement rate | Day14 Improvement rate |
Number | Day7 Improvement rate | Day14 Improvement rate |
Day7 | Day14 | |
| Fever | 29 | 29 (100.0) | 29 (100.0) | 32 | 32 (100.0) | 32 (100.0) | 28 | 28 (100.0) | 28 (100.0) | – | – |
| Diarrhoea | 10 | 8 (80.0) | 10 (100.0) | 14 | 11 (78.6) | 14 (100.0) | 14 | 13 (92.9) | 14 (100.0) | 0.625 | – |
| Nausea and vomiting | 18 | 15 (83.3) | 17 (94.4) | 16 | 15 (93.8) | 16 (100.0) | 11 | 9 (81.8) | 11 (100.0) | 0.628 | 1.000 |
| Sore limbs | 22 | 20 (90.9) | 20 (90.9) | 19 | 16 (84.2) | 18 (94.7) | 20 | 15 (75.0) | 18 (90.0) | 0.424 | 1.000 |
| Loss of appetite | 35 | 25 (71.4) | 34 (97.1) | 45 | 36 (80.0) | 41 (91.1) | 29 | 23 (79.3) | 26 (89.7) | 0.628 | 0.420 |
| Tired | 53 | 40 (75.5) | 45 (84.9) | 50 | 38 (76.0) | 45 (90.0) | 50 | 35 (70.0) | 41 (82.0) | 0.750 | 0.513 |
| Chest tightness and shortness of breath | 47 | 32 (68.1) | 37 (78.7) | 44 | 30 (68.2) | 38 (86.4) | 34 | 18 (52.9) | 28 (82.4) | 0.290 | 0.633 |
| Cough | 52 | 32 (61.5) | 43 (82.7) | 49 | 31 (63.3) | 35 (71.4) | 54 | 31 (57.4) | 47 (87.0) | 0.821 | 0.121 |
Table 4 presents the improvement in clinical symptoms of diagnosed patients alone. There was no significant difference in the improvement rate of clinical symptoms among the three groups before and after treatment (P > 0.05). All treatment schemes could improve these patients' fever and diarrhea. Treatment utilizing Huoxiang Zhengqi dropping pills combined with Lianhua Qingwen granules had an advantage in the treatment of anorexia, limb pain, fatigue, chest tightness, and shortness of breath over western medicine alone.
Table 4.
Improvement of clinical symptoms in diagnosed patients.
| Term | LHQW (N = 58) |
LHQW + HXZQ (N = 61) |
WM (N = 63) |
P-value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Day7 Improvement rate | Day14 Improvement rate |
Number | Day7 Improvement rate | Day14 Improvement rate |
Number | Day7 Improvement rate | Day14 Improvement rate |
Day7 | Day14 | |
| Loss of appetite | 21 | 16 (76.2) | 20 (95.2) | 27 | 25 (92.6) | 27 (100.0) | 17 | 15 (88.2) | 15 (88.2) | 0.254 | 0.111 |
| Fever | 24 | 24 (100.0) | 24 (100.0) | 25 | 25 (100.0) | 25 (100.0) | 18 | 18 (100.0) | 18 (100.0) | – | – |
| Nausea and vomiting | 15 | 12 (80.0) | 14 (93.3) | 13 | 12 (92.3) | 13 (100.0) | 6 | 6 (100.0) | 6 (100.0) | 0.495 | 1.000 |
| Diarrhoea | 8 | 7 (87.5) | 8 (100.0) | 11 | 9 (81.8) | 11 (100.0) | 8 | 7 (87.5) | 8 (100.0) | 1.000 | – |
| Sore limbs | 20 | 18 (90.0) | 18 (90.0) | 11 | 8 (72.7) | 11 (100.0) | 14 | 10 (71.4) | 12 (85.7) | 0.320 | 0.669 |
| Tired | 32 | 24 (75.0) | 27 (84.4) | 30 | 22 (73.3) | 27 (90.0) | 30 | 22 (73.3) | 24 (80.0) | 0.985 | 0.587 |
| Chest tightness and shortness of breath | 33 | 22 (66.7) | 25 (75.8) | 26 | 18 (69.2) | 22 (84.6) | 23 | 13 (56.5) | 18 (78.3) | 0.618 | 0.700 |
| Cough | 32 | 20 (62.5) | 25 (78.1) | 32 | 21 (65.6) | 25 (78.1) | 34 | 20 (58.8) | 31 (91.2) | 0.850 | 0.267 |
3.3. Medication after treatment
The use of western medicines in diagnosed and suspected patients after treatment is shown in Table 5 . Both diagnosed and suspected patients were treated with antiviral drugs. There was a significant difference in the use of macrolides among the three treatment groups (P < 0.05). The utilization rate of anti-infective drugs in the western medicine group was significantly higher than the other two groups. The medication use of diagnosed patients after treatment is presented in Table 6 . All diagnosed patients were treated with antiviral drugs. In the use of anti-infective drugs, there is a significant difference in the use of macrolide antibiotics among the three groups (P < 0.05). The utilization rate of anti-infective drugs in the western medicine group was significantly higher than the other two groups.
Table 5.
Medication status of diagnosed and suspected patients after treatment.
| Medication after treatment | LHQW (N = 94) | LHQW + HXZQ (N = 95) | WM (N = 94) | P-value |
|---|---|---|---|---|
| Antiviral drugs | 94 (100.0) | 95 (100.0) | 94 (100.0) | – |
| Oseltamivir | 76 (80.9) | 78 (82.1) | 93 (98.9) | <.001 |
| Arbidol | 29 (30.9) | 28 (29.5) | 10 (10.6) | <.001 |
| Others | 1 (1.1) | 0 (0.0) | 0 (0.0) | – |
| Antibiotic drugs | 42 (44.7) | 34 (35.8) | 93 (98.9) | <.001 |
| Macrolide antibiotics | 22 (23.4) | 21 (22.1) | 77 (81.9) | <.001 |
| Ofloxacin | 14 (14.9) | 14 (14.7) | 17 (18.1) | 0.778 |
| Cephalosporins | 3 (3.2) | 3 (3.2) | 4 (4.3) | 0.926 |
| Penicillins | 3 (3.2) | 1 (1.1) | 1 (1.1) | 0.541 |
Table 6.
Medication status of diagnosed patients after treatment.
| Medication after treatment | LHQW (N = 58) | LHQW + HXZQ (N = 61) | WM (N = 63) | P-value |
|---|---|---|---|---|
| Antiviral drugs | 58 (100.0) | 61 (100.0) | 63 (100.0) | – |
| Oseltamivir | 49 (84.5) | 53 (86.9) | 62 (98.4) | 0.022 |
| Arbidol | 19 (32.8) | 19 (31.1) | 8 (12.7) | 0.016 |
| Others | 1 (1.7) | 0 (0.0) | 0 (0.0) | – |
| Anti-infective drug | 30 (51.7) | 25 (41.0) | 62 (98.4) | <.001 |
| Macrolide antibiotics | 14 (24.1) | 15 (24.6) | 50 (79.4) | <.001 |
| Ofloxacin | 11 (19.0) | 9 (14.8) | 13 (20.6) | 0.683 |
| Cephalosporins | 2 (3.4) | 1 (1.6) | 3 (4.8) | 0.694 |
| Penicillins | 3 (5.2) | 1 (1.6) | 1 (1.6) | 0.449 |
3.4. Prognosis
The time spent taking medication and prognosis of the 182 diagnosed patients who completed the trial are presented in Table 7 . Time spent taking medication ranged from 4 to 14 days. There were 13 patients who progressed to a severe status, including 1 case in Huoxiang + Lianhua group (1.6 %), 5 cases in the Lianhua group (8.6 %), and 7 cases in western medicine group (11.1 %). There was no significant statistical difference in the proportion of severe disease status among the three groups (P > 0.05). While not reaching statistical significance, the proportion of patients who progressed to severe status is lowest in the Huoxiang + Lianhua group, while that in western medicine group is relatively high, suggesting that the combination of TCM has a potential advantage in improving prognosis of patients with COVID-19.
Table 7.
Prognosis of diagnosed patients who completed the trial.
| Term | LHQW (N = 58) | LHQW + HXZQ (N = 61) | WM (N = 63) | P-value |
|---|---|---|---|---|
| Medication time [day, ( ± s)] | 12.47 ± 3.16 | 12.79 ± 2.94 | 13.14 ± 2.54 | 0.834 |
| Aggravation of disease [number(%)] | 5 (8.6) | 1 (1.6) | 7 (11.1) | 0.089 |
| Rate difference and bilateral 95 %CI (LHQW - LHQW + HXZQ) |
6.981 % (-0.914 %, 14.876 %) | |||
| Rate difference and bilateral 95 %CI (LHQW - WM) |
−2.490% (-13.092 %, 8.111 %) | |||
| Rate difference and bilateral 95 %CI (LHQW + HXZQ - WM) |
−9.472% (-17.861 %, -1.083 %) |
4. Discussion
For patients with COVID-19, fever, cough, fatigue, diarrhea, chest tightness, and shortness of breath are common initial symptoms, and could be classified as a "cold-dampness epidemic" in TCM theory. The prescription of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules was based on the experience of TCM in the prevention and treatment of infectious disease. The study provides clinical reference for relieving clinical symptoms, improving prognosis, and reducing the utilization rate of anti-infective drugs in COVID-19 using TCM.
All of the treatment schemes in this study had therapeutic effects on fever, fatigue, and cough in patients with diagnosed and suspected COVID-19. Although there was no statistical significance among the three groups, the subjects in Huoxiang + Lianhua group have the highest improvement and resolution rates of clinical symptoms, especially for fatigue, nausea and vomiting, chest tightness, shortness of breath, and limb soreness. In TCM theory, cold-dampness toxin caused the epidemic, which is most likely to trap the spleen and cause spleen-yang stagnation, developing into symptoms such as fatigue, nausea and vomiting, chest tightness, shortness of breath, and sore limbs. Huo Xiang Zhengqi dropping pills could dissolve dampness and regulate the organism, while Lianhua Qingwen granules could clear plague and induce detoxification. The combination of both medications could clear the cold dampness and improve clinical symptoms. Clinical trials have demonstrated that Lianhua Qingwen granules has a good clinical effect on reducing fever and relieving symptoms such as cough, sore throat, body pain and fatigue in the treatment of type A H1N1 influenza [15]. Furthermore, Huo Xiang Zhengqi dropping pills also play an important role in the treatment of acute respiratory infectious diseases caused by influenza virus such as fever, muscle soreness, and fatigue among other symptoms [16]. The results of this study also demonstrated that, with the lack of specific antiviral drugs for COVID-19, therapeutic characteristics and rational use of TCM have an important clinical value for the prevention and control of respiratory symptoms caused by the virus, confirming the scientific nature of the "cold-damp epidemic" theory and the potential of Lianhua Qingwen granules and Huoxiang Zhengqi dropping pills as treatment.
Lianhua Qingwen granules has been widely involved and highly recognized in the fight against SARS and influenza in China. Previous pharmacodynamic studies have confirmed that Lianhua Qingwen granules can significantly inhibit the SARS-CoV activity cultured in vitro [17]. A recent study by the State Key Laboratory of Respiratory Diseases of the First Affiliated Hospital of Guangzhou Medical University found that Lianhua Qingwen granules could significantly stall the activity of a novel coronavirus, reducing the content of the virus in the cell membrane and cytoplasm and inhibiting excessive activation of cytokines [[18], [19], [20]]. At the same time, Huoxiang Zhengqi dropping pills have also achieved initial results in the battle against this epidemic [22,23]. For instance, application of Huoxiang Zhengqi dropping pills in treating SARS alleviated the inflammatory response and injury to the lung [22], and Huoxiang Zhengqi caspules reduced the release of inflammatory cytokines stimulated by lipopolysaccharides [23]. One of the main components of Huoxiang Zhengqi, Radix Isatidis, has the following effects: anti-inflammation, anti-viral, antipyretic, and immunity-enhancing. Its antiviral effect is due to competitive adsorption with the virus on the cell surface, preventing invasion of normal, healthy cells. In addition, Herba Houttuyniae can block Madin-Darby Canine Kidney cells (MDCK) apoptosis induced by the influenza virus. Finally, patchouli ketone and patchouli alcohol in patchouli have an obvious inhibitory effect on adeno-, influenza A, and coxsackieviruses [24,25].
This study demonstrated no significant difference in the improvement of clinical symptoms among the three treatment groups. However, utilization rate of anti-infective drugs and the proportion of patients who progressed to severe status was significantly higher in the western medicine group than the two groups treated with TCM. While this may suggest that the combination of TCM treatment had no significant advantage in the improvement of clinical symptoms in a short amount of time, TCM could reduce the utilization rate of anti-infective drugs. Furthermore, TCM has potential advantages in preventing aggravation while improving prognosis of the disease.
This study has some limitations. There was a lack of viral nucleic acid detection data support, and a larger sample size and regular follow-up intervention for in-depth verification is required.
5. Conclusion
Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules combined with western medicine treatment has potential clinical advantages for COVID-19 patients with improving clinical symptoms, not just by reducing the utilization rate of anti-infective drugs, but also by improving the prognosis of patients. This finding could set the precedence for complementary medicine treatment in COVID-19.
Funding
This work was funded by the Special Project for Emergency of the Ministry of Science and Technology (2020YFC0845000) and the Traditional Chinese Medicine Special Project for COVID-19 Emergency of National Administration of Traditional Chinese Medicine (2020ZYLCYJ04-1, 2020ZYLCYJ04-3).
Declaration of Competing Interest
The authors report no declarations of interest. TASLY Holding Group and Yiling Pharmaceutical Co., Ltd. provided the medications for the study, while they did not participate in research design, data collection, data analysis, data interpretation, nor article writing.
References
- 1.Beijing News Network . 2020. World Health Organization (WHO) Declared Novel Coronavirus’s Epidemic a Global Pandemic, and Guterres Called for Action.http://www.bjnews.com.cn/world/2020/03/12/702593.html [Google Scholar]
- 2.World Health Organization . 2020. Coronavirus Disease (COVID-19) Situation Dashboard.https://experience.arcgis.com/experience/62c28590b5ae41ef920e4d5a4128504a [Google Scholar]
- 3.World Health Organization . 2020. Statement on the Second Meeting of the International Health Regulations (2005) Emergency Committee Regarding the Outbreak of Novel Coronavirus (2019-nCoV)https://www.who.int/zh/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov) [Google Scholar]
- 4.World Health Organization . 2020. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19 - 3 April 2020.https://www.who.int/zh/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19--3-april-2020 [Google Scholar]
- 5.Hou N.N., Chen Y., Ren J.S., Li W.J., Li H., Luo X.H. The origin and detection method of novel coronavirus (SARS-CoV-2) Drug Eval. Res. 2020;43(04):620–623. [Google Scholar]
- 6.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization . 2020. Q&A On Coronaviruses.https://www.who.int/news-room/q-a-detail/q-a-coronaviruses (Accessed 04-04 2020) [Google Scholar]
- 8.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg. Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of the People’s Republic of China . 2020. Guideline on Diagnosis and Treatment of COVID-19 (Trial 6th Edition) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of the People’s Republic of China . 2020. Transcript of the Press Conference on February 17, 2020.http://www.nhc.gov.cn/xcs/fkdt/202002/f12a62d10c2a48c6895cedf2faea6e1f.shtml [Google Scholar]
- 11.Tong X., Li A., Zhang Z., Duan J., Chen X., Hua C., Zhao D., Xu Y., Shi X., Li P., Tian X., Lin F., Cao Y., Jin L., Chang M., Wang Y. TCM treatment of infectious atypical pneumonia--a report of 16 cases. J. Tradit. Chin. Med. 2004;24(4):266–269. [PubMed] [Google Scholar]
- 12.Wang L., Zhang R.M., Liu G.Y., Wei B.L., Wang Y., Cai H.Y., Li F.S., Xu Y.L., Zheng S.P., Wang G. Chinese herbs in treatment of influenza: a randomized, double-blind, placebo-controlled trial. Respir. Med. 2010;104(9):1362–1369. doi: 10.1016/j.rmed.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C., Cao B., Liu Q.Q., Zou Z.Q., Liang Z.A., Gu L., Dong J.P., Liang L.R., Li X.W., Hu K., He X.S., Sun Y.H., An Y., Yang T., Cao Z.X., Guo Y.M., Wen X.M., Wang Y.G., Liu Y.L., Jiang L.D. Oseltamivir compared with the Chinese traditional therapy maxingshigan-yinqiaosan in the treatment of H1N1 influenza: a randomized trial. Ann. Intern. Med. 2011;155(4):217–225. doi: 10.7326/0003-4819-155-4-201108160-00005. [DOI] [PubMed] [Google Scholar]
- 14.Luo H., Tang Q.L., Shang Y.X., Liang S.B., Yang M., Robinson N., Liu J.P. Can chinese medicine Be used for prevention of corona virus disease 2019 (COVID-19)? A review of historical classics, research evidence and current prevention programs. Chin. J. Integr. Med. 2020;26(4):243–250. doi: 10.1007/s11655-020-3192-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao P., Yang H.Z., Lv H.Y., Wei Z.M. Efficacy of Lianhuaqingwen capsule compared with oseltamivir for influenza A virus infection: a meta-analysis of randomized, controlled trials. Altern. Ther. Health Med. 2014;20(2):25–30. [PubMed] [Google Scholar]
- 16.Han X.P. Efficacy of oseltamivir phosphate in the treatment of influenza with Huoxiang Zhengqi liquid. Chin. J. Modern Drug Appl. 2016;10(18):139–141. [Google Scholar]
- 17.Zhu S.Y., Li X.Y., Wei Y.L., Yang P.Y., Qin E.D. Preliminary study on the inhibitory effect of three traditional Chinese medicine prescriptions on SARS-associated coronavirus in vitro. Lett. Biotechnol. 2003;(05):390–392. [Google Scholar]
- 18.Jia Z.H., Li H.R., Chang L.P., Wei C. Historical review and reflection on the response of traditional Chinese Medicine to epidemic Diseases. Chin. J. Exp. Tradit. Med. Formul. 2020;26(11):1–7. [Google Scholar]
- 19.Ding Y., Zeng L., Li R., Chen Q., Zhou B., Chen Q., Cheng P.L., Yutao W., Zheng J., Yang Z., Zhang F. The Chinese prescription lianhuaqingwen capsule exerts anti-influenza activity through the inhibition of viral propagation and impacts immune function. BMC Complement. Altern. Med. 2017;17(1):130. doi: 10.1186/s12906-017-1585-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Runfeng L., Yunlong H., Jicheng H., Weiqi P., Qinhai M., Yongxia S., Chufang L., Jin Z., Zhenhua J., Haiming J., Kui Z., Shuxiang H., Jun D., Xiaobo L., Xiaotao H., Lin W., Nanshan Z., Zifeng Y. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol. Res. 2020;156 doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H., Lu C.Z., Tang K.C. Clinical observation on treatment of SARS with combination of chaihu droplet pill and huoxiang zhengqi droplet pill. Chin. J. Integr. Tradit. Western Med. 2004;24(4):321–324. [PubMed] [Google Scholar]
- 23.Wang H.M., Lv Y.Z., Liu L.N., Zhou J.M., Xu Z.L., Zhou J., Wang Z.Z., Xiao W. Different formulas of Jiawei Huoxiang Zhengqi Soft Capsule on expression of inflammatory factors induced by lipopolysaccharide in primary murine bone marrow macrophages. Modern. Tradit. Chin. Med. Mater. Med.-World Sci. Technol. 2016;18(03):476–481. [Google Scholar]
- 24.Li H.R., Chang L.P., Wei C., Jia Z.H. Theoretical research basis and clinical efficacy of Lianhua Qingwen in the treatment of COVID-19. World Chin. Med. 2020;15(03):332–336. [Google Scholar]
- 25.Xiong W., Ran J.Y., Xie X.J., Xia Y.H., Lan B., Wang M.D., Shi C.Y., Fang J.G., Wang W.Q. Pharmacological effects and clinical application of proprietary Chinese medicine in the treatment of COVID-19. Herald Med. 2020;39(04):465–476. [Google Scholar]